Refine search
Actions for selected content:
106116 results in Materials Science
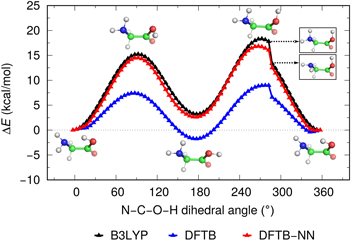
Artificial neural network correction for density-functional tight-binding molecular dynamics simulations
-
- Journal:
- MRS Communications / Volume 9 / Issue 3 / September 2019
- Published online by Cambridge University Press:
- 28 June 2019, pp. 867-873
- Print publication:
- September 2019
-
- Article
- Export citation
JMR volume 34 issue 12 Cover and Front matter
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 12 / 28 June 2019
- Published online by Cambridge University Press:
- 28 June 2019, pp. f1-f5
- Print publication:
- 28 June 2019
-
- Article
-
- You have access
- Export citation
Laser-induced structure transition of diamond-like carbon coated on cemented carbide and formation of reduced graphene oxide
-
- Journal:
- MRS Communications / Volume 9 / Issue 3 / September 2019
- Published online by Cambridge University Press:
- 28 June 2019, pp. 910-915
- Print publication:
- September 2019
-
- Article
- Export citation
Loading and release of a model high-molecular-weight protein from temperature-sensitive micro-structured hydrogels
-
- Journal:
- MRS Communications / Volume 9 / Issue 3 / September 2019
- Published online by Cambridge University Press:
- 28 June 2019, pp. 1041-1045
- Print publication:
- September 2019
-
- Article
- Export citation
JMR volume 34 issue 12 Cover and Back matter
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 12 / 28 June 2019
- Published online by Cambridge University Press:
- 28 June 2019, pp. b1-b5
- Print publication:
- 28 June 2019
-
- Article
-
- You have access
- Export citation
Effect of Gd3+ doping on structural, morphological, optical, dielectric, and nonlinear optical properties of high-quality PbI2 thin films for optoelectronic applications
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 16 / 28 August 2019
- Published online by Cambridge University Press:
- 28 June 2019, pp. 2765-2774
- Print publication:
- 28 August 2019
-
- Article
- Export citation
Powder X-ray structural studies and reference diffraction patterns for three forms of porous aluminum terephthalate, MIL-53(A1)
-
- Journal:
- Powder Diffraction / Volume 34 / Issue 3 / September 2019
- Published online by Cambridge University Press:
- 27 June 2019, pp. 216-226
-
- Article
- Export citation
Simulation studies of Sn-based perovskites with Cu back-contact for non-toxic and non-corrosive devices
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 16 / 28 August 2019
- Published online by Cambridge University Press:
- 24 June 2019, pp. 2789-2795
- Print publication:
- 28 August 2019
-
- Article
- Export citation
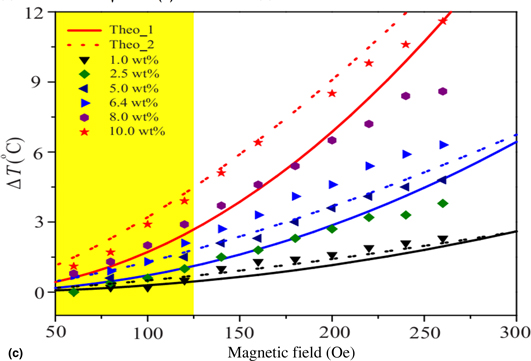
Role of nanoparticle interaction in magnetic heating
-
- Journal:
- MRS Communications / Volume 9 / Issue 3 / September 2019
- Published online by Cambridge University Press:
- 21 June 2019, pp. 1034-1040
- Print publication:
- September 2019
-
- Article
- Export citation
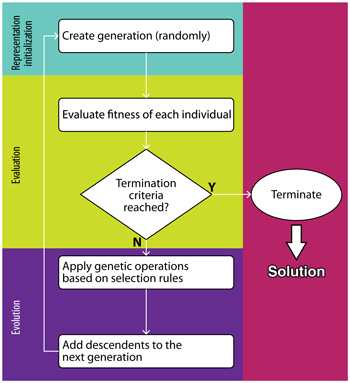
Symbolic regression in materials science
-
- Journal:
- MRS Communications / Volume 9 / Issue 3 / September 2019
- Published online by Cambridge University Press:
- 21 June 2019, pp. 793-805
- Print publication:
- September 2019
-
- Article
- Export citation
Chapter 5 - Molecular Modeling Applications in Crystallization
-
-
- Book:
- Handbook of Industrial Crystallization
- Published online:
- 14 June 2019
- Print publication:
- 20 June 2019, pp 136-171
-
- Chapter
- Export citation
Chapter 9 - Melt Crystallization
-
-
- Book:
- Handbook of Industrial Crystallization
- Published online:
- 14 June 2019
- Print publication:
- 20 June 2019, pp 266-289
-
- Chapter
- Export citation
Corn stover–derived biochar for efficient adsorption of oxytetracycline from wastewater
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 17 / 16 September 2019
- Published online by Cambridge University Press:
- 20 June 2019, pp. 3050-3060
- Print publication:
- 16 September 2019
-
- Article
- Export citation
Chapter 14 - Crystallization of Proteins
-
-
- Book:
- Handbook of Industrial Crystallization
- Published online:
- 14 June 2019
- Print publication:
- 20 June 2019, pp 414-459
-
- Chapter
- Export citation
Chapter 3 - Crystal Nucleation
-
-
- Book:
- Handbook of Industrial Crystallization
- Published online:
- 14 June 2019
- Print publication:
- 20 June 2019, pp 76-114
-
- Chapter
- Export citation
Chapter 10 - Crystallizer Mixing
-
-
- Book:
- Handbook of Industrial Crystallization
- Published online:
- 14 June 2019
- Print publication:
- 20 June 2019, pp 290-312
-
- Chapter
- Export citation
A novel and facile way to synthesize diamondoids nanowire cluster array
-
- Journal:
- Journal of Materials Research / Volume 34 / Issue 17 / 16 September 2019
- Published online by Cambridge University Press:
- 20 June 2019, pp. 2976-2982
- Print publication:
- 16 September 2019
-
- Article
- Export citation
Chapter 15 - Crystallization in Foods
-
-
- Book:
- Handbook of Industrial Crystallization
- Published online:
- 14 June 2019
- Print publication:
- 20 June 2019, pp 460-478
-
- Chapter
- Export citation
Chapter 1 - Solutions and Solution Properties
-
-
- Book:
- Handbook of Industrial Crystallization
- Published online:
- 14 June 2019
- Print publication:
- 20 June 2019, pp 1-31
-
- Chapter
- Export citation
Contents
-
- Book:
- Handbook of Industrial Crystallization
- Published online:
- 14 June 2019
- Print publication:
- 20 June 2019, pp v-vii
-
- Chapter
- Export citation