Refine search
Actions for selected content:
106116 results in Materials Science
Cooperative self-assembly of porphyrins and derivatives
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 3 / March 2019
- Published online by Cambridge University Press:
- 11 March 2019, pp. 178-182
- Print publication:
- March 2019
-
- Article
- Export citation
Energy Focus: Functionalized-carbon-supported Pt-Co alloy nanoparticle catalyst yields reduced-cost fuel cells
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 3 / March 2019
- Published online by Cambridge University Press:
- 11 March 2019, pp. 153-154
- Print publication:
- March 2019
-
- Article
-
- You have access
- HTML
- Export citation
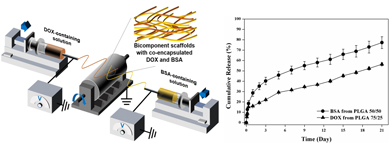
Bicomponent nanofibrous scaffolds with dual release of anticancer drugs and biomacromolecules
-
- Journal:
- MRS Communications / Volume 9 / Issue 1 / March 2019
- Published online by Cambridge University Press:
- 04 March 2019, pp. 413-420
- Print publication:
- March 2019
-
- Article
- Export citation
Size- and shape-dependent photocatalysis of porphyrin nanocrystals
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 3 / March 2019
- Published online by Cambridge University Press:
- 11 March 2019, pp. 172-177
- Print publication:
- March 2019
-
- Article
- Export citation
Research Highlights: Perovskites
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 3 / March 2019
- Published online by Cambridge University Press:
- 11 March 2019, pp. 149-150
- Print publication:
- March 2019
-
- Article
-
- You have access
- HTML
- Export citation
MRC volume 9 issue 1 Cover and Front matter
-
- Journal:
- MRS Communications / Volume 9 / Issue 1 / March 2019
- Published online by Cambridge University Press:
- 18 March 2019, pp. f1-f7
- Print publication:
- March 2019
-
- Article
-
- You have access
- Export citation
Van Swygenhoven-Moens to present Kavli lecture during 2019 MRS Spring Meeting plenary session
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 3 / March 2019
- Published online by Cambridge University Press:
- 11 March 2019, p. 217
- Print publication:
- March 2019
-
- Article
-
- You have access
- HTML
- Export citation
MRS volume 44 issue 3 Cover and Back matter
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 3 / March 2019
- Published online by Cambridge University Press:
- 11 March 2019, pp. b1-b2
- Print publication:
- March 2019
-
- Article
-
- You have access
- Export citation
Porphyrin-based nanocomposites for tumor photodynamic therapy
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 3 / March 2019
- Published online by Cambridge University Press:
- 11 March 2019, pp. 189-194
- Print publication:
- March 2019
-
- Article
- Export citation
In Memoriam: Siegfried Bauer (1961–2018)
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 3 / March 2019
- Published online by Cambridge University Press:
- 11 March 2019, p. 220
- Print publication:
- March 2019
-
- Article
-
- You have access
- HTML
- Export citation
Analysis of life-cycle costs and benefits of hydrogen fuel cells
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 3 / March 2019
- Published online by Cambridge University Press:
- 11 March 2019, p. 161
- Print publication:
- March 2019
-
- Article
-
- You have access
- HTML
- Export citation
Materials challenges in the hydrogen cycle
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 3 / March 2019
- Published online by Cambridge University Press:
- 11 March 2019, pp. 164-166
- Print publication:
- March 2019
-
- Article
-
- You have access
- HTML
- Export citation
13th New Diamond and Nano Carbons Conference to be held in Taiwan
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 3 / March 2019
- Published online by Cambridge University Press:
- 11 March 2019, p. 222
- Print publication:
- March 2019
-
- Article
-
- You have access
- HTML
- Export citation
MRS volume 44 issue 3 Cover and Front matter
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 3 / March 2019
- Published online by Cambridge University Press:
- 11 March 2019, pp. f1-f6
- Print publication:
- March 2019
-
- Article
-
- You have access
- Export citation
Binary ionic porphyrin self-assembly: Structures, and electronic and light-harvesting properties
-
- Journal:
- MRS Bulletin / Volume 44 / Issue 3 / March 2019
- Published online by Cambridge University Press:
- 11 March 2019, pp. 183-188
- Print publication:
- March 2019
-
- Article
- Export citation
13 - Topology and Berry Phase
-
- Book:
- Modern Condensed Matter Physics
- Published online:
- 26 February 2019
- Print publication:
- 28 February 2019, pp 331-361
-
- Chapter
- Export citation
Preface
-
- Book:
- Modern Condensed Matter Physics
- Published online:
- 26 February 2019
- Print publication:
- 28 February 2019, pp xvii-xviii
-
- Chapter
- Export citation
Appendix I - Low-Energy Effective Hamiltonians
-
- Book:
- Modern Condensed Matter Physics
- Published online:
- 26 February 2019
- Print publication:
- 28 February 2019, pp 675-679
-
- Chapter
- Export citation
JMR volume 34 issue 4 Cover and Front matter
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 4 / 28 February 2019
- Published online by Cambridge University Press:
- 28 February 2019, pp. f1-f5
- Print publication:
- 28 February 2019
-
- Article
-
- You have access
- Export citation
Frontmatter
-
- Book:
- Modern Condensed Matter Physics
- Published online:
- 26 February 2019
- Print publication:
- 28 February 2019, pp i-iv
-
- Chapter
- Export citation