Article contents
Enhancing ionic conductivity with fluorination in organosilyl solvents for lithium-ion battery electrolytes
Published online by Cambridge University Press: 26 September 2019
Abstract
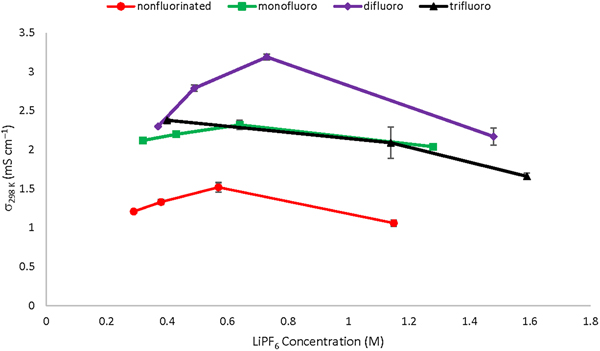
Increasing fluorination of organosilyl nitrile solvents improves ionic conductivities of lithium salt electrolytes, resulting from higher values of salt dissociation. Ionic conductivities at 298 K range from 1.5 to 3.2 mS/cm for LiPF6 salt concentrations at 0.6 or 0.7 M. The authors also report on solvent blend electrolytes where the fluoroorganosilyl (FOS) nitrile solvent is mixed with ethylene carbonate and diethyl carbonate. Ionic conductivities of the FOS solvent/carbonate blend electrolytes increase achieving ionic conductivities at 298 K of 5.5–6.3 mS/cm and salt dissociation values ranging from 0.42 to 0.45. Salt dissociation generally decreases with increasing temperature.
Information
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 3
- Cited by


