Refine search
Actions for selected content:
106116 results in Materials Science
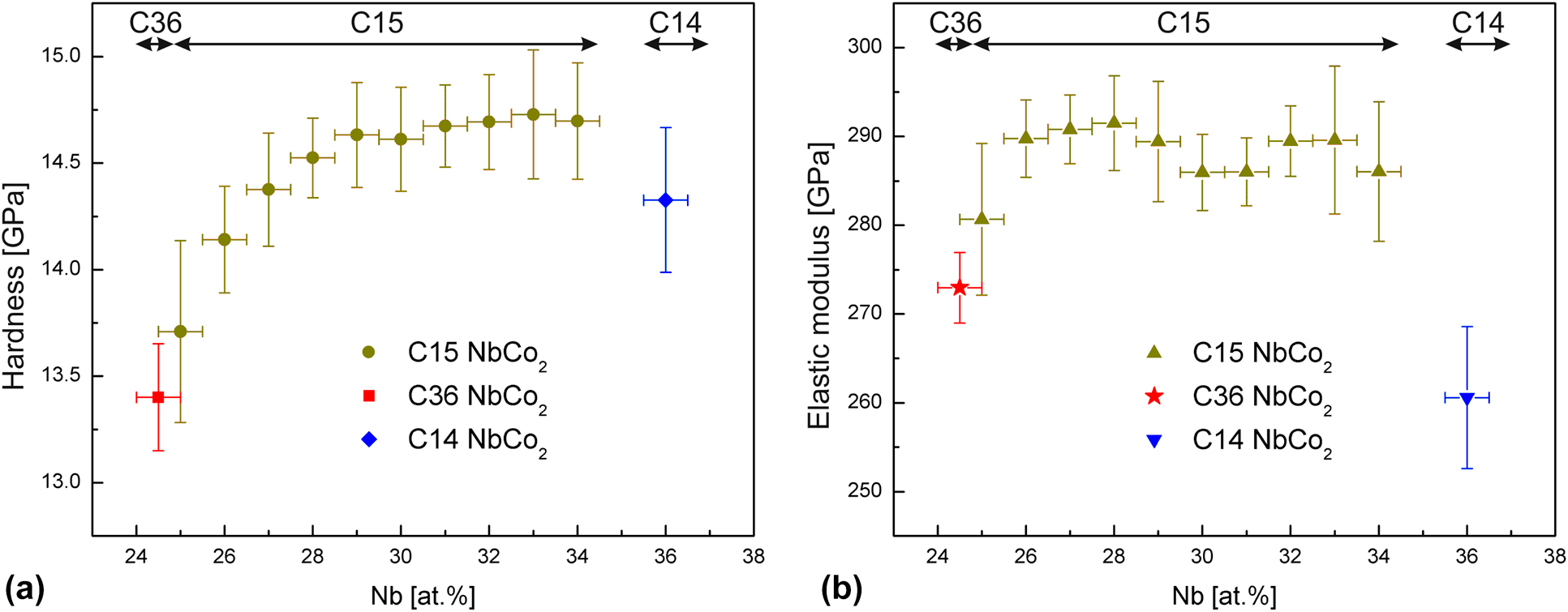
Composition dependence of hardness and elastic modulus of the cubic and hexagonal NbCo2 Laves phase polytypes studied by nanoindentation
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 2 / 28 January 2020
- Published online by Cambridge University Press:
- 28 January 2020, pp. 185-195
- Print publication:
- 28 January 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
JMR volume 35 issue 2 Cover and Front matter
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 2 / 28 January 2020
- Published online by Cambridge University Press:
- 28 January 2020, pp. f1-f5
- Print publication:
- 28 January 2020
-
- Article
-
- You have access
- Export citation
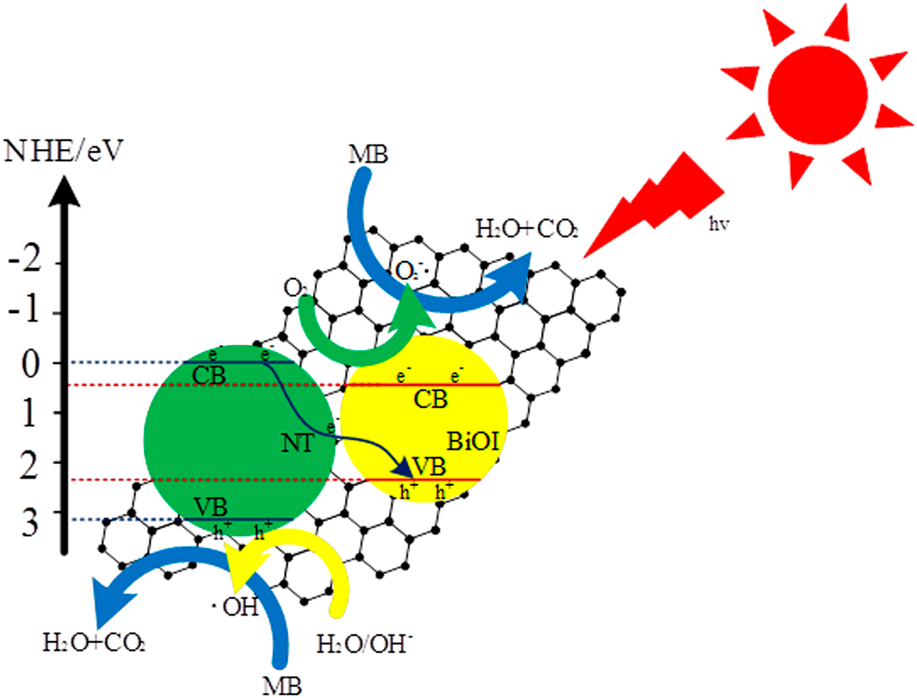
Synthesis of N-TiO2/BiOI/RGO composites with significantly enhanced visible light photocatalytic activity
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 2 / 28 January 2020
- Published online by Cambridge University Press:
- 28 January 2020, pp. 153-161
- Print publication:
- 28 January 2020
-
- Article
- Export citation
JMR volume 35 issue 2 Cover and Back matter
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 2 / 28 January 2020
- Published online by Cambridge University Press:
- 28 January 2020, pp. b1-b2
- Print publication:
- 28 January 2020
-
- Article
-
- You have access
- Export citation
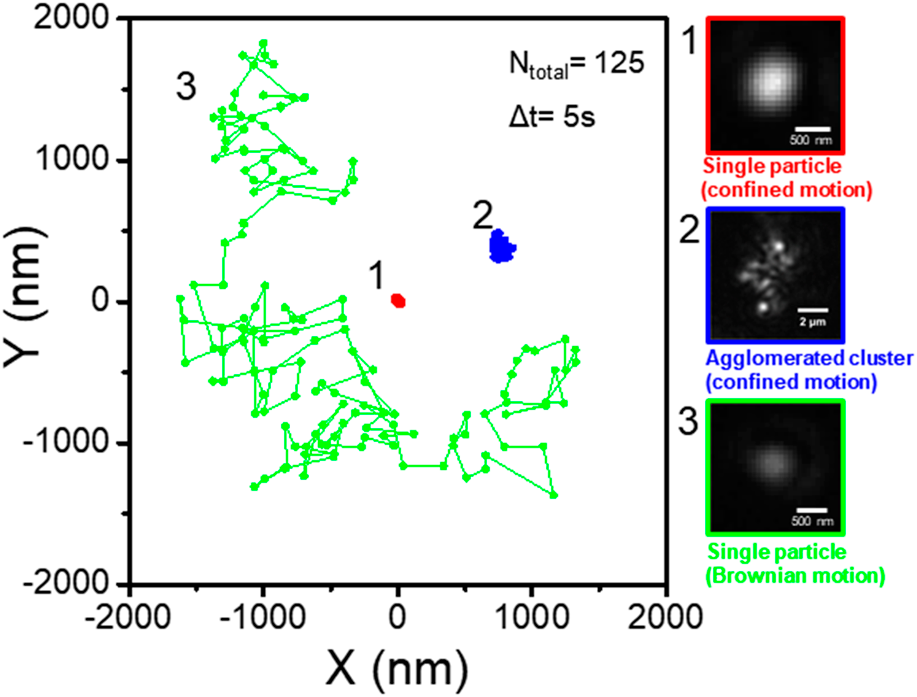
Trajectories, diffusion, and interactions of single ceria particles on a glass surface observed by evanescent wave microscopy
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 3 / 14 February 2020
- Published online by Cambridge University Press:
- 27 January 2020, pp. 321-331
- Print publication:
- 14 February 2020
-
- Article
- Export citation
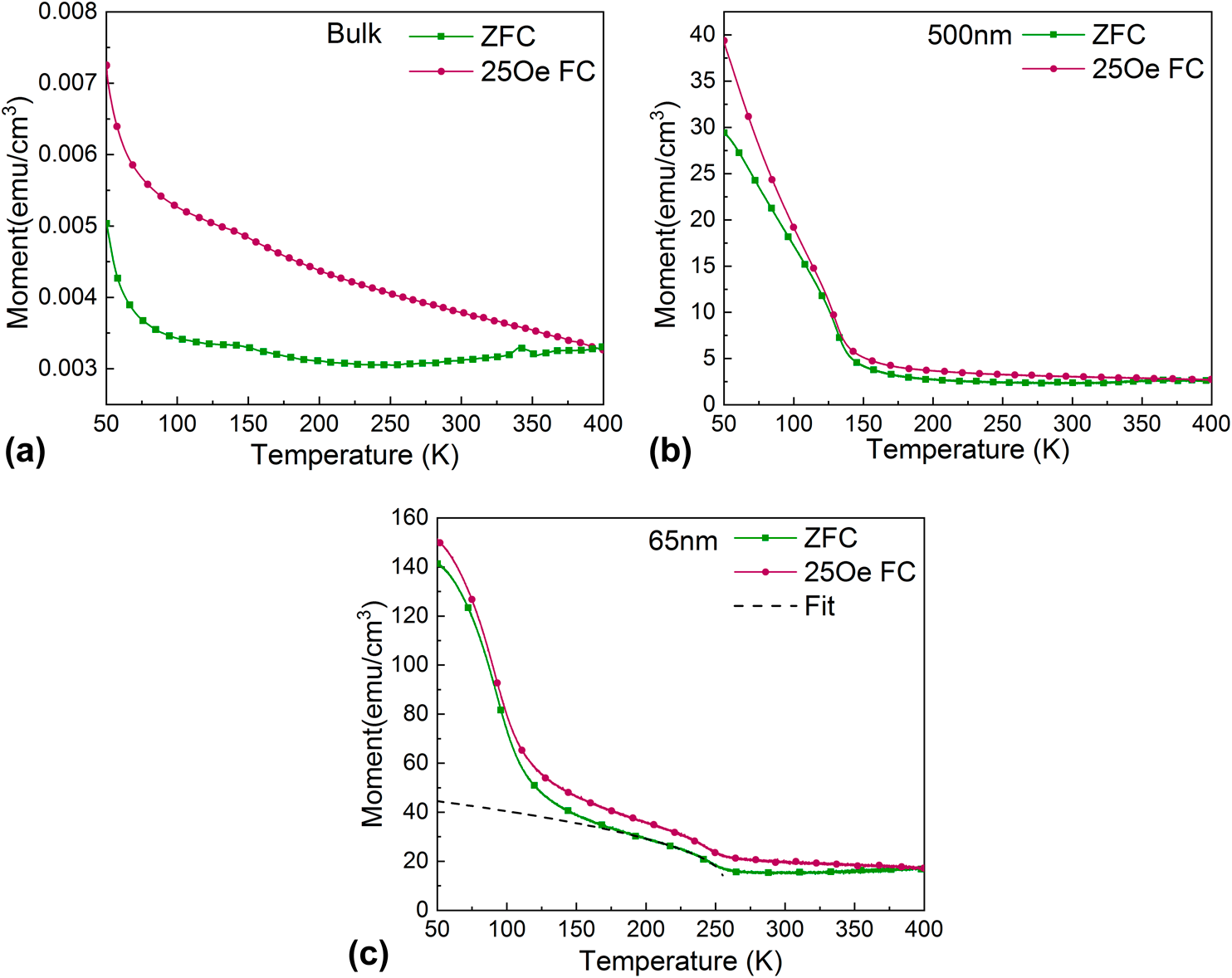
Structural and magnetic properties of FeCoMnCrSi multi-principal alloy
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 8 / 28 April 2020
- Published online by Cambridge University Press:
- 27 January 2020, pp. 981-989
- Print publication:
- 28 April 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
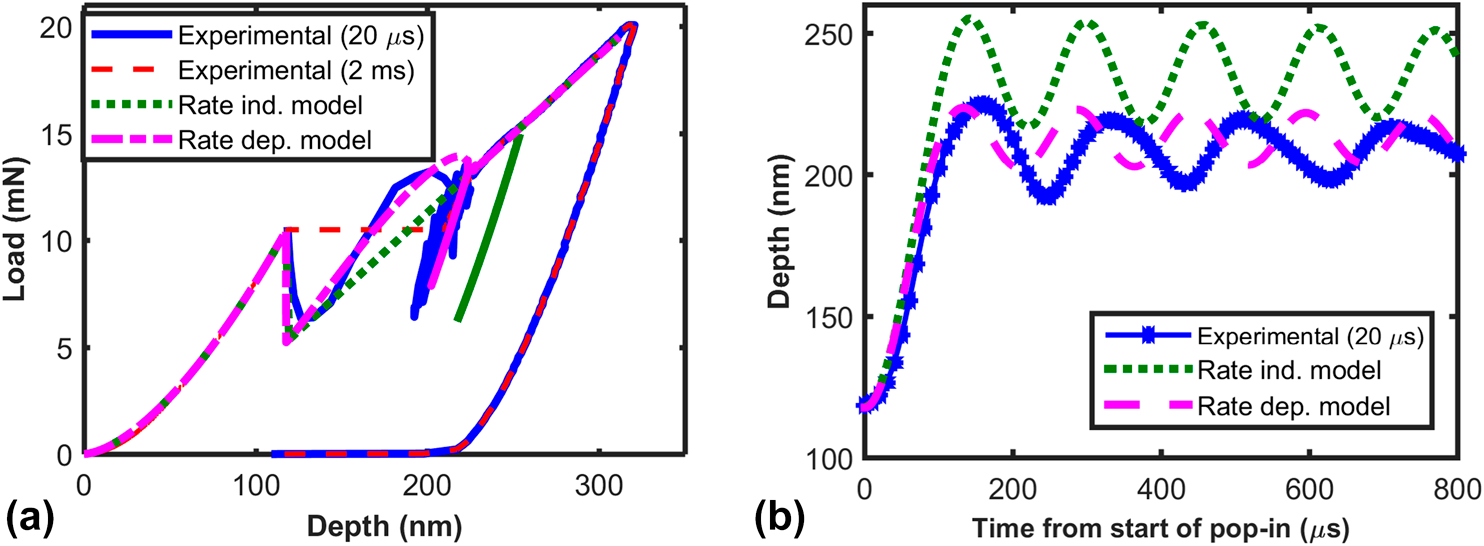
Critical examination of experimental data on strain bursts (pop-in) during spherical indentation
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 8 / 28 April 2020
- Published online by Cambridge University Press:
- 27 January 2020, pp. 1028-1036
- Print publication:
- 28 April 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
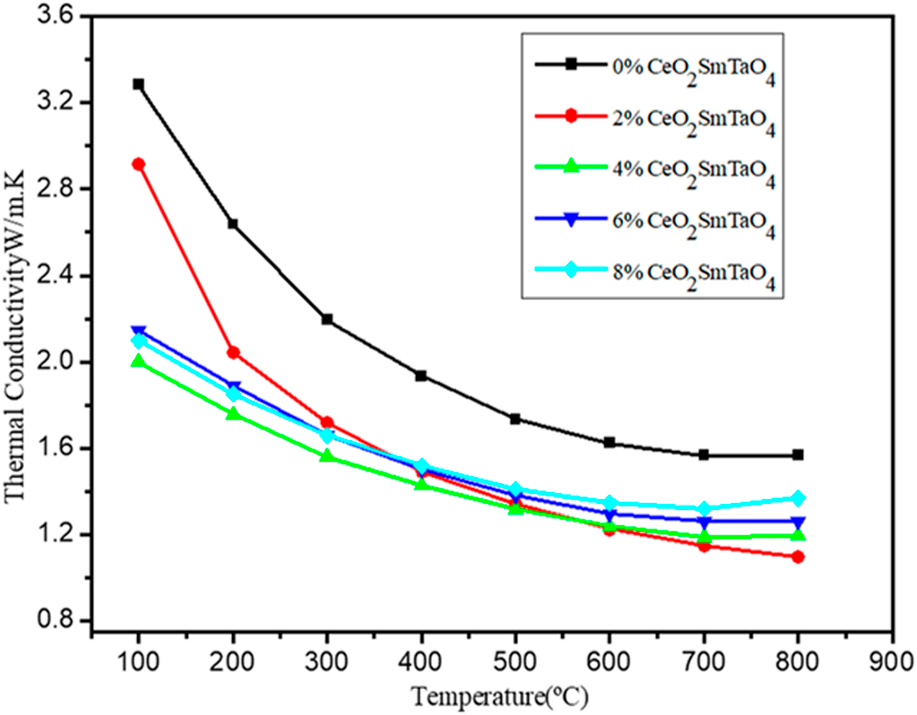
Microstructure and thermophysical properties of CeO2-doped SmTaO4 ceramics for thermal barrier coatings
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 3 / 14 February 2020
- Published online by Cambridge University Press:
- 27 January 2020, pp. 242-251
- Print publication:
- 14 February 2020
-
- Article
- Export citation
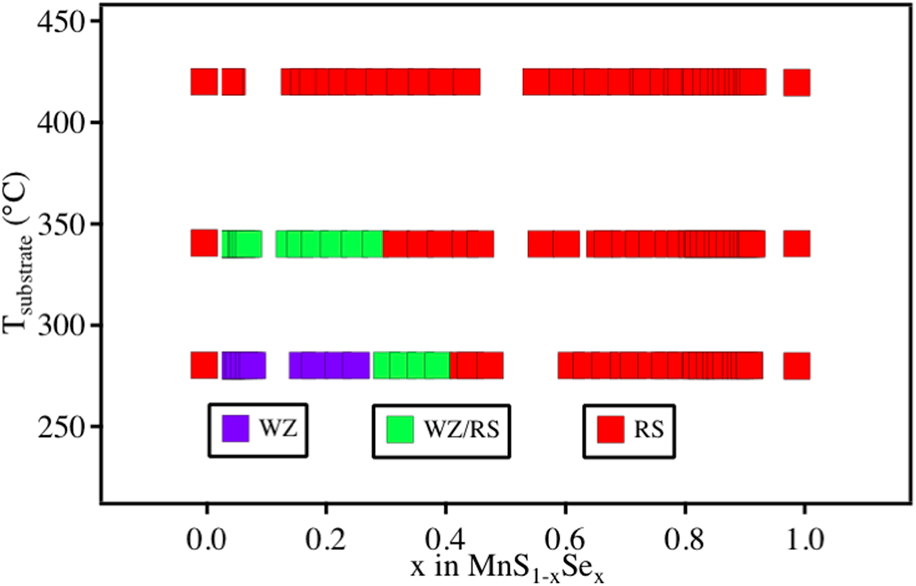
Wurtzite materials in alloys of rock salt compounds
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 8 / 28 April 2020
- Published online by Cambridge University Press:
- 24 January 2020, pp. 972-980
- Print publication:
- 28 April 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
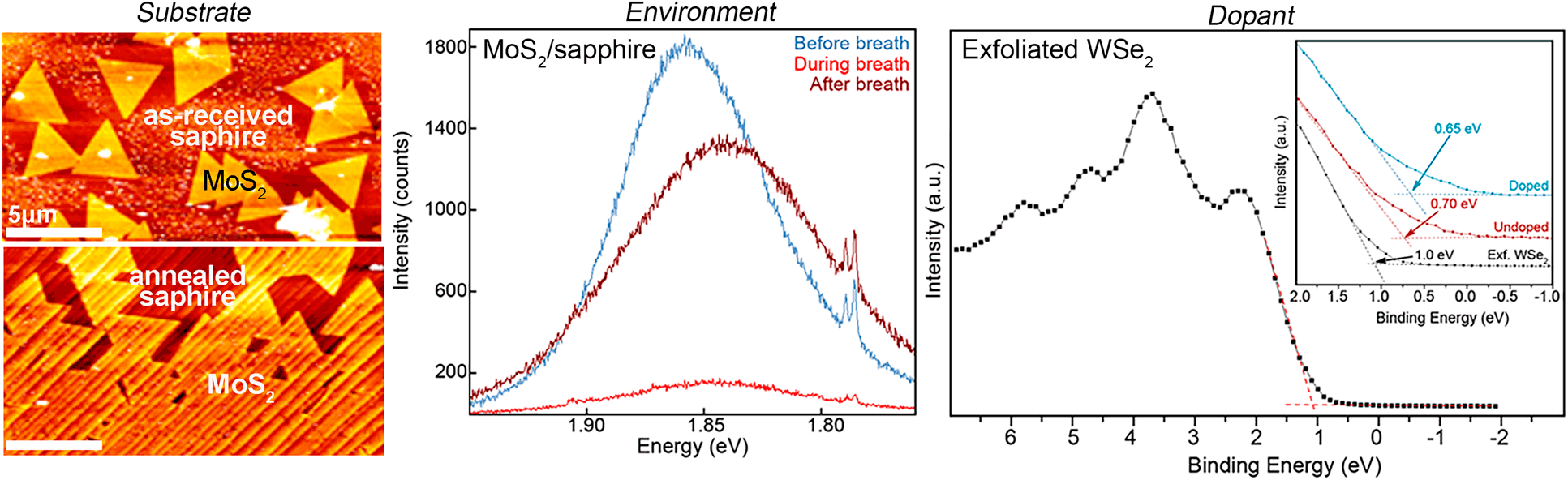
Caveats in obtaining high-quality 2D materials and property characterization
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 8 / 28 April 2020
- Published online by Cambridge University Press:
- 23 January 2020, pp. 855-863
- Print publication:
- 28 April 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
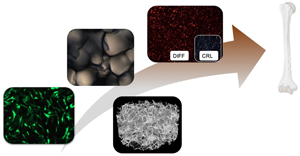
Biomimetic and electroactive 3D scaffolds for human neural crest-derived stem cell expansion and osteogenic differentiation
-
- Journal:
- MRS Communications / Volume 10 / Issue 1 / March 2020
- Published online by Cambridge University Press:
- 23 January 2020, pp. 179-187
- Print publication:
- March 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Hot deformation behavior of a new tailored cobalt-based superalloy for turbine discs
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 6 / 30 March 2020
- Published online by Cambridge University Press:
- 22 January 2020, pp. 633-643
- Print publication:
- 30 March 2020
-
- Article
- Export citation
Crystal structure of pantoprazole sodium sesquihydrate Form I, C16H14F2N3O4SNa(H2O)1.5
-
- Journal:
- Powder Diffraction / Volume 35 / Issue 1 / March 2020
- Published online by Cambridge University Press:
- 20 January 2020, pp. 53-60
-
- Article
- Export citation
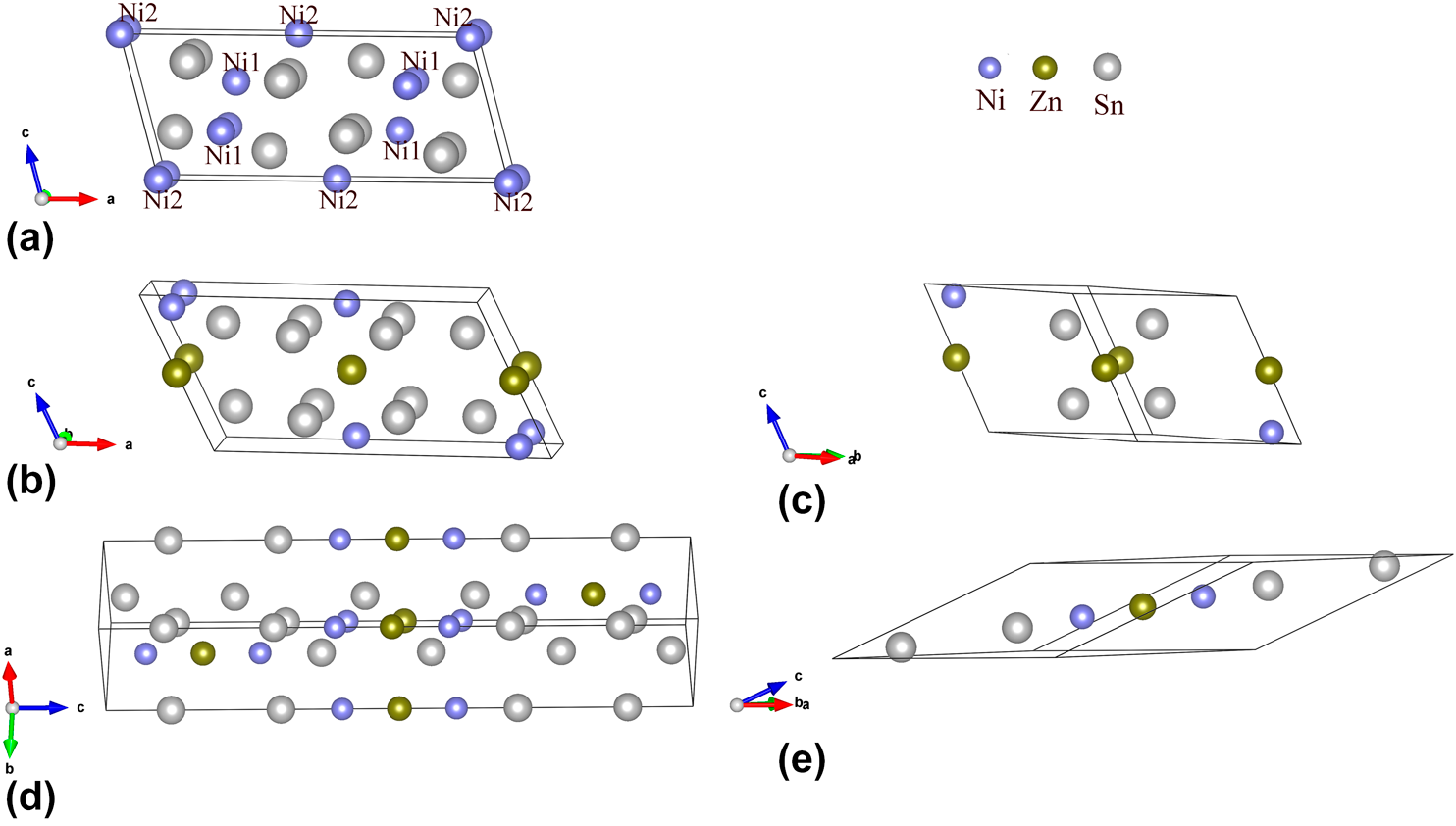
Exploring the structural, mechanical, thermodynamic, and electronic properties of (Ni0.66, Zn0.33)3Sn4 ternary intermetallic compounds by the first-principles study
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 3 / 14 February 2020
- Published online by Cambridge University Press:
- 20 January 2020, pp. 263-271
- Print publication:
- 14 February 2020
-
- Article
- Export citation
Crystal structure of cloxacillin sodium monohydrate, C19H17CIN3O5SNa(H2O) — ERRATUM
-
- Journal:
- Powder Diffraction / Volume 35 / Issue 1 / March 2020
- Published online by Cambridge University Press:
- 20 January 2020, p. 79
-
- Article
-
- You have access
- HTML
- Export citation
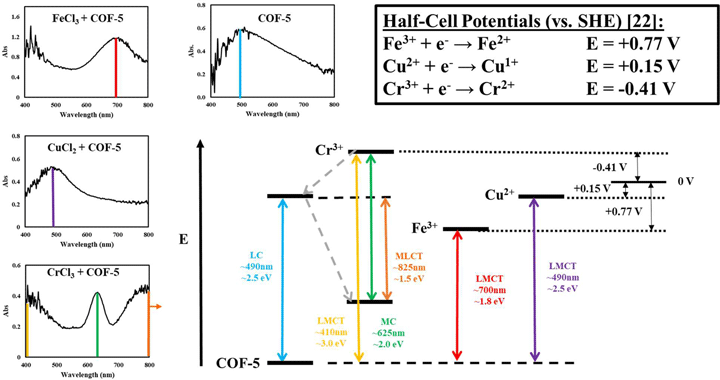
Electronic charge transfer properties of COF-5 solutions and films with intercalated metal ions
-
- Journal:
- MRS Communications / Volume 10 / Issue 1 / March 2020
- Published online by Cambridge University Press:
- 20 January 2020, pp. 91-97
- Print publication:
- March 2020
-
- Article
- Export citation
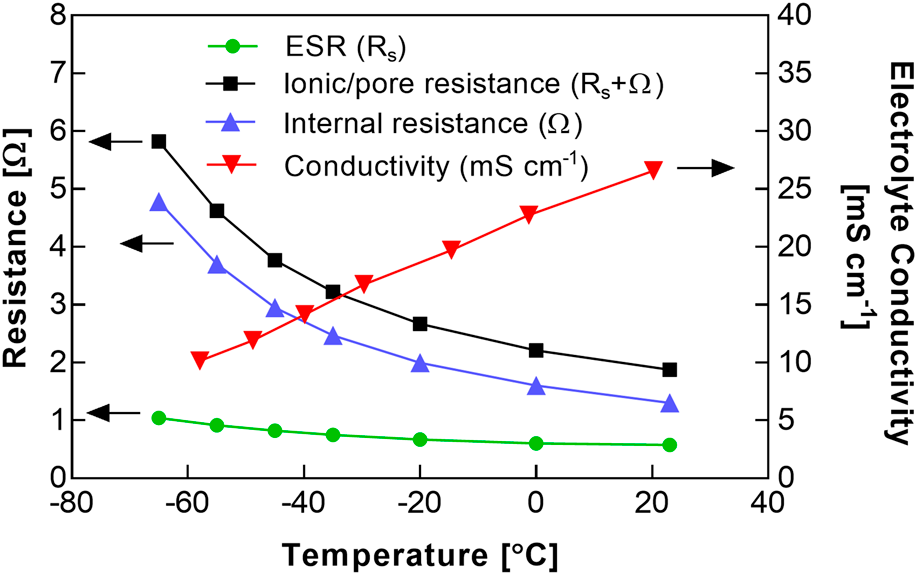
Low-temperature performance of electrochemical capacitors using acetonitrile/methyl formate electrolytes and activated carbon fabric electrodes
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 2 / 28 January 2020
- Published online by Cambridge University Press:
- 20 January 2020, pp. 113-121
- Print publication:
- 28 January 2020
-
- Article
- Export citation
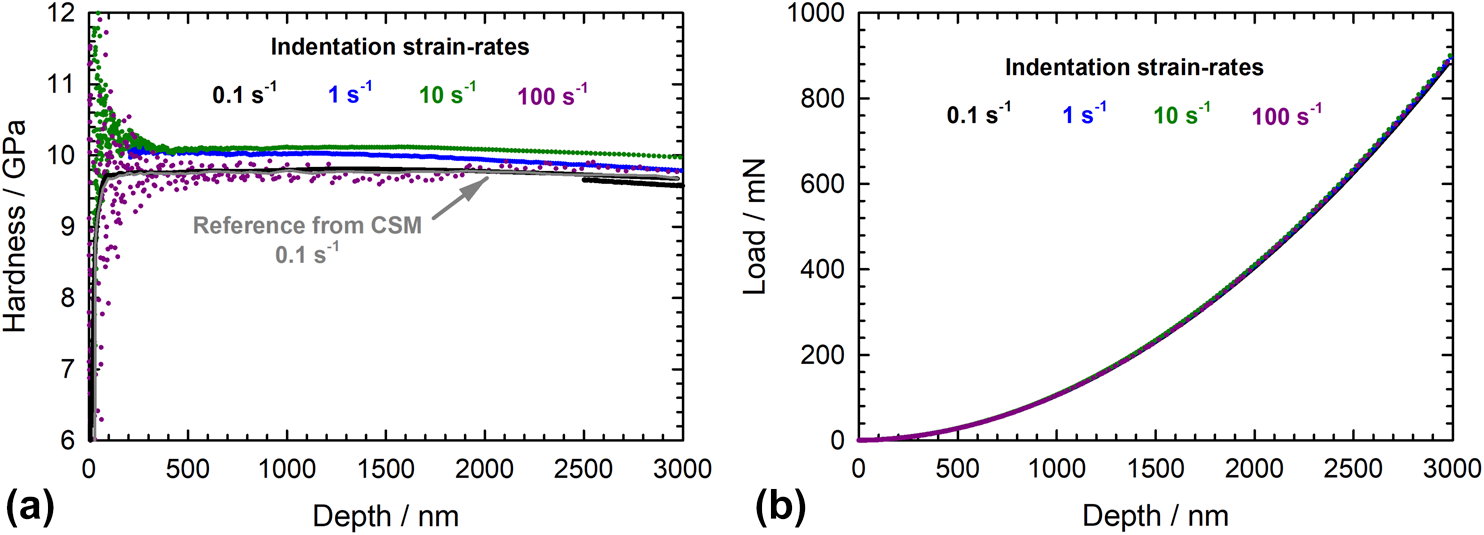
Extending the range of constant strain rate nanoindentation testing
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 4 / 28 February 2020
- Published online by Cambridge University Press:
- 20 January 2020, pp. 343-352
- Print publication:
- 28 February 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Crystal structure of ipratropium bromide monohydrate, C20H30NO3Br(H2O)
-
- Journal:
- Powder Diffraction / Volume 35 / Issue 1 / March 2020
- Published online by Cambridge University Press:
- 20 January 2020, pp. 61-66
-
- Article
- Export citation
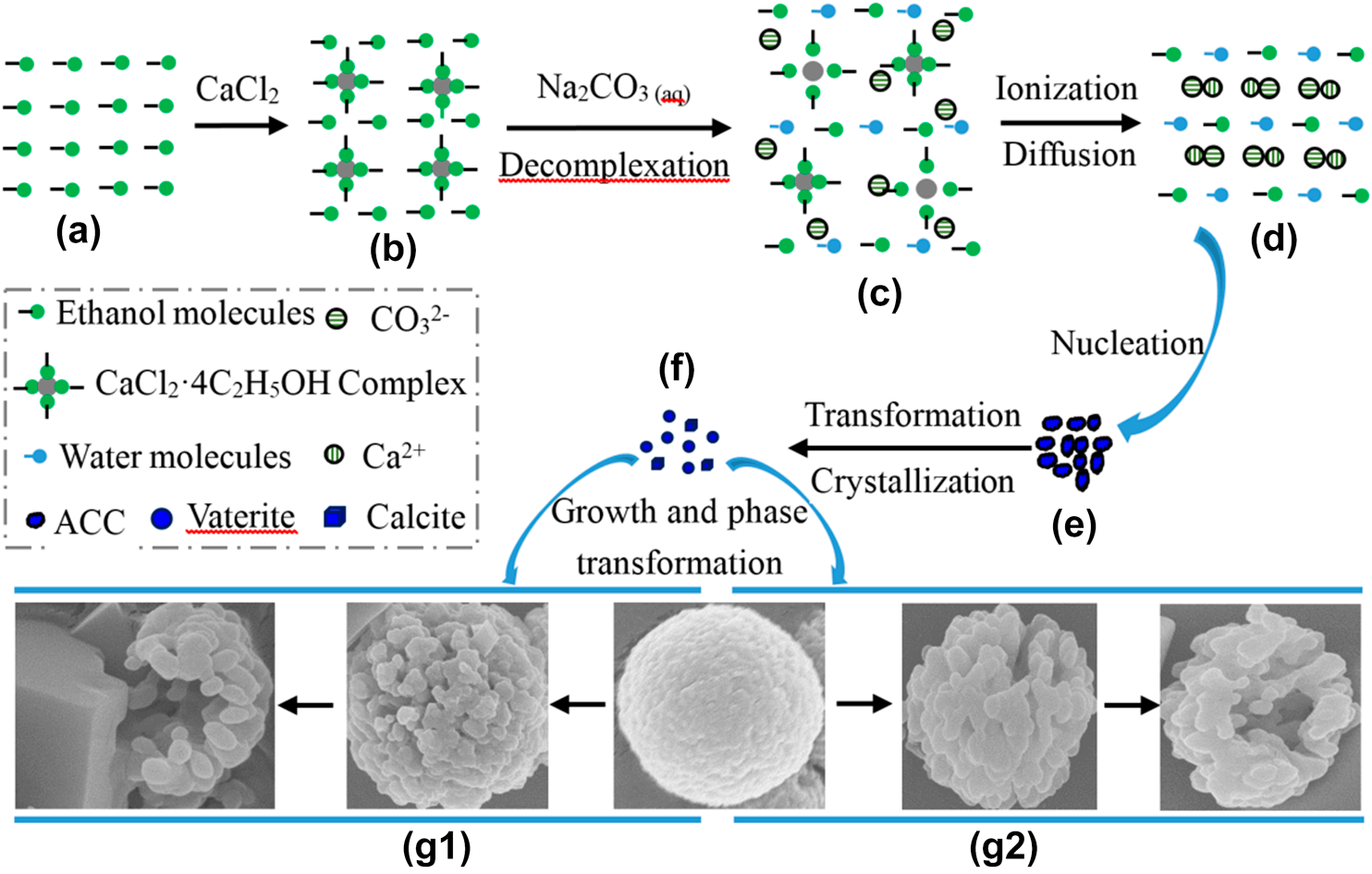
The advantage of alcohol–calcium method on the formation and the stability of vaterite against ethanol–water binary solvent method
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 3 / 14 February 2020
- Published online by Cambridge University Press:
- 17 January 2020, pp. 289-298
- Print publication:
- 14 February 2020
-
- Article
- Export citation