Refine search
Actions for selected content:
106116 results in Materials Science
Hot-carrier dynamics in catalysis
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 1 / January 2020
- Published online by Cambridge University Press:
- 10 January 2020, pp. 32-36
- Print publication:
- January 2020
-
- Article
- Export citation
The future of MRS Governance
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 1 / January 2020
- Published online by Cambridge University Press:
- 10 January 2020, p. 5
- Print publication:
- January 2020
-
- Article
-
- You have access
- HTML
- Export citation
Keeping current: How advancements in electricity storage can save money for consumers- ADDENDUM
-
- Journal:
- MRS Energy & Sustainability / Volume 7 / 2020
- Published online by Cambridge University Press:
- 20 April 2020, E15
- Print publication:
- 2020
-
- Article
- Export citation
New horizons for MRS Bulletin
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 1 / January 2020
- Published online by Cambridge University Press:
- 10 January 2020, p. 6
- Print publication:
- January 2020
-
- Article
-
- You have access
- HTML
- Export citation
Markus J. Buehler appointed MRS Bulletin Impact Editor
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 1 / January 2020
- Published online by Cambridge University Press:
- 10 January 2020, p. 65
- Print publication:
- January 2020
-
- Article
-
- You have access
- HTML
- Export citation
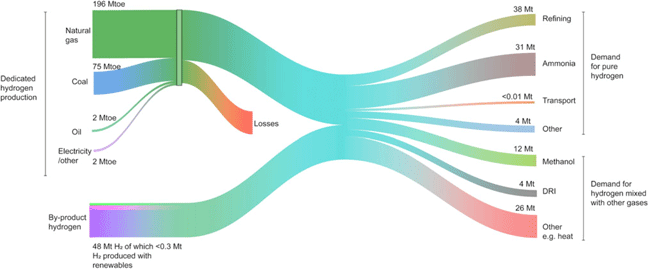
Renewable hydrogen for the chemical industry
- Part of
-
- Journal:
- MRS Energy & Sustainability / Volume 7 / 2020
- Published online by Cambridge University Press:
- 25 September 2020, E33
- Print publication:
- 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
MRS Journal Highlights
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 1 / January 2020
- Published online by Cambridge University Press:
- 10 January 2020, p. 17
- Print publication:
- January 2020
-
- Article
-
- You have access
- HTML
- Export citation
High-rate lithium ion energy storage to facilitate increased penetration of photovoltaic systems in electricity grids - ADDENDUM
-
- Journal:
- MRS Energy & Sustainability / Volume 7 / 2020
- Published online by Cambridge University Press:
- 20 April 2020, E6
- Print publication:
- 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Why nonconventional materials are answers for sustainable agriculture - ADDENDUM
-
- Journal:
- MRS Energy & Sustainability / Volume 7 / 2020
- Published online by Cambridge University Press:
- 20 April 2020, E9
- Print publication:
- 2020
-
- Article
- Export citation
Within the global circular economy: A special case of Turkey towards energy transition
- Part of
-
- Journal:
- MRS Energy & Sustainability / Volume 7 / 2020
- Published online by Cambridge University Press:
- 01 September 2020, E24
- Print publication:
- 2020
-
- Article
- Export citation
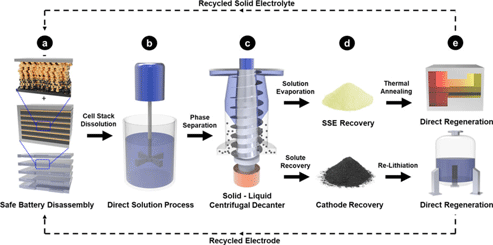
Sustainable design of fully recyclable all solid-state batteries
-
- Journal:
- MRS Energy & Sustainability / Volume 7 / 2020
- Published online by Cambridge University Press:
- 21 August 2020, E23
- Print publication:
- 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Energy Focus: High-performance, long-lasting battery comes with test protocol
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 1 / January 2020
- Published online by Cambridge University Press:
- 10 January 2020, pp. 12-13
- Print publication:
- January 2020
-
- Article
-
- You have access
- HTML
- Export citation
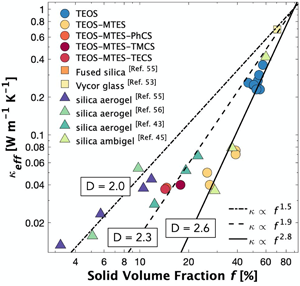
Engineering mesoporous silica for superior optical and thermal properties
-
- Journal:
- MRS Energy & Sustainability / Volume 7 / 2020
- Published online by Cambridge University Press:
- 16 November 2020, E39
- Print publication:
- 2020
-
- Article
- Export citation
Enabling sustainable critical materials for battery storage through efficient recycling and improved design: A perspective
-
- Journal:
- MRS Energy & Sustainability / Volume 7 / 2020
- Published online by Cambridge University Press:
- 09 September 2020, E27
- Print publication:
- 2020
-
- Article
- Export citation
Valuation and cost reduction of behind-the-meter hydrogen production in Hawaii
-
- Journal:
- MRS Energy & Sustainability / Volume 7 / 2020
- Published online by Cambridge University Press:
- 07 September 2020, E26
- Print publication:
- 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Materials matter in phosphorus sustainability
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 1 / January 2020
- Published online by Cambridge University Press:
- 10 January 2020, pp. 7-10
- Print publication:
- January 2020
-
- Article
-
- You have access
- HTML
- Export citation
How to treat energy storage as a transmission asset?
-
- Journal:
- MRS Energy & Sustainability / Volume 7 / 2020
- Published online by Cambridge University Press:
- 12 August 2020, E20
- Print publication:
- 2020
-
- Article
- Export citation
Porphyrin-based photocatalysts for hydrogen production
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 1 / January 2020
- Published online by Cambridge University Press:
- 10 January 2020, pp. 49-56
- Print publication:
- January 2020
-
- Article
- Export citation
Hydrogen technologies for energy storage: A perspective - ERRATUM
-
- Journal:
- MRS Energy & Sustainability / Volume 7 / 2020
- Published online by Cambridge University Press:
- 18 December 2020, E43
- Print publication:
- 2020
-
- Article
-
- You have access
- HTML
- Export citation
Does electrifying organic synthesis pay off? The energy efficiency of electro-organic conversions
- Part of
-
- Journal:
- MRS Energy & Sustainability / Volume 7 / 2020
- Published online by Cambridge University Press:
- 10 December 2020, E42
- Print publication:
- 2020
-
- Article
- Export citation