Refine search
Actions for selected content:
106116 results in Materials Science
8 - X-Ray Tomography
-
- Book:
- X-ray Microscopy
- Published online:
- 28 October 2019
- Print publication:
- 19 December 2019, pp 321-349
-
- Chapter
- Export citation
3 - X-Ray Physics
-
- Book:
- X-ray Microscopy
- Published online:
- 28 October 2019
- Print publication:
- 19 December 2019, pp 23-70
-
- Chapter
- Export citation
1 - X-Ray Microscopes: a Short Introduction
-
- Book:
- X-ray Microscopy
- Published online:
- 28 October 2019
- Print publication:
- 19 December 2019, pp 1-4
-
- Chapter
- Export citation
Contributors page
-
- Book:
- X-ray Microscopy
- Published online:
- 28 October 2019
- Print publication:
- 19 December 2019, pp xii-xii
-
- Chapter
- Export citation
Appendix A X-Ray Data Tabulations
-
- Book:
- X-ray Microscopy
- Published online:
- 28 October 2019
- Print publication:
- 19 December 2019, pp 515-518
-
- Chapter
- Export citation
9 - X-Ray Spectromicroscopy
-
- Book:
- X-ray Microscopy
- Published online:
- 28 October 2019
- Print publication:
- 19 December 2019, pp 350-389
-
- Chapter
- Export citation
5 - X-Ray Focusing Optics
-
- Book:
- X-ray Microscopy
- Published online:
- 28 October 2019
- Print publication:
- 19 December 2019, pp 199-240
-
- Chapter
- Export citation
10 - Coherent Imaging
-
- Book:
- X-ray Microscopy
- Published online:
- 28 October 2019
- Print publication:
- 19 December 2019, pp 390-456
-
- Chapter
- Export citation
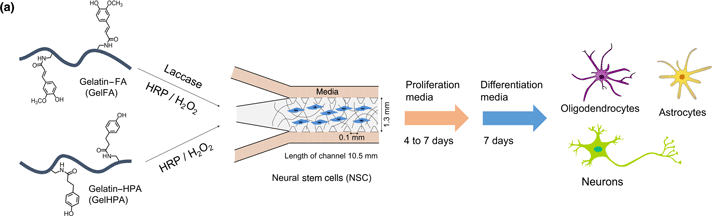
Response of neuroglia to hypoxia-induced oxidative stress using enzymatically crosslinked hydrogels
-
- Journal:
- MRS Communications / Volume 10 / Issue 1 / March 2020
- Published online by Cambridge University Press:
- 18 December 2019, pp. 83-90
- Print publication:
- March 2020
-
- Article
- Export citation

Intermediate Solid Mechanics
-
- Published online:
- 16 December 2019
- Print publication:
- 09 January 2020
Flexible, stretchable, conformal electronics, and smart textiles: environmental life cycle considerations for emerging applications
-
- Journal:
- MRS Communications / Volume 10 / Issue 1 / March 2020
- Published online by Cambridge University Press:
- 16 December 2019, pp. 69-82
- Print publication:
- March 2020
-
- Article
- Export citation
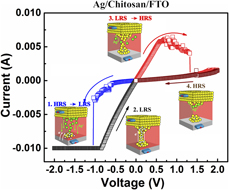
Influence of top electrode on resistive switching effect of chitosan thin films
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 23 / 16 December 2019
- Published online by Cambridge University Press:
- 16 December 2019, pp. 3899-3906
- Print publication:
- 16 December 2019
-
- Article
- Export citation
JMR volume 34 issue 23 Cover and Back matter
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 23 / 16 December 2019
- Published online by Cambridge University Press:
- 16 December 2019, pp. b1-b4
- Print publication:
- 16 December 2019
-
- Article
-
- You have access
- Export citation
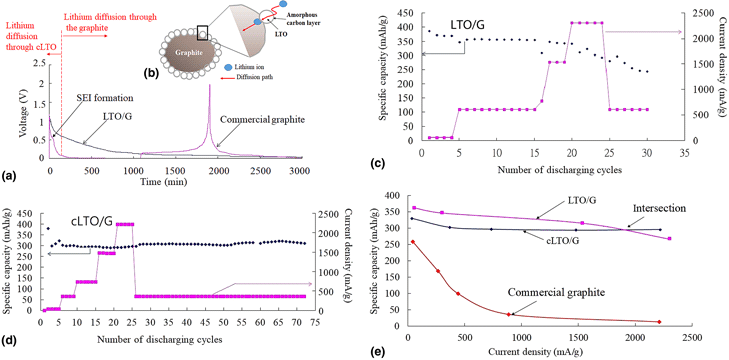
Commercial carbon anode material surface-modified by spinel lithium titanate for fast lithium-ion interaction
-
- Journal:
- MRS Communications / Volume 10 / Issue 1 / March 2020
- Published online by Cambridge University Press:
- 16 December 2019, pp. 141-146
- Print publication:
- March 2020
-
- Article
- Export citation
JMR volume 34 issue 23 Cover and Front matter
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 23 / 16 December 2019
- Published online by Cambridge University Press:
- 16 December 2019, pp. f1-f5
- Print publication:
- 16 December 2019
-
- Article
-
- You have access
- Export citation
Type-II WS2–ReSe2 heterostructure and its charge-transfer properties
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 11 / 15 June 2020
- Published online by Cambridge University Press:
- 16 December 2019, pp. 1417-1423
- Print publication:
- 15 June 2020
-
- Article
- Export citation
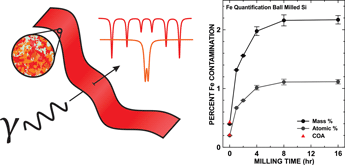
Quantitative composition determination by Mössbauer spectroscopy
-
- Journal:
- MRS Communications / Volume 10 / Issue 1 / March 2020
- Published online by Cambridge University Press:
- 16 December 2019, pp. 123-128
- Print publication:
- March 2020
-
- Article
- Export citation
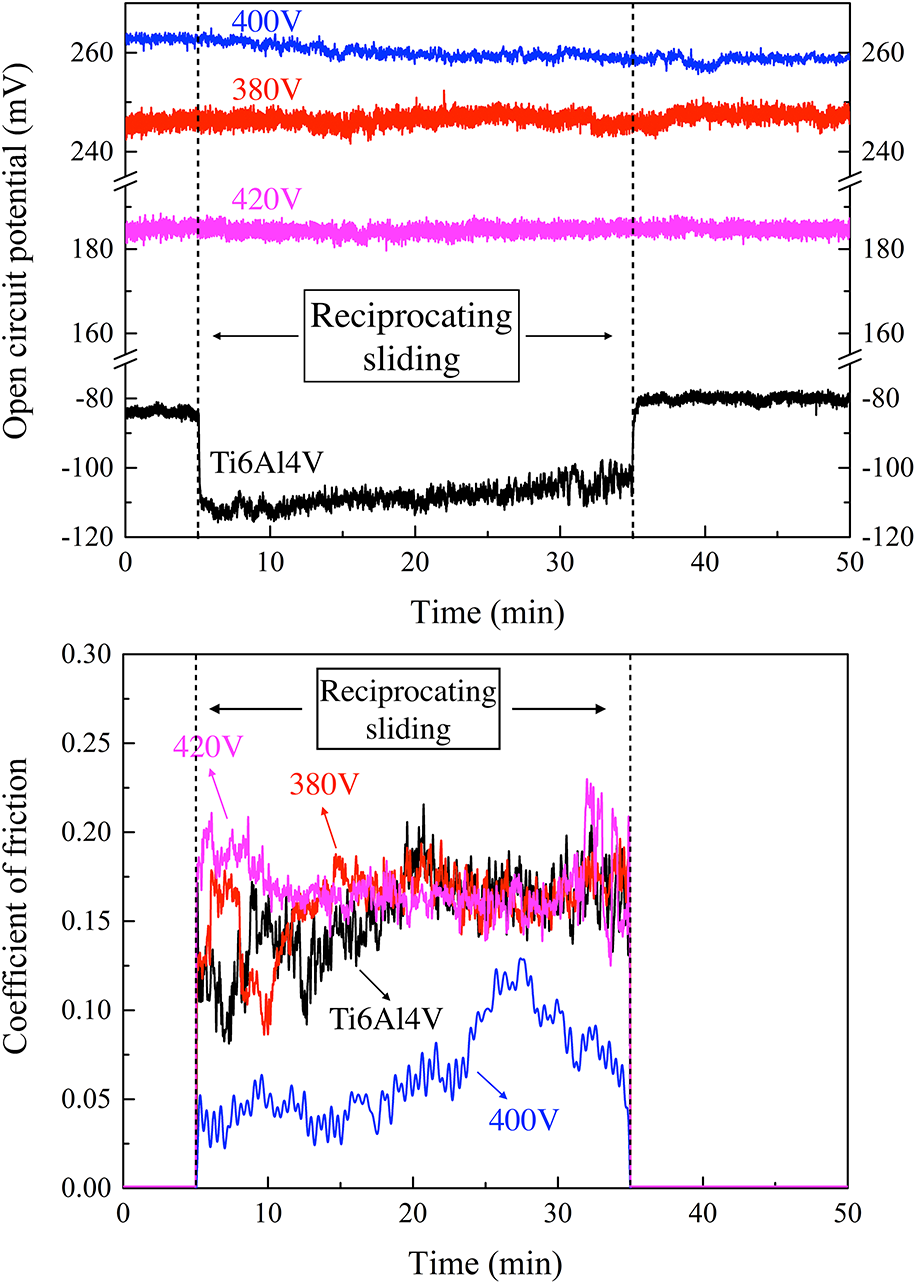
Tribocorrosion behavior of Ca–P MAO coatings on Ti6Al4V alloy at various applied voltages
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 5 / 16 March 2020
- Published online by Cambridge University Press:
- 16 December 2019, pp. 444-453
- Print publication:
- 16 March 2020
-
- Article
- Export citation
Effect of gravity in the Cassie-to-Wenzel transition on a micropatterned surface
-
- Journal:
- MRS Communications / Volume 10 / Issue 1 / March 2020
- Published online by Cambridge University Press:
- 13 December 2019, pp. 129-134
- Print publication:
- March 2020
-
- Article
- Export citation
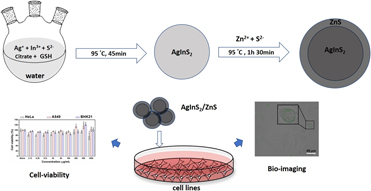
Cell viability assessments of green synthesized water-soluble AgInS2/ZnS core/shell quantum dots against different cancer cell lines
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 24 / 30 December 2019
- Published online by Cambridge University Press:
- 09 December 2019, pp. 4037-4044
- Print publication:
- 30 December 2019
-
- Article
- Export citation