Refine search
Actions for selected content:
106116 results in Materials Science
JMR volume 34 issue 18 Cover and Back matter
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 18 / 30 September 2019
- Published online by Cambridge University Press:
- 27 September 2019, pp. b1-b3
- Print publication:
- 30 September 2019
-
- Article
-
- You have access
- Export citation
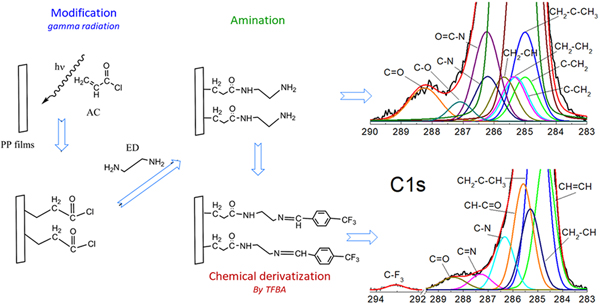
Amine modifications of polypropylene films by gamma radiation to be applied in cell cultures
-
- Journal:
- MRS Communications / Volume 9 / Issue 4 / December 2019
- Published online by Cambridge University Press:
- 26 September 2019, pp. 1323-1330
- Print publication:
- December 2019
-
- Article
- Export citation
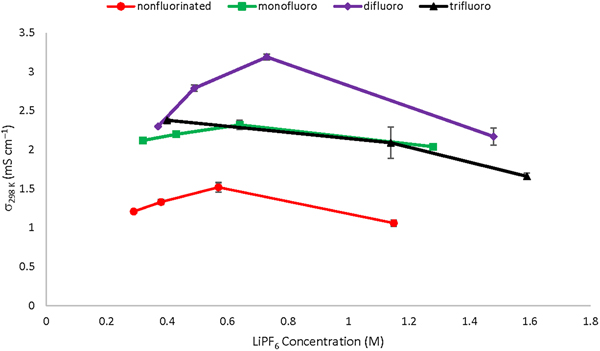
Enhancing ionic conductivity with fluorination in organosilyl solvents for lithium-ion battery electrolytes
-
- Journal:
- MRS Communications / Volume 9 / Issue 4 / December 2019
- Published online by Cambridge University Press:
- 26 September 2019, pp. 1200-1205
- Print publication:
- December 2019
-
- Article
- Export citation
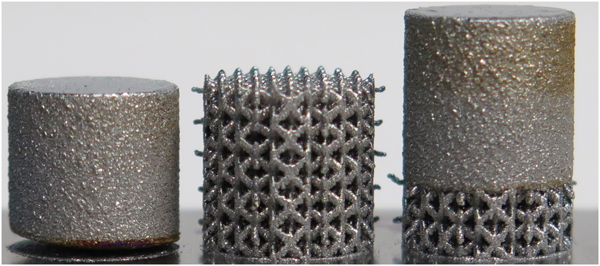
Scalable laser powder bed fusion processing of nitinol shape memory alloy
-
- Journal:
- MRS Communications / Volume 9 / Issue 4 / December 2019
- Published online by Cambridge University Press:
- 26 September 2019, pp. 1214-1220
- Print publication:
- December 2019
-
- Article
- Export citation
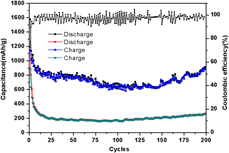
In situ hydrothermal synthesis of rGO-wrapped Fe1−xS particles for lithium storage
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 18 / 30 September 2019
- Published online by Cambridge University Press:
- 23 September 2019, pp. 3186-3194
- Print publication:
- 30 September 2019
-
- Article
- Export citation
Structural and optical properties of Ba3(Nb6−xTax)Si4O26 (x = 0.6, 1.8, 3.0, 4.2, 5.4)
-
- Journal:
- Powder Diffraction / Volume 34 / Issue 4 / December 2019
- Published online by Cambridge University Press:
- 23 September 2019, pp. 331-338
-
- Article
- Export citation
Immiscibility between two rutile phases in the GeO2–TiO2 system and application as a temperature sensor in high-pressure experiments
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 19 / 14 October 2019
- Published online by Cambridge University Press:
- 20 September 2019, pp. 3368-3376
- Print publication:
- 14 October 2019
-
- Article
- Export citation
Crystal structure of bisoprolol fumarate Form I, (C18H32NO4) (C4H2O4)0.5
-
- Journal:
- Powder Diffraction / Volume 35 / Issue 1 / March 2020
- Published online by Cambridge University Press:
- 20 September 2019, pp. 34-40
-
- Article
- Export citation
Detection of low-level humic acid in water using room temperature-synthesized copper (I) oxide colloids
-
- Journal:
- MRS Communications / Volume 9 / Issue 4 / December 2019
- Published online by Cambridge University Press:
- 20 September 2019, pp. 1317-1322
- Print publication:
- December 2019
-
- Article
- Export citation
A comparative analysis of sound absorption performance of ZL104/aluminum fiber composite foam
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 21 / 14 November 2019
- Published online by Cambridge University Press:
- 20 September 2019, pp. 3717-3724
- Print publication:
- 14 November 2019
-
- Article
- Export citation
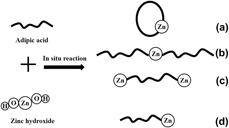
A novel approach of preparing zinc adipate as β-nucleating agent for polypropylene engineering
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 21 / 14 November 2019
- Published online by Cambridge University Press:
- 20 September 2019, pp. 3654-3665
- Print publication:
- 14 November 2019
-
- Article
- Export citation
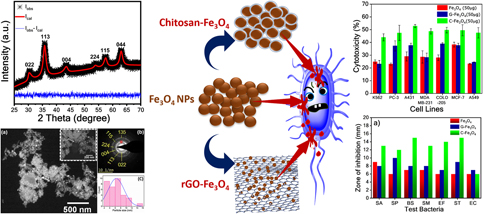
Graphene/chitosan-functionalized iron oxide nanoparticles for biomedical applications
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 20 / 28 October 2019
- Published online by Cambridge University Press:
- 20 September 2019, pp. 3389-3399
- Print publication:
- 28 October 2019
-
- Article
- Export citation
Crystal structure of atropine sulfate monohydrate, (C17H24NO3)2(SO4)·(H2O)
-
- Journal:
- Powder Diffraction / Volume 34 / Issue 4 / December 2019
- Published online by Cambridge University Press:
- 20 September 2019, pp. 389-395
-
- Article
- Export citation
Study of the dielectric properties of ACu3Ti4O12 (A = Eu2/3, Tb2/3, and Na1/2Eu1/2)
-
- Journal:
- Powder Diffraction / Volume 34 / Issue 4 / December 2019
- Published online by Cambridge University Press:
- 20 September 2019, pp. 345-351
-
- Article
- Export citation
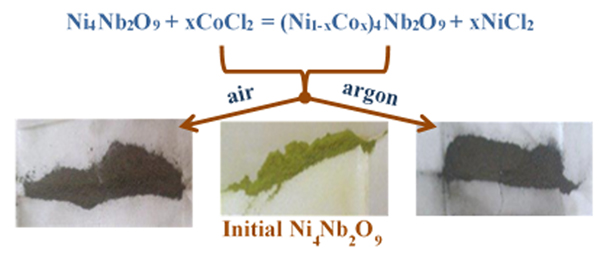
Modifying chemical composition of the fine Ni4Nb2O9 powders using chloride melts as reaction medium
-
- Journal:
- MRS Communications / Volume 9 / Issue 4 / December 2019
- Published online by Cambridge University Press:
- 20 September 2019, pp. 1300-1305
- Print publication:
- December 2019
-
- Article
- Export citation
Recent advances in experimental thermodynamics of metal–organic frameworks
-
- Journal:
- Powder Diffraction / Volume 34 / Issue 4 / December 2019
- Published online by Cambridge University Press:
- 20 September 2019, pp. 297-301
-
- Article
- Export citation
Exploring the electronic, mechanical, and anisotropy properties of novel tetragonal B2CO phase
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 21 / 14 November 2019
- Published online by Cambridge University Press:
- 20 September 2019, pp. 3617-3626
- Print publication:
- 14 November 2019
-
- Article
- Export citation
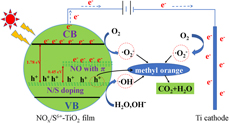
Electro-assisted ammonium persulfate activation to promote the introduction of N and S into TiO2 film: Enhancing its photoelectrocatalytic performance under solar
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 20 / 28 October 2019
- Published online by Cambridge University Press:
- 20 September 2019, pp. 3573-3582
- Print publication:
- 28 October 2019
-
- Article
- Export citation
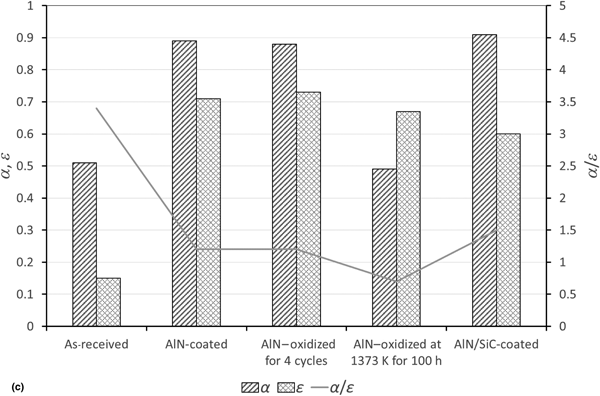
Multilayer multifunctional advanced coatings for receivers of concentrated solar power plants
-
- Journal:
- MRS Communications / Volume 9 / Issue 4 / December 2019
- Published online by Cambridge University Press:
- 19 September 2019, pp. 1193-1199
- Print publication:
- December 2019
-
- Article
- Export citation
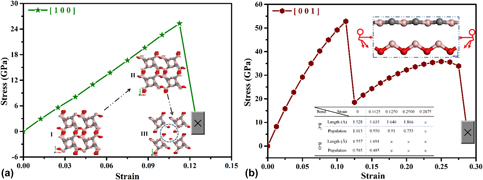
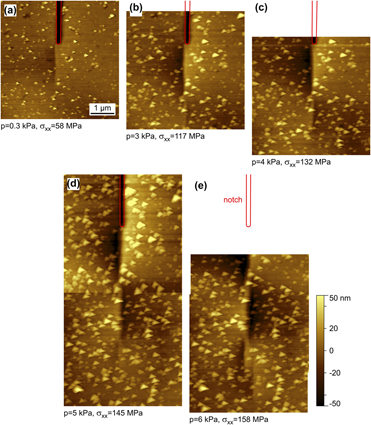
Microstructural dependence of the fracture toughness of metallic thin films: A bulge test and atomistic simulation study on single-crystalline and polycrystalline silver films
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 20 / 28 October 2019
- Published online by Cambridge University Press:
- 18 September 2019, pp. 3483-3494
- Print publication:
- 28 October 2019
-
- Article
-
- You have access
- Open access
- HTML
- Export citation