Refine listing
Actions for selected content:
12321 results in Parasitology
Analysis of the mitochondrial genome to determine the origins and pathways of entry of Angiostrongylus cantonensis in continental Europe (Valencia, Spain) - CORRIGENDUM
-
- Journal:
- Parasitology / Volume 152 / Issue 2 / February 2025
- Published online by Cambridge University Press:
- 19 May 2025, p. 229
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Trypanosoma tertium n. sp.: prevalences in natural hosts and development in the mosquito vector
-
- Journal:
- Parasitology / Volume 152 / Issue 5 / April 2025
- Published online by Cambridge University Press:
- 28 April 2025, pp. 487-496
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Helminths assemblage in two opossum’s species, Didelphis albiventris and Didelphis aurita (Mammalia: Didelphimorphia), from the Atlantic Forest of Argentina
-
- Journal:
- Parasitology / Volume 152 / Issue 5 / April 2025
- Published online by Cambridge University Press:
- 24 April 2025, pp. 551-562
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
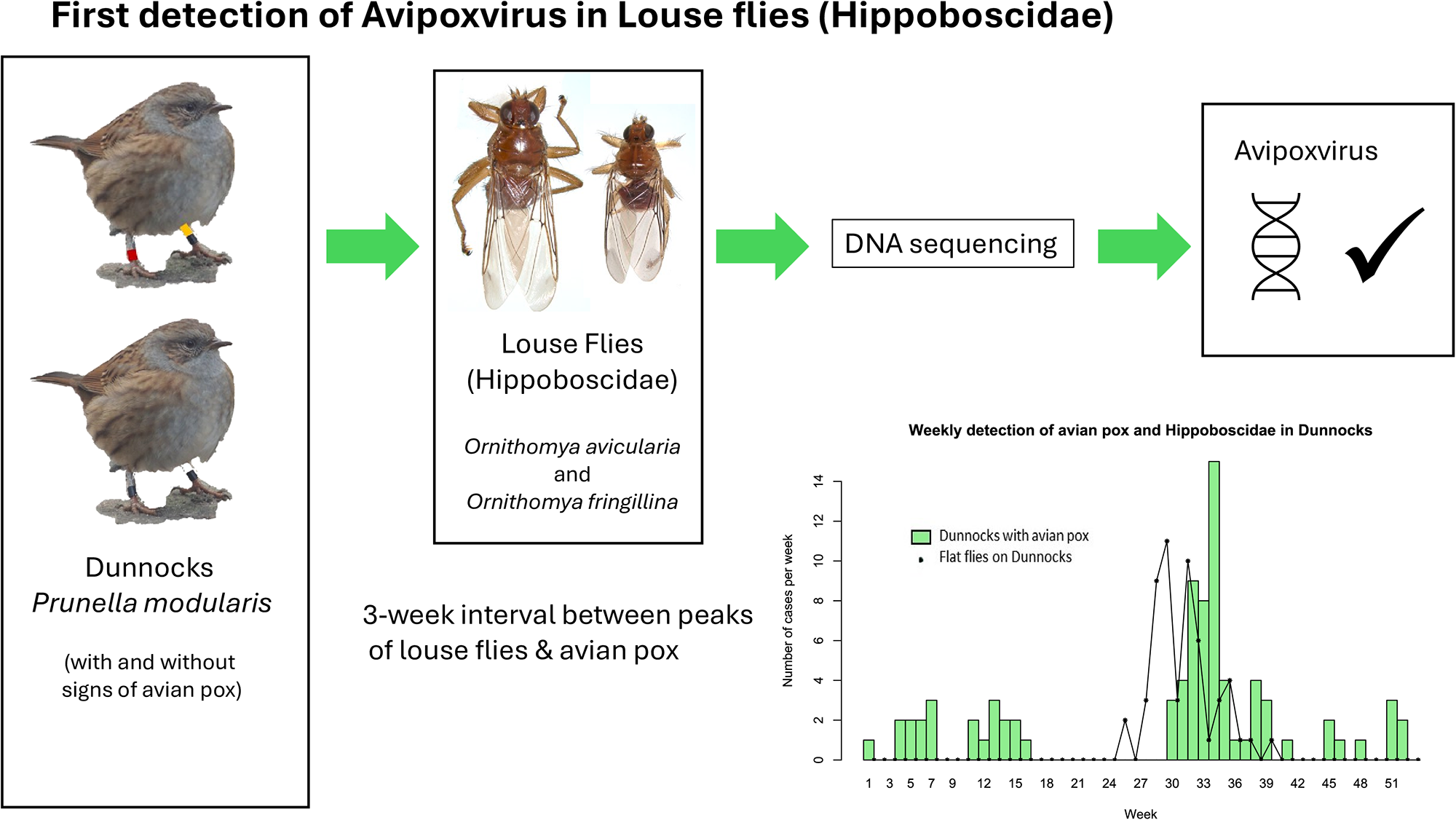
A first report of the detection of Avipoxvirus genomic sequences in louse flies (Diptera: Hippoboscidae)
-
- Journal:
- Parasitology / Volume 152 / Issue 5 / April 2025
- Published online by Cambridge University Press:
- 22 April 2025, pp. 522-530
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
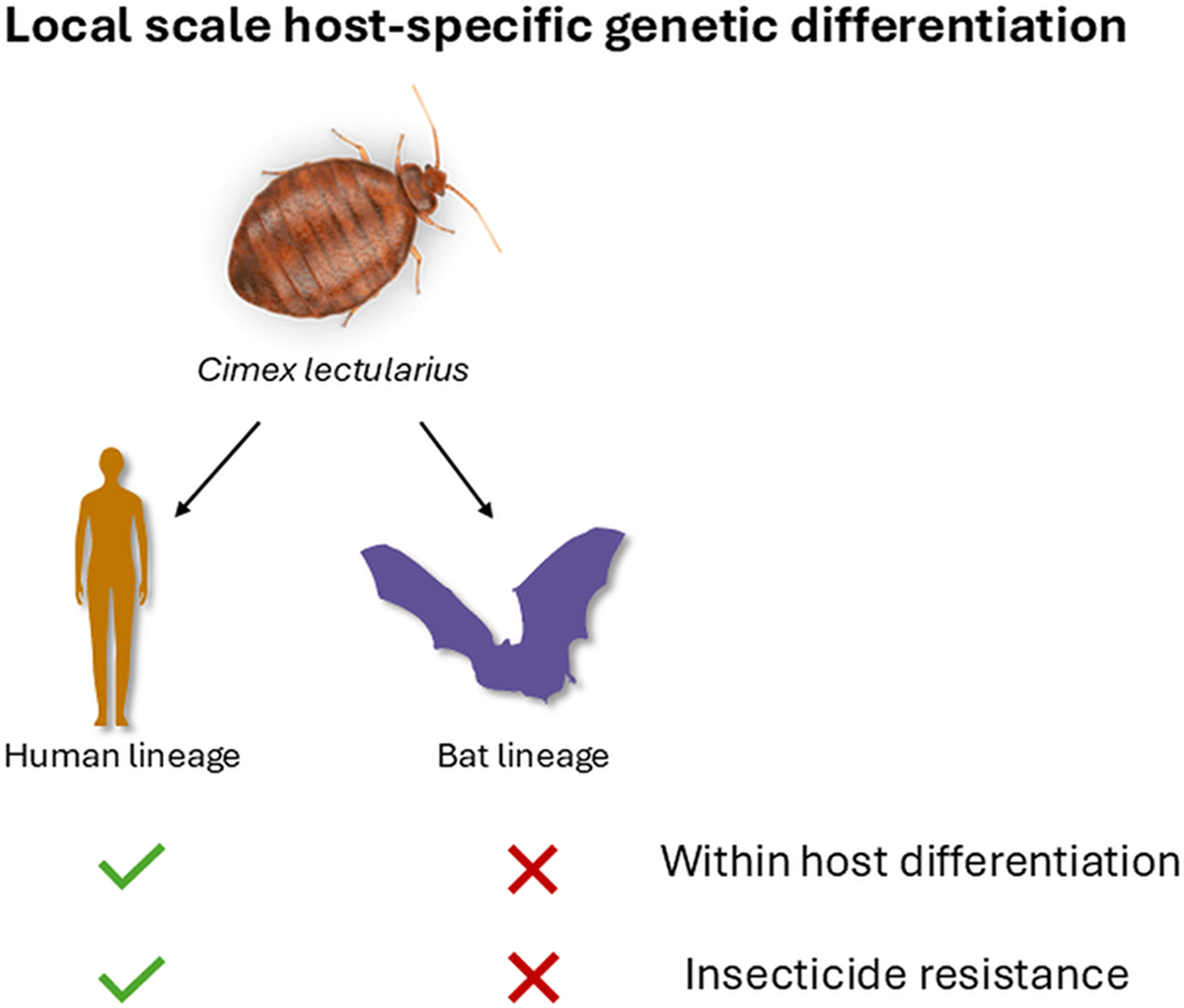
Genetic characterization of the bat and human lineages of the common bed bug (Cimex lectularius) at a local scale
-
- Journal:
- Parasitology / Volume 152 / Issue 5 / April 2025
- Published online by Cambridge University Press:
- 21 April 2025, pp. 510-521
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Molecular characterization of Spirometra isolates across the USA
-
- Journal:
- Parasitology / Volume 152 / Issue 5 / April 2025
- Published online by Cambridge University Press:
- 16 April 2025, pp. 477-486
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Angiopoietins as biomarkers of schistosomiasis severity: a cross-sectional study
-
- Journal:
- Parasitology / Volume 152 / Issue 3 / March 2025
- Published online by Cambridge University Press:
- 16 April 2025, pp. 330-337
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Toxoplasma gondii non-archetypal strain induces lung inflammation during acute and early chronic infection in mice
-
- Journal:
- Parasitology / Volume 152 / Issue 5 / April 2025
- Published online by Cambridge University Press:
- 14 April 2025, pp. 497-509
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
What’s so special about special issues: Highlighting a central role of parasitology to support specific innovations and advance progress within our discipline
-
- Journal:
- Parasitology / Volume 152 / Issue 1 / January 2025
- Published online by Cambridge University Press:
- 14 April 2025, pp. 1-5
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Highlights of Toxoplasma gondii research papers published in Parasitology in the last 5 decades: personal perspective
-
- Journal:
- Parasitology / Volume 152 / Issue 3 / March 2025
- Published online by Cambridge University Press:
- 11 April 2025, pp. 231-238
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Intestinal parasite infection in non-human primates from The Gambia, West Africa, and their relationship to human activity
-
- Journal:
- Parasitology / Volume 152 / Issue 4 / April 2025
- Published online by Cambridge University Press:
- 10 April 2025, pp. 469-476
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Molecular and morphological characterization of one known and three new species of fish parasitic Trypanosoma Gruby, 1972 from the south coast of South Africa
-
- Journal:
- Parasitology / Volume 152 / Issue 5 / April 2025
- Published online by Cambridge University Press:
- 08 April 2025, pp. 531-550
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Morphological and molecular characterization of the trematodes (Digenea: Acanthocolpidae and Cryptogonimidae) of the black-spotted croaker (Protonibea diacanthus) (Teleostei: Sciaenidae) in northern Australia
-
- Journal:
- Parasitology / Volume 152 / Issue 4 / April 2025
- Published online by Cambridge University Press:
- 04 April 2025, pp. 453-468
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Hybridization and introgression of the mitochondrial genome between the two species Anisakis pegreffii and A. simplex (s.s.) using a wide genotyping approach: evolutionary and ecological implications
-
- Journal:
- Parasitology / Volume 152 / Issue 3 / March 2025
- Published online by Cambridge University Press:
- 04 April 2025, pp. 293-313
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Uncovering human Dirofilaria repens infections: new cases in Southern Italy
-
- Journal:
- Parasitology / Volume 152 / Issue 4 / April 2025
- Published online by Cambridge University Press:
- 04 April 2025, pp. 399-408
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
A meta-analysis on Dirofilaria immitis and Dirofilaria repens in countries of North Africa and the Middle East
-
- Journal:
- Parasitology / Volume 152 / Issue 4 / April 2025
- Published online by Cambridge University Press:
- 01 April 2025, pp. 347-365
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Molecular analysis of Sarcoptes scabiei infecting wild and domestic South American camelids in Argentina
-
- Journal:
- Parasitology / Volume 152 / Issue 4 / April 2025
- Published online by Cambridge University Press:
- 28 March 2025, pp. 409-418
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Zoogonids (trematoda) infecting Indo-West Pacific damselfishes (Pomacentridae), including the proposal of a new genus and two new species
-
- Journal:
- Parasitology / Volume 152 / Issue 4 / April 2025
- Published online by Cambridge University Press:
- 28 March 2025, pp. 436-452
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Infection with Angiostrongylus cantonensis reveals up- and down-regulation of the protein profile in the mucus of infected slugs
-
- Journal:
- Parasitology / Volume 152 / Issue 4 / April 2025
- Published online by Cambridge University Press:
- 28 March 2025, pp. 395-398
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Spatial metabolomics to profile metabolic reprogramming of liver in Schistosoma japonicum-infected mice
-
- Journal:
- Parasitology , First View
- Published online by Cambridge University Press:
- 28 March 2025, pp. 1-9
-
- Article
-
- You have access
- Open access
- HTML
- Export citation