Refine search
Actions for selected content:
106116 results in Materials Science
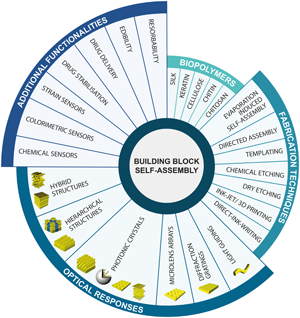
N-dimensional optics with natural materials
-
- Journal:
- MRS Communications / Volume 10 / Issue 2 / June 2020
- Published online by Cambridge University Press:
- 22 April 2020, pp. 201-214
- Print publication:
- June 2020
-
- Article
- Export citation
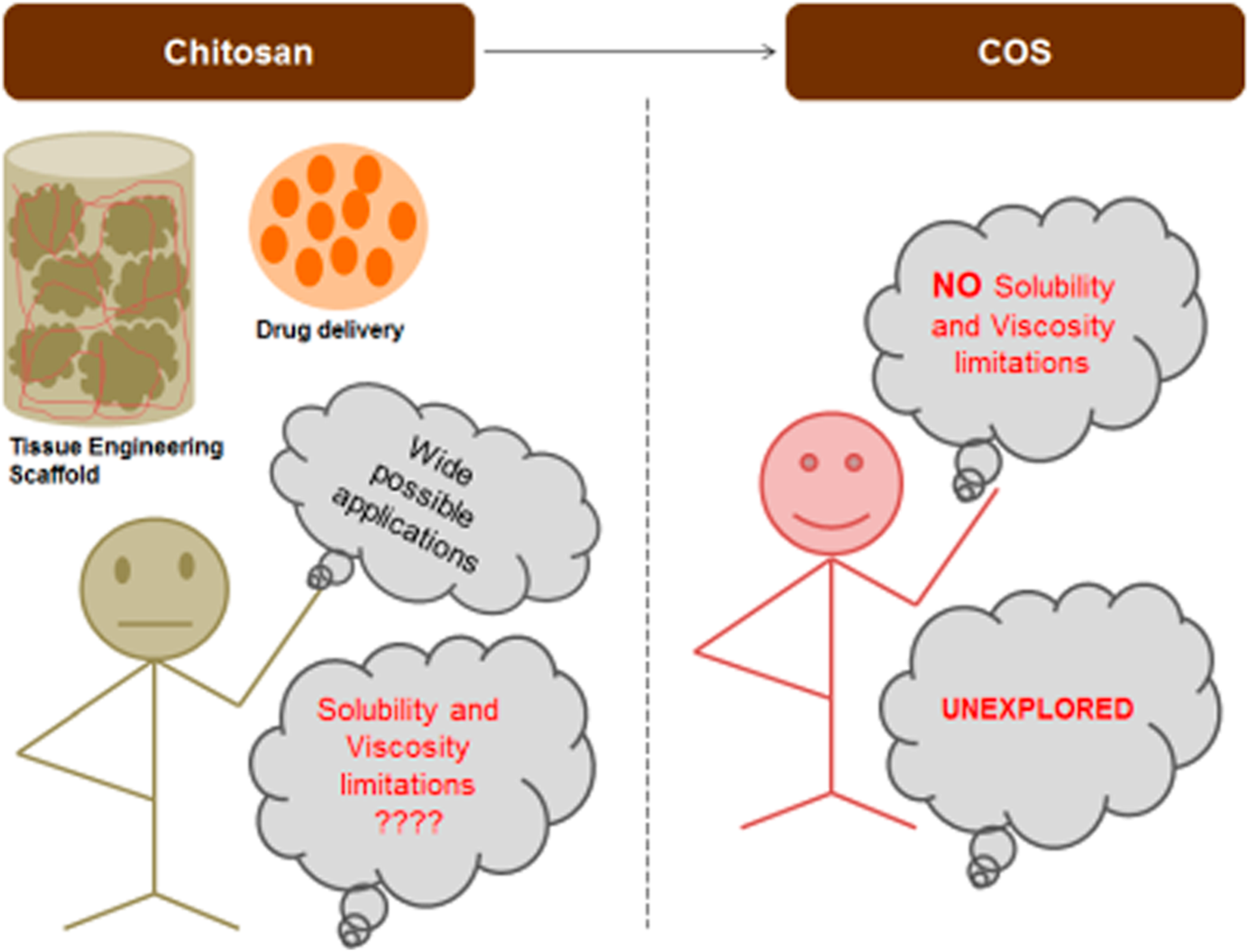
The virtuous potential of chitosan oligosaccharide for promising biomedical applications
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 9 / 14 May 2020
- Published online by Cambridge University Press:
- 21 April 2020, pp. 1123-1134
- Print publication:
- 14 May 2020
-
- Article
- Export citation
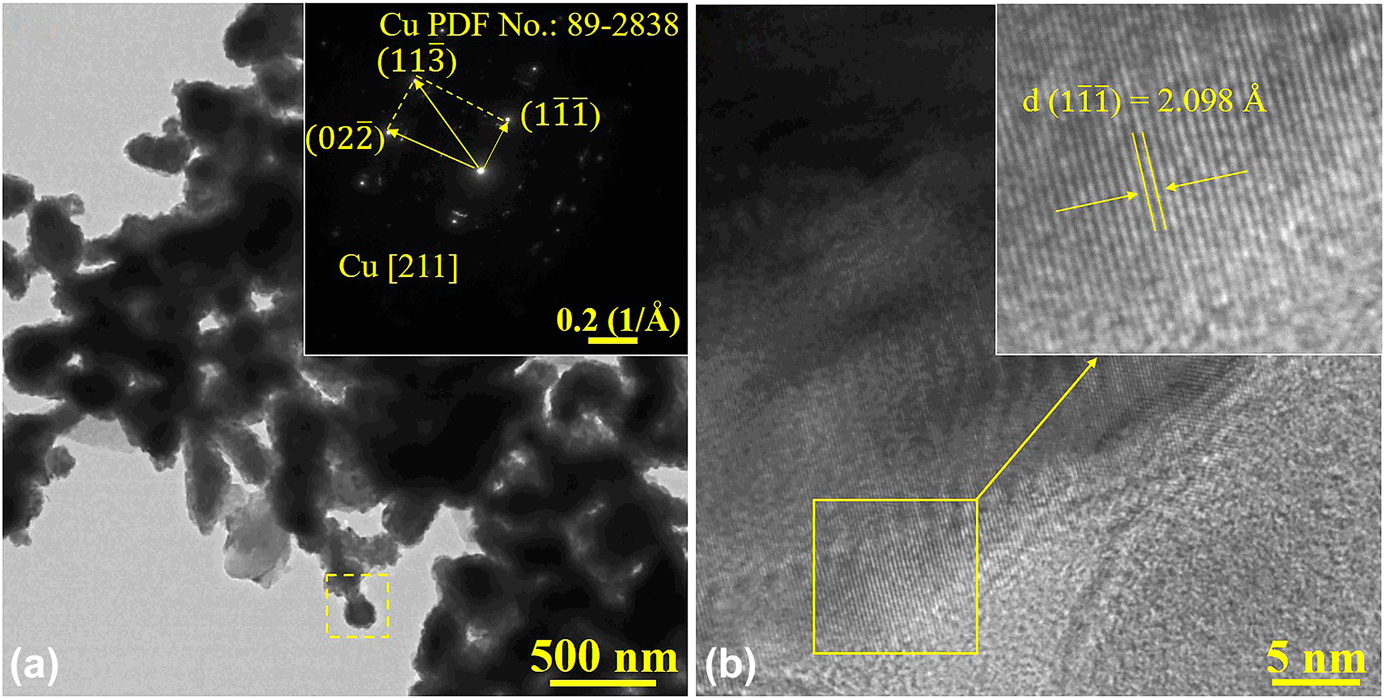
Influence of dealloying solution on the microstructure of nanoporous copper through chemical dealloying of Al75Cu25 ribbons
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 19 / 14 October 2020
- Published online by Cambridge University Press:
- 20 April 2020, pp. 2610-2619
- Print publication:
- 14 October 2020
-
- Article
- Export citation
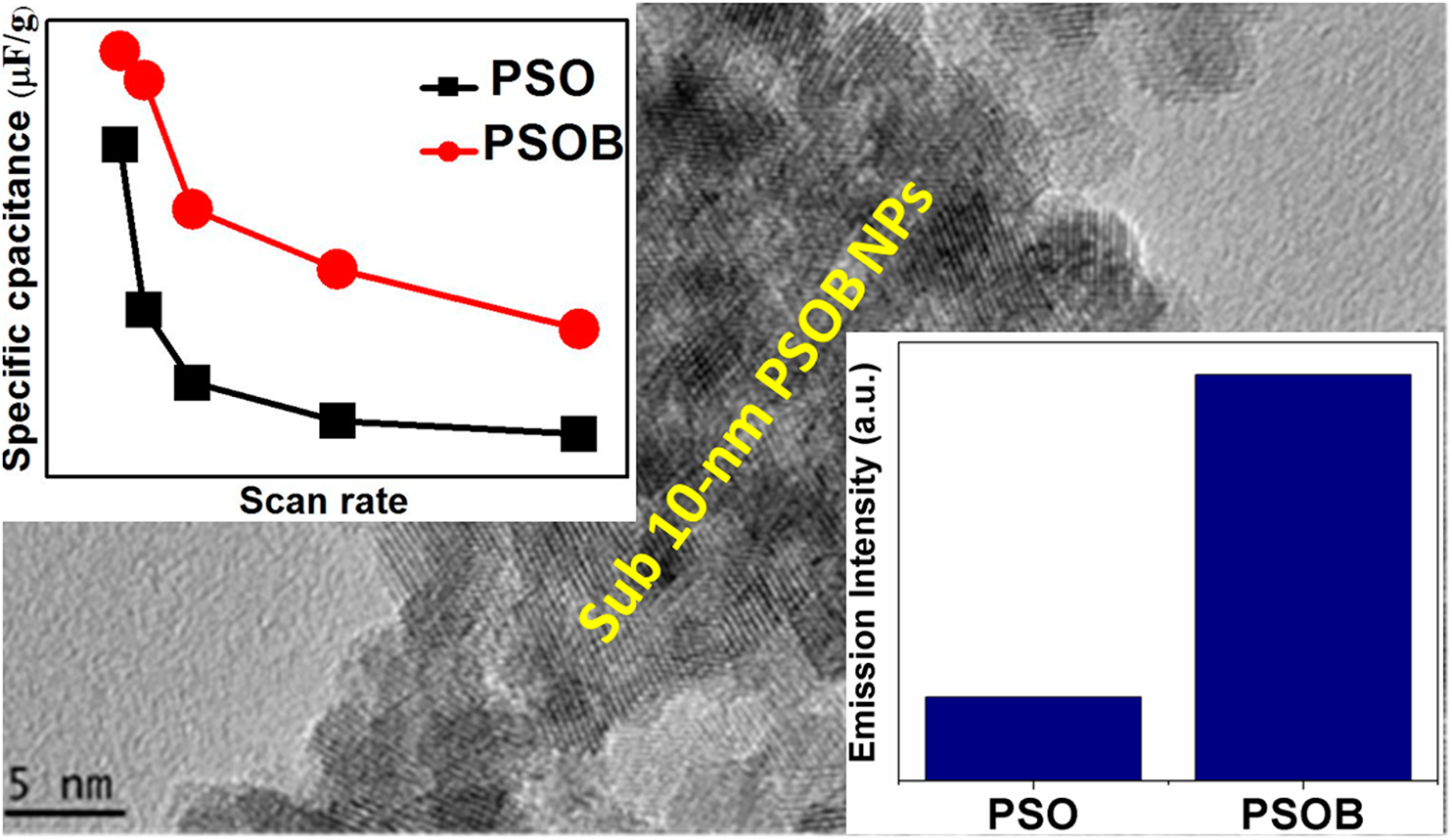
Defect-induced optical and electrochemical properties of Pr2Sn2O7 nanoparticles enhanced by Bi3+ doping
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 9 / 14 May 2020
- Published online by Cambridge University Press:
- 20 April 2020, pp. 1214-1224
- Print publication:
- 14 May 2020
-
- Article
- Export citation
Printing composite nanofilaments for use in a simple and low-cost 3D pen
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 9 / 14 May 2020
- Published online by Cambridge University Press:
- 20 April 2020, pp. 1154-1162
- Print publication:
- 14 May 2020
-
- Article
- Export citation
Microstructure and properties of a novel wear- and corrosion-resistant stainless steel fabricated by laser melting deposition
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 15 / 14 August 2020
- Published online by Cambridge University Press:
- 17 April 2020, pp. 2006-2015
- Print publication:
- 14 August 2020
-
- Article
- Export citation
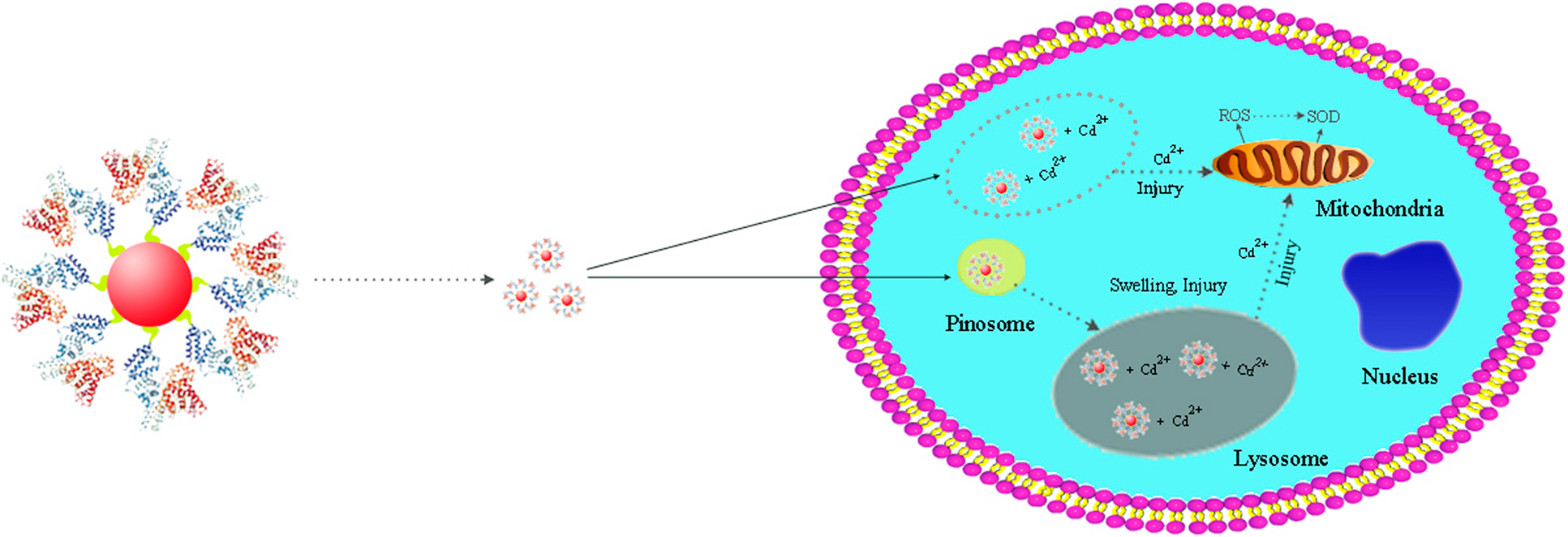
Study on cytotoxicity of polyethylene glycol and albumin bovine serum molecule–modified quantum dots prepared by hydrothermal method
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 9 / 14 May 2020
- Published online by Cambridge University Press:
- 17 April 2020, pp. 1135-1142
- Print publication:
- 14 May 2020
-
- Article
- Export citation
How to use and how not to use certified reference materials in industrial chemical metrology laboratories
-
- Journal:
- Powder Diffraction / Volume 35 / Issue 2 / June 2020
- Published online by Cambridge University Press:
- 15 April 2020, pp. 104-111
-
- Article
- Export citation
On the S-phase precipitates in 2024 aluminum alloy: An atomic-scale investigation using high-angle annular dark-field scanning transmission electron microscopy
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 12 / 29 June 2020
- Published online by Cambridge University Press:
- 15 April 2020, pp. 1582-1589
- Print publication:
- 29 June 2020
-
- Article
- Export citation
Preparation, microstructure, and microhardness of selective laser-melted W–3Ta sample
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 15 / 14 August 2020
- Published online by Cambridge University Press:
- 15 April 2020, pp. 2016-2024
- Print publication:
- 14 August 2020
-
- Article
- Export citation
X-ray diffraction as a major tool for the analysis of PM2.5 and PM10 aerosols
-
- Journal:
- Powder Diffraction / Volume 35 / Issue 2 / June 2020
- Published online by Cambridge University Press:
- 15 April 2020, pp. 98-103
-
- Article
- Export citation
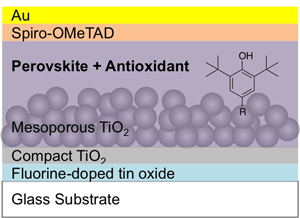
Phenolic antioxidant-incorporated durable perovskite layers and their application for a solar cell
-
- Journal:
- MRS Communications / Volume 10 / Issue 2 / June 2020
- Published online by Cambridge University Press:
- 15 April 2020, pp. 312-316
- Print publication:
- June 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Atomic-level study of twin–twin interactions in hexagonal metals
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 13 / 14 July 2020
- Published online by Cambridge University Press:
- 14 April 2020, pp. 1647-1659
- Print publication:
- 14 July 2020
-
- Article
- Export citation
Crystal and molecular structure of fenspiride, C15H20N2O2
-
- Journal:
- Powder Diffraction / Volume 35 / Issue 2 / June 2020
- Published online by Cambridge University Press:
- 14 April 2020, pp. 144-146
-
- Article
- Export citation
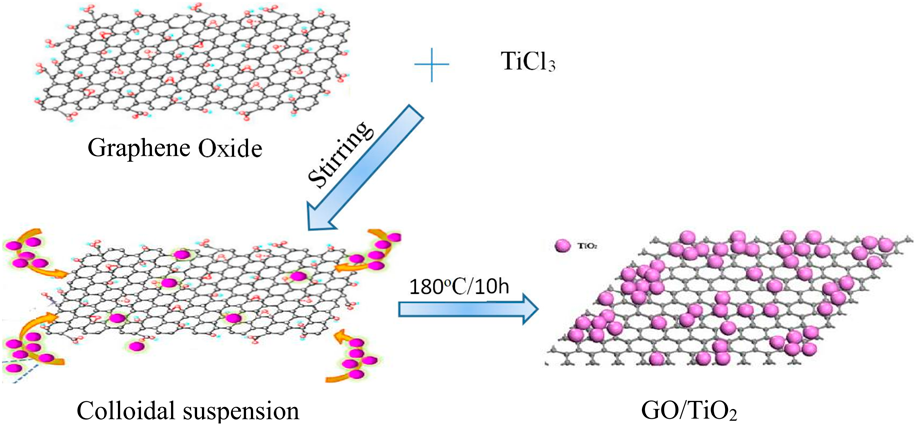
GO/TiO2 composites as a highly active photocatalyst for the degradation of methyl orange
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 10 / 28 May 2020
- Published online by Cambridge University Press:
- 14 April 2020, pp. 1307-1315
- Print publication:
- 28 May 2020
-
- Article
- Export citation
JMR volume 35 issue 7 Cover and Back matter
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 7 / 14 April 2020
- Published online by Cambridge University Press:
- 15 April 2020, pp. b1-b2
- Print publication:
- 14 April 2020
-
- Article
-
- You have access
- Export citation
JMR volume 35 issue 7 Cover and Front matter
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 7 / 14 April 2020
- Published online by Cambridge University Press:
- 15 April 2020, pp. f1-f5
- Print publication:
- 14 April 2020
-
- Article
-
- You have access
- Export citation
Introduction
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 7 / 14 April 2020
- Published online by Cambridge University Press:
- 15 April 2020, p. 655
- Print publication:
- 14 April 2020
-
- Article
-
- You have access
- HTML
- Export citation
Functionalization of single-layer TaS2 and formation of ultrathin Janus structures
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 11 / 15 June 2020
- Published online by Cambridge University Press:
- 08 April 2020, pp. 1397-1406
- Print publication:
- 15 June 2020
-
- Article
- Export citation
Growth characteristics of BaxSr(1−x)TiO3 thin films produced by micro-arc oxidation
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 13 / 14 July 2020
- Published online by Cambridge University Press:
- 07 April 2020, pp. 1703-1714
- Print publication:
- 14 July 2020
-
- Article
- Export citation