Refine search
Actions for selected content:
106116 results in Materials Science
Crystal structure of cloxacillin sodium monohydrate, C19H17ClN3O5SNa(H2O)
-
- Journal:
- Powder Diffraction / Volume 34 / Issue 4 / December 2019
- Published online by Cambridge University Press:
- 02 September 2019, pp. 374-378
-
- Article
- Export citation
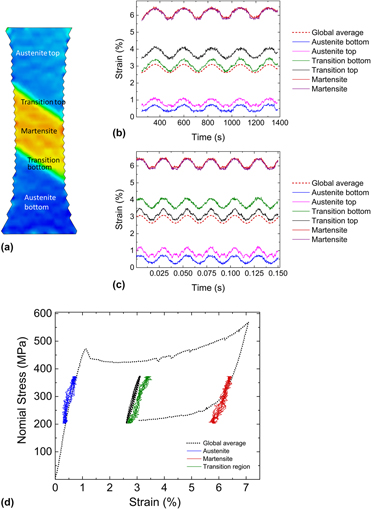
Cyclic response and fatigue failure of Nitinol under tension–tension loading
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 20 / 28 October 2019
- Published online by Cambridge University Press:
- 02 September 2019, pp. 3504-3522
- Print publication:
- 28 October 2019
-
- Article
- Export citation
Ovonic threshold switching selectors for three-dimensional stackable phase-change memory
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 9 / September 2019
- Published online by Cambridge University Press:
- 05 September 2019, pp. 715-720
- Print publication:
- September 2019
-
- Article
- Export citation
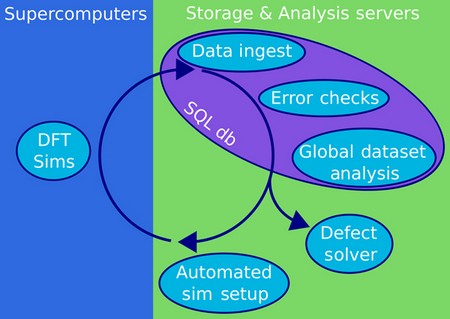
An informatics software stack for point defect-derived opto-electronic properties: the Asphalt Project
-
- Journal:
- MRS Communications / Volume 9 / Issue 3 / September 2019
- Published online by Cambridge University Press:
- 02 September 2019, pp. 839-845
- Print publication:
- September 2019
-
- Article
- Export citation
US Department of Energy funds R&D for fusion
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 9 / September 2019
- Published online by Cambridge University Press:
- 05 September 2019, pp. 679-680
- Print publication:
- September 2019
-
- Article
-
- You have access
- HTML
- Export citation
Electrolysis for hydrogen production
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 9 / September 2019
- Published online by Cambridge University Press:
- 05 September 2019, pp. 684-685
- Print publication:
- September 2019
-
- Article
-
- You have access
- HTML
- Export citation
Phase-change materials: Empowered by an unconventional bonding mechanism
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 9 / September 2019
- Published online by Cambridge University Press:
- 05 September 2019, pp. 699-704
- Print publication:
- September 2019
-
- Article
- Export citation
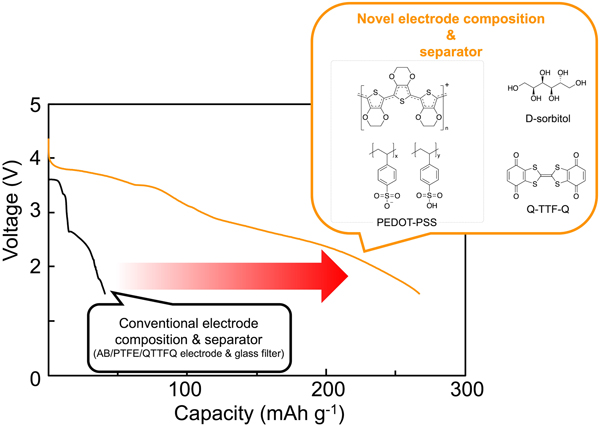
Conductive polymer binder and separator for high energy density lithium organic battery
-
- Journal:
- MRS Communications / Volume 9 / Issue 3 / September 2019
- Published online by Cambridge University Press:
- 02 September 2019, pp. 979-984
- Print publication:
- September 2019
-
- Article
- Export citation
Phase-change materials in electronics and photonics
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 9 / September 2019
- Published online by Cambridge University Press:
- 05 September 2019, pp. 686-690
- Print publication:
- September 2019
-
- Article
-
- You have access
- HTML
- Export citation
Nacre-inspired composites display optical transparency, fracture toughness
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 9 / September 2019
- Published online by Cambridge University Press:
- 05 September 2019, pp. 674-675
- Print publication:
- September 2019
-
- Article
-
- You have access
- HTML
- Export citation
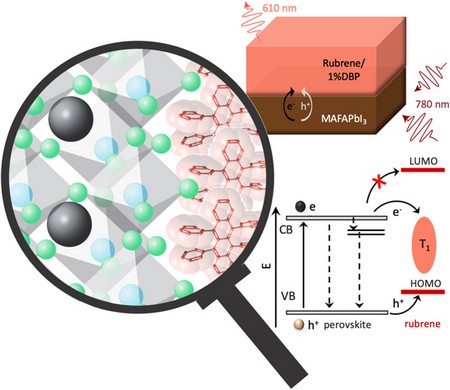
A perspective on triplet fusion upconversion: triplet sensitizers beyond quantum dots
-
- Journal:
- MRS Communications / Volume 9 / Issue 3 / September 2019
- Published online by Cambridge University Press:
- 02 September 2019, pp. 924-935
- Print publication:
- September 2019
-
- Article
- Export citation
Time is critical for fuel-cell resurgence
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 9 / September 2019
- Published online by Cambridge University Press:
- 05 September 2019, p. 681
- Print publication:
- September 2019
-
- Article
-
- You have access
- HTML
- Export citation
Proteins designed to bind to a specific surface
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 9 / September 2019
- Published online by Cambridge University Press:
- 05 September 2019, pp. 676-677
- Print publication:
- September 2019
-
- Article
-
- You have access
- HTML
- Export citation
MRS University Chapter Special Project Grants offer learning and collaboration
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 9 / September 2019
- Published online by Cambridge University Press:
- 05 September 2019, pp. 728-730
- Print publication:
- September 2019
-
- Article
-
- You have access
- HTML
- Export citation
MRS Journal Highlights
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 9 / September 2019
- Published online by Cambridge University Press:
- 05 September 2019, pp. 671-672
- Print publication:
- September 2019
-
- Article
-
- You have access
- HTML
- Export citation
South Korea strengthens international ties for Industry 4.0
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 9 / September 2019
- Published online by Cambridge University Press:
- 05 September 2019, p. 679
- Print publication:
- September 2019
-
- Article
-
- You have access
- HTML
- Export citation
The puzzle of water solubilities of polyethers solved
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 9 / September 2019
- Published online by Cambridge University Press:
- 05 September 2019, pp. 675-676
- Print publication:
- September 2019
-
- Article
-
- You have access
- HTML
- Export citation
MRS volume 44 issue 9 Cover and Back matter
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 9 / September 2019
- Published online by Cambridge University Press:
- 05 September 2019, pp. b1-b2
- Print publication:
- September 2019
-
- Article
-
- You have access
- Export citation
Enhanced lithium-ion transport in organosilyl electrolytes for lithium-ion battery applications
-
- Journal:
- MRS Communications / Volume 9 / Issue 3 / September 2019
- Published online by Cambridge University Press:
- 30 September 2019, pp. 985-991
- Print publication:
- September 2019
-
- Article
- Export citation
MRC volume 9 issue 3 Cover and Front matter
-
- Journal:
- MRS Communications / Volume 9 / Issue 3 / September 2019
- Published online by Cambridge University Press:
- 30 September 2019, pp. f1-f6
- Print publication:
- September 2019
-
- Article
-
- You have access
- Export citation