Refine search
Actions for selected content:
106116 results in Materials Science
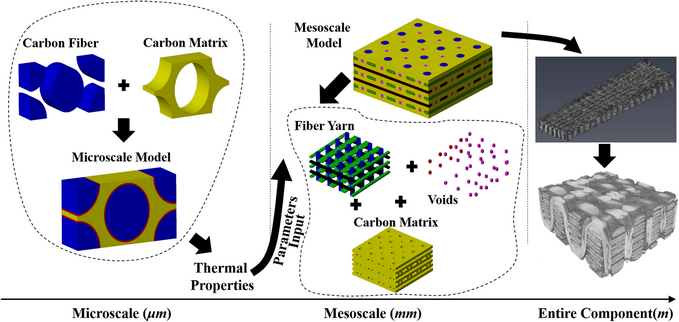
Numerical analysis of out-of-plane thermal conductivity of C/C composites by flexible oriented 3D weaving process considering voids and fiber volume fractions
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 14 / 28 July 2020
- Published online by Cambridge University Press:
- 22 July 2020, pp. 1888-1897
- Print publication:
- 28 July 2020
-
- Article
- Export citation
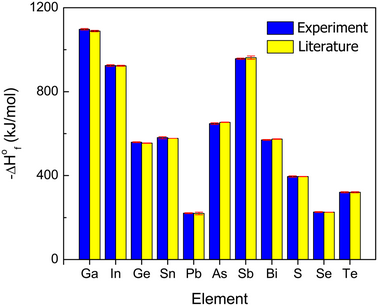
Development of high-temperature oxide melt solution calorimetry for p-block element containing materials
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 16 / 28 August 2020
- Published online by Cambridge University Press:
- 22 July 2020, pp. 2239-2246
- Print publication:
- 28 August 2020
-
- Article
- Export citation
Social Jetlag is Independently Associated with Chronotype and Poor Memory for Extinguished Fear
-
- Journal:
- Experimental Results / Volume 1 / 2020
- Published online by Cambridge University Press:
- 20 July 2020, e22
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
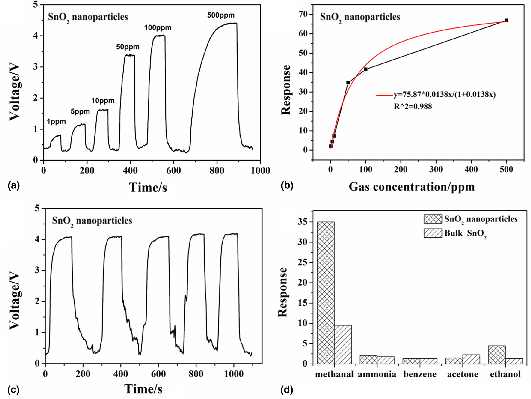
Synthesis of SnO2 nanoparticles for formaldehyde detection with high sensitivity and good selectivity
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 16 / 28 August 2020
- Published online by Cambridge University Press:
- 20 July 2020, pp. 2208-2217
- Print publication:
- 28 August 2020
-
- Article
- Export citation
g-C3N4 nanoparticle@porous g-C3N4 composite photocatalytic materials with significantly enhanced photo-generated carrier separation efficiency
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 16 / 28 August 2020
- Published online by Cambridge University Press:
- 20 July 2020, pp. 2148-2157
- Print publication:
- 28 August 2020
-
- Article
- Export citation
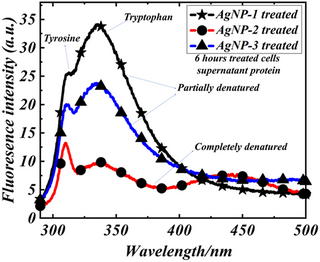
Molecular weight of polyethylenimine-dependent transfusion and selective antimicrobial activity of functional silver nanoparticles
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 18 / 28 September 2020
- Published online by Cambridge University Press:
- 20 July 2020, pp. 2405-2415
- Print publication:
- 28 September 2020
-
- Article
- Export citation
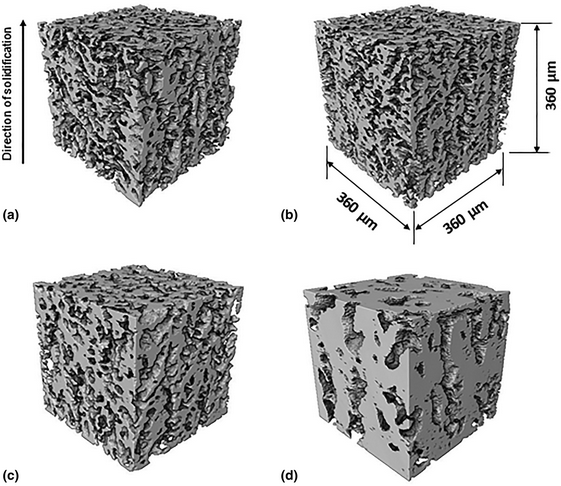
Structure–processing relationships of freeze-cast iron foams fabricated with various solidification rates and post-casting heat treatment
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 19 / 14 October 2020
- Published online by Cambridge University Press:
- 20 July 2020, pp. 2587-2596
- Print publication:
- 14 October 2020
-
- Article
- Export citation
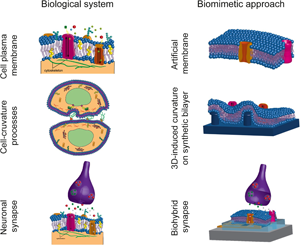
Towards biomimetic electronics that emulate cells
-
- Journal:
- MRS Communications / Volume 10 / Issue 3 / September 2020
- Published online by Cambridge University Press:
- 20 July 2020, pp. 398-412
- Print publication:
- September 2020
-
- Article
- Export citation
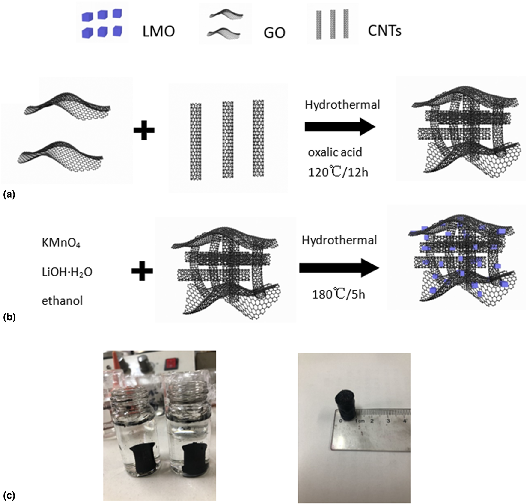
In situ low-temperature hydrothermal synthesis of LiMn2O4 nanocomposites based on graphene oxide/carbon nanotubes hydrogel and its capacities
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 18 / 28 September 2020
- Published online by Cambridge University Press:
- 17 July 2020, pp. 2516-2527
- Print publication:
- 28 September 2020
-
- Article
- Export citation
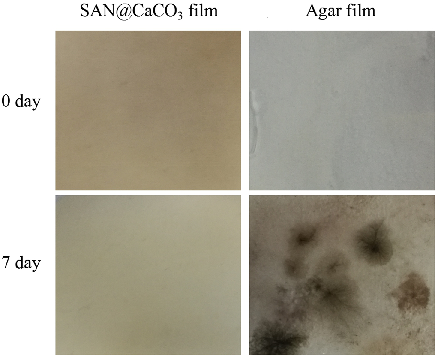
Biomimetic synthesis of vaterite CaCO3 microspheres under threonine for preparation of pH-responsive antibacterial biofilm
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 18 / 28 September 2020
- Published online by Cambridge University Press:
- 17 July 2020, pp. 2427-2440
- Print publication:
- 28 September 2020
-
- Article
- Export citation
Fabrication and characteristics of solar-driven phase change microcapsules with crystalline TiO2/CuS hybrid shell for solar energy conversion and storage
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 16 / 28 August 2020
- Published online by Cambridge University Press:
- 15 July 2020, pp. 2126-2137
- Print publication:
- 28 August 2020
-
- Article
- Export citation
Complications of using thin film geometries for nanocrystalline thermal stability investigations
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 16 / 28 August 2020
- Published online by Cambridge University Press:
- 15 July 2020, pp. 2087-2097
- Print publication:
- 28 August 2020
-
- Article
- Export citation
Application of deconvolutional treatment to powder diffraction data collected with a Bragg-Brentano diffractometer with a contaminated Cu target and a Ni filter
-
- Journal:
- Powder Diffraction / Volume 35 / Issue 3 / September 2020
- Published online by Cambridge University Press:
- 15 July 2020, pp. 166-177
-
- Article
- Export citation
Microstructures and mechanical properties of Ti–44Al–5Nb–3Cr–1.5Zr–xMo–yB alloys
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 20 / 28 October 2020
- Published online by Cambridge University Press:
- 15 July 2020, pp. 2756-2764
- Print publication:
- 28 October 2020
-
- Article
- Export citation
JMR volume 35 issue 13 Cover and Front matter
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 13 / 14 July 2020
- Published online by Cambridge University Press:
- 14 July 2020, pp. f1-f5
- Print publication:
- 14 July 2020
-
- Article
-
- You have access
- Export citation
JMR volume 35 issue 13 Cover and Back matter
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 13 / 14 July 2020
- Published online by Cambridge University Press:
- 14 July 2020, pp. b1-b3
- Print publication:
- 14 July 2020
-
- Article
-
- You have access
- Export citation
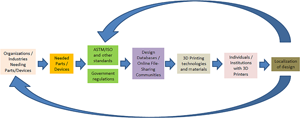
Additive manufacturing for COVID-19: devices, materials, prospects, and challenges
-
- Journal:
- MRS Communications / Volume 10 / Issue 3 / September 2020
- Published online by Cambridge University Press:
- 14 July 2020, pp. 413-427
- Print publication:
- September 2020
-
- Article
-
- You have access
- Open access
- HTML
- Export citation

Many-Body Theory of Condensed Matter Systems
- An Introductory Course
-
- Published online:
- 13 July 2020
- Print publication:
- 30 July 2020
Crystal structure and X-ray absorption spectroscopy of trimethylarsine oxide dihydrate, (CH3)3AsO⋅2H2O
-
- Journal:
- Powder Diffraction / Volume 35 / Issue 3 / September 2020
- Published online by Cambridge University Press:
- 13 July 2020, pp. 190-196
-
- Article
- Export citation
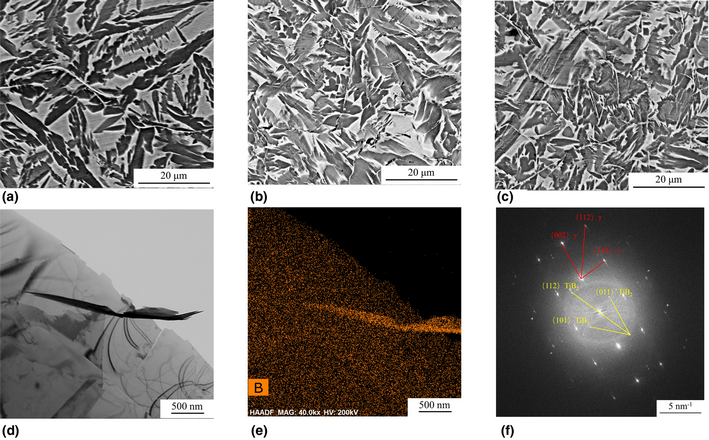
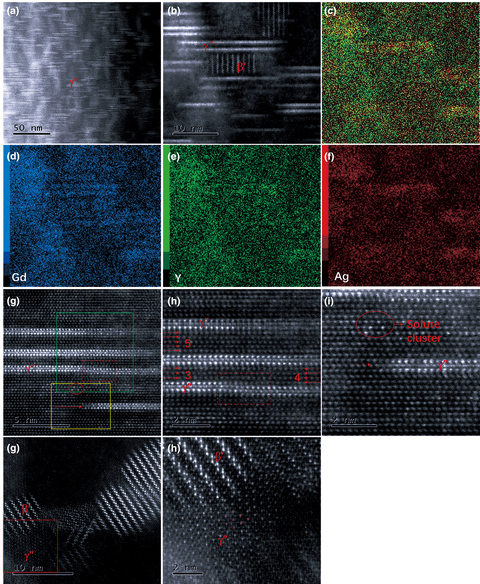
Atomic-scale insights on the plate-shaped γ″ phase in Mg–Gd–Y–Ag–Zr alloy
-
- Journal:
- Journal of Materials Research / Volume 35 / Issue 14 / 28 July 2020
- Published online by Cambridge University Press:
- 13 July 2020, pp. 1837-1845
- Print publication:
- 28 July 2020
-
- Article
- Export citation