Refine search
Actions for selected content:
106116 results in Materials Science
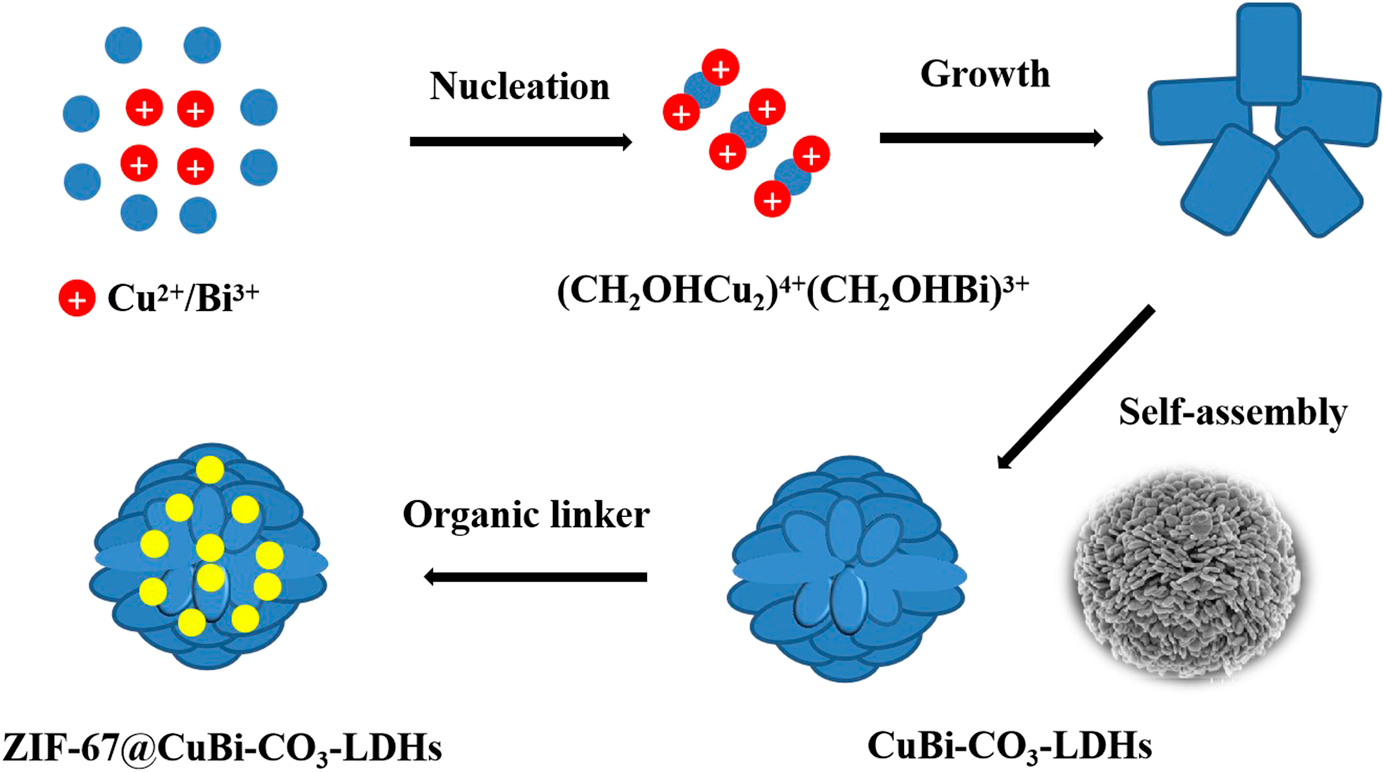
Synthesis of metal–organic framework nanocrystals immobilized with 3D flowerlike Cu–Bi-layered double hydroxides for iodine efficient removal
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 3 / 14 February 2020
- Published online by Cambridge University Press:
- 07 February 2020, pp. 299-311
- Print publication:
- 14 February 2020
-
- Article
- Export citation
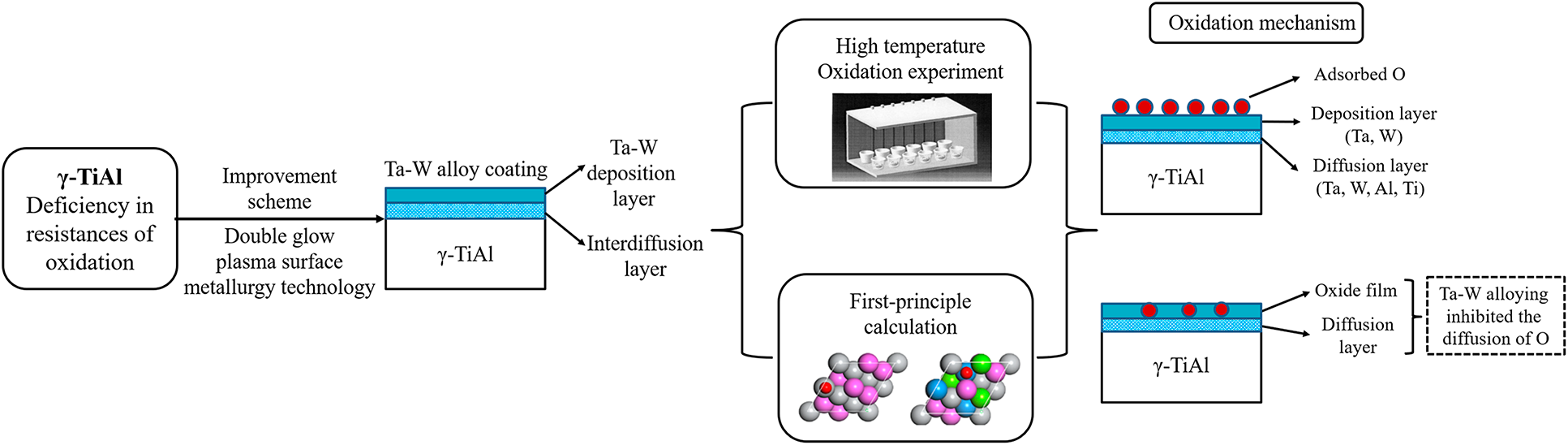
A combined experimental and first-principle study on the effect of plasma surface Ta–W co-alloying on the oxidation behavior of γ-TiAl at 900 °C
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 5 / 16 March 2020
- Published online by Cambridge University Press:
- 07 February 2020, pp. 516-526
- Print publication:
- 16 March 2020
-
- Article
- Export citation
Powder X-ray diffraction of trimethoprim Form I, C14H18N4O3
-
- Journal:
- Powder Diffraction / Volume 35 / Issue 1 / March 2020
- Published online by Cambridge University Press:
- 06 February 2020, pp. 69-70
-
- Article
- Export citation
Manufacturing strategies for wafer-scale two-dimensional transition metal dichalcogenide heterolayers
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 11 / 15 June 2020
- Published online by Cambridge University Press:
- 05 February 2020, pp. 1350-1368
- Print publication:
- 15 June 2020
-
- Article
- Export citation
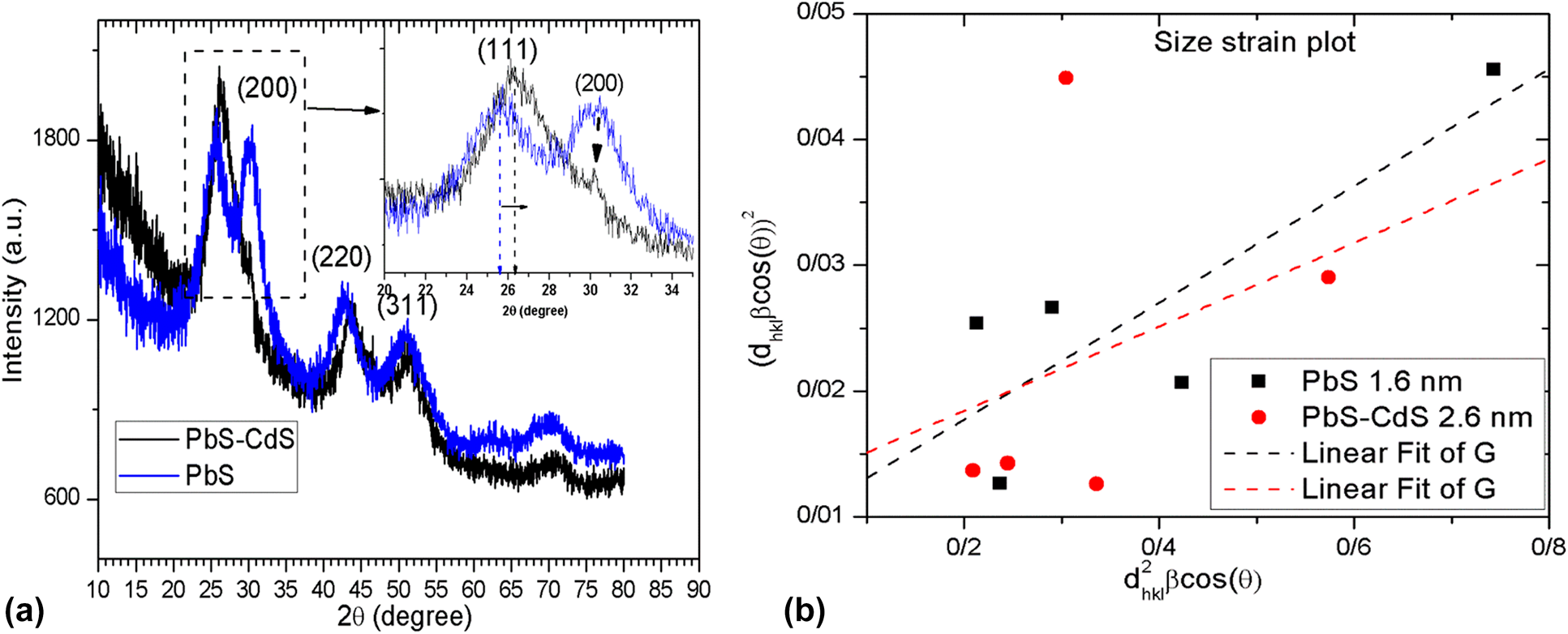
PbS and PbS/CdS quantum dots: Synthesized by photochemical approach, structural, linear and nonlinear response properties, and optical limiting
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 4 / 28 February 2020
- Published online by Cambridge University Press:
- 04 February 2020, pp. 401-409
- Print publication:
- 28 February 2020
-
- Article
- Export citation
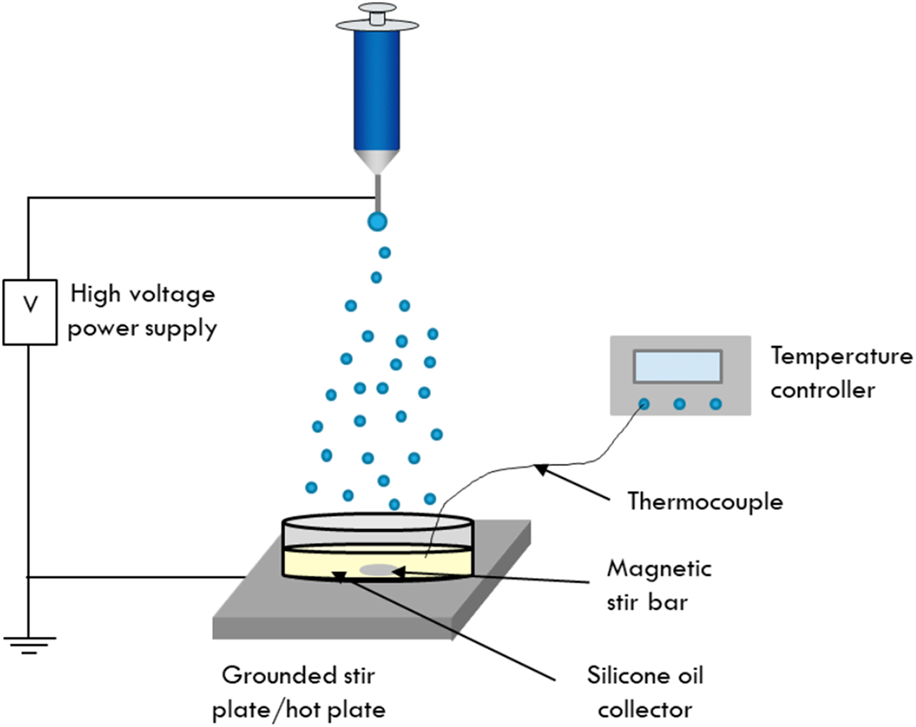
Synthesis of cobalt ferrite nanoparticles via electrospraying into a liquid collector
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 8 / 28 April 2020
- Published online by Cambridge University Press:
- 03 February 2020, pp. 864-871
- Print publication:
- 28 April 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
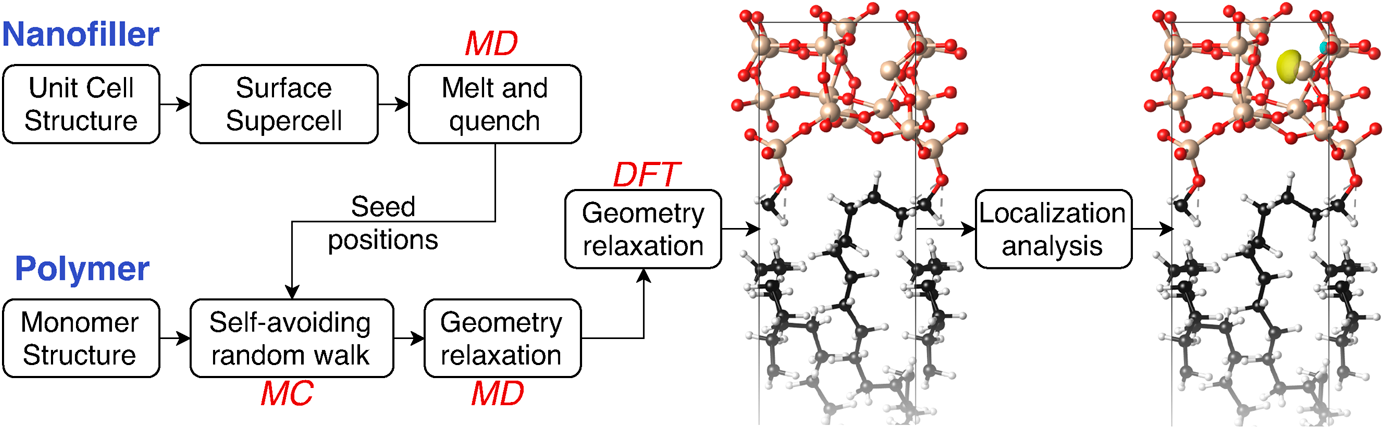
First-principles identification of localized trap states in polymer nanocomposite interfaces
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 8 / 28 April 2020
- Published online by Cambridge University Press:
- 03 February 2020, pp. 931-939
- Print publication:
- 28 April 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
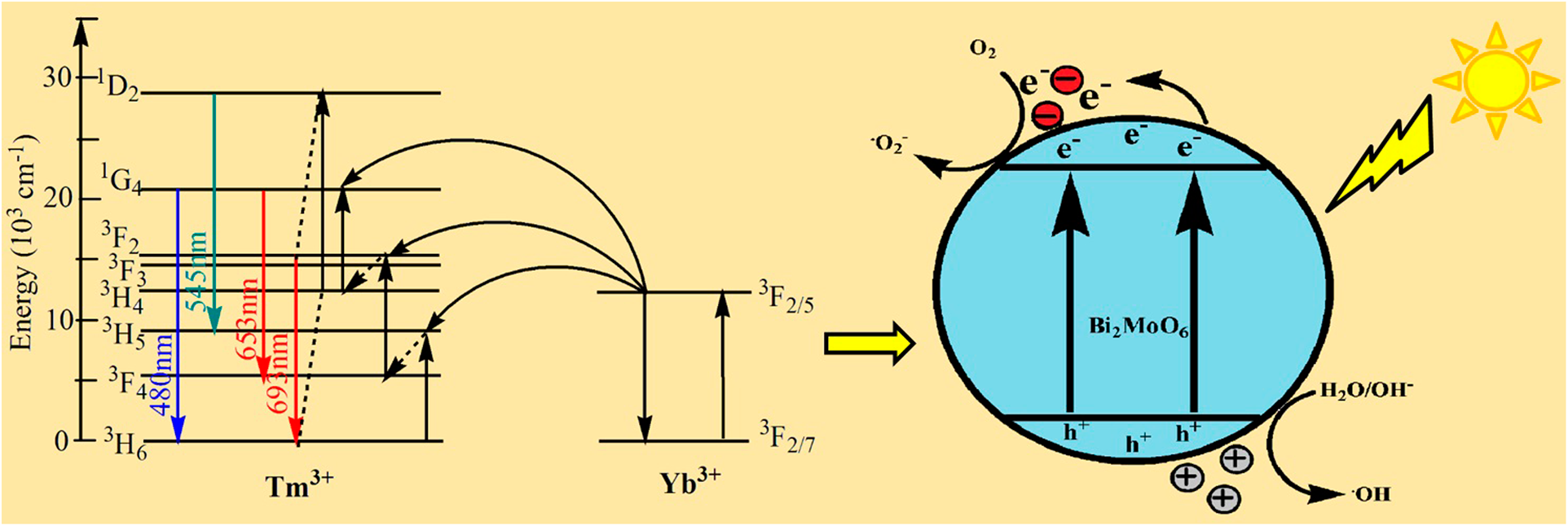
Novel Tm3+/Yb3+–co-doped Bi2MoO6: Synthesis, characterization, and enhanced photocatalytic activity under visible-light irradiation
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 3 / 14 February 2020
- Published online by Cambridge University Press:
- 03 February 2020, pp. 312-320
- Print publication:
- 14 February 2020
-
- Article
- Export citation
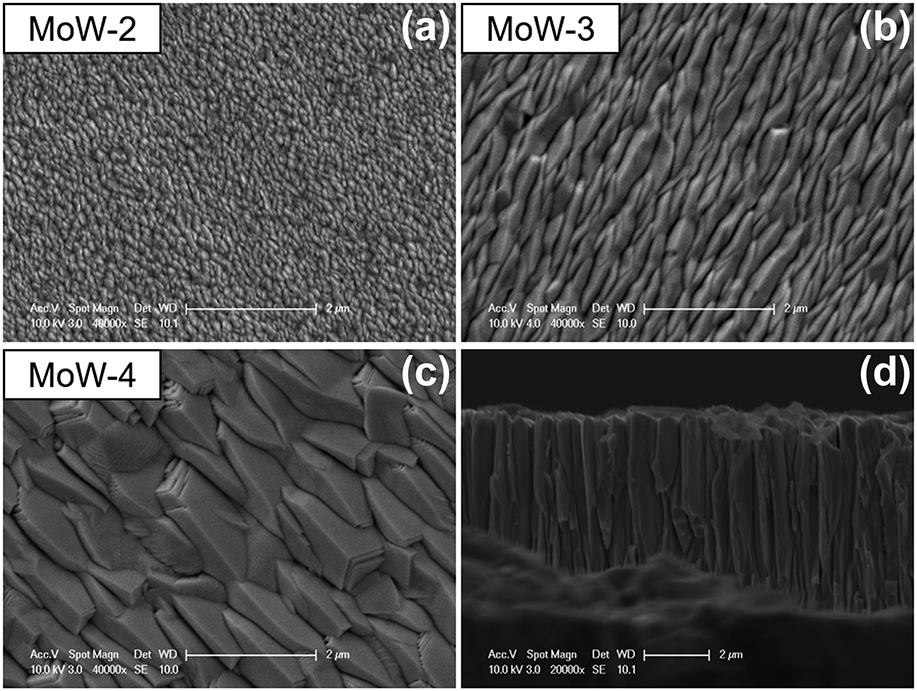
The effect of MoW interlayer thickness on diamond growth on steel substrates
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 5 / 16 March 2020
- Published online by Cambridge University Press:
- 03 February 2020, pp. 491-499
- Print publication:
- 16 March 2020
-
- Article
- Export citation
PCAST reincarnation brings promise for US materials science community
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 2 / February 2020
- Published online by Cambridge University Press:
- 10 February 2020, pp. 82-83
- Print publication:
- February 2020
-
- Article
-
- You have access
- HTML
- Export citation
Bio Focus: X-ray polarization reveals the secret to enamel’s toughness
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 2 / February 2020
- Published online by Cambridge University Press:
- 10 February 2020, p. 79
- Print publication:
- February 2020
-
- Article
-
- You have access
- HTML
- Export citation
US role in nuclear nonproliferation
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 2 / February 2020
- Published online by Cambridge University Press:
- 10 February 2020, p. 77
- Print publication:
- February 2020
-
- Article
-
- You have access
- HTML
- Export citation
MRS invites nominations for awards program
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 2 / February 2020
- Published online by Cambridge University Press:
- 10 February 2020, pp. 149-150
- Print publication:
- February 2020
-
- Article
-
- You have access
- HTML
- Export citation
Oxide glass exhibits plasticity without fracture at room temperature
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 2 / February 2020
- Published online by Cambridge University Press:
- 10 February 2020, pp. 78-79
- Print publication:
- February 2020
-
- Article
-
- You have access
- HTML
- Export citation
Biodegradable and stretchable polymeric materials for transient electronic devices
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 2 / February 2020
- Published online by Cambridge University Press:
- 10 February 2020, pp. 96-102
- Print publication:
- February 2020
-
- Article
- Export citation
Bio Focus: 2D carbon-network nanomaterial shows promise as an antibacterial agent
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 2 / February 2020
- Published online by Cambridge University Press:
- 10 February 2020, pp. 80-81
- Print publication:
- February 2020
-
- Article
-
- You have access
- HTML
- Export citation
2019 MRS Fall Meeting featured a variety of outreach, career, and professional development offerings
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 2 / February 2020
- Published online by Cambridge University Press:
- 10 February 2020, pp. 143-145
- Print publication:
- February 2020
-
- Article
-
- You have access
- HTML
- Export citation
MRS volume 45 issue 2 Cover and Front matter
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 2 / February 2020
- Published online by Cambridge University Press:
- 10 February 2020, pp. f1-f6
- Print publication:
- February 2020
-
- Article
-
- You have access
- Export citation
BREWing better broader impacts
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 2 / February 2020
- Published online by Cambridge University Press:
- 10 February 2020, pp. 84-86
- Print publication:
- February 2020
-
- Article
-
- You have access
- HTML
- Export citation
CAREER CENTRAL
-
- Journal:
- MRS Bulletin / Volume 45 / Issue 2 / February 2020
- Published online by Cambridge University Press:
- 10 February 2020, p. 151
- Print publication:
- February 2020
-
- Article
-
- You have access
- Export citation