Chronic constipation is a common bowel disorder characterised by unsatisfactory defecation that results from infrequent bowel movements, difficult stool passage, or both(1). It affects approximately 10 % of the global population, although prevalence varies among countries and the diagnostic criteria used(Reference Barberio, Judge and Savarino2). Chronic constipation is commonly diagnosed using the symptom-based Rome IV criteria, according to which two or more of the following symptoms need to be present for the past three months: hard/lumpy stools, straining, a sense of incomplete evacuation, use of manual manoeuvres to facilitate stool passage, a sense of anorectal obstruction and having less than three bowel movements per week(Reference Mearin, Lacy and Chang3). However, people with constipation also report a wide range of other symptoms, such as no sensation of needing a bowel movement and frequent toilet trips without a successful bowel movement, that are burdensome and often require clinical care(Reference Dimidi, Cox and Grant4,Reference Dimidi, Dibley and Cotterill5) . Chronic constipation negatively affects patients’ quality of life and also leads to a considerable financial burden to patients and healthcare systems(Reference Belsey, Greenfield and Candy6–Reference Peery, Crockett and Murphy8). There are several treatment strategies for constipation, including dietary and lifestyle, pharmacological and behavioural treatments and more rarely, surgery. However, despite these, patients still report high dissatisfaction rates over the treatment options available to them primarily due to inadequate relief of their symptoms, emphasising the need for better access to evidence-based and effective management strategies(Reference Johanson and Kralstein9).
Diet plays an important role in the management of chronic constipation, and offers a cost-effective treatment strategy, compared to laxatives(Reference Han, Iragorri and Clement10). Several randomized controlled trials (RCT) have been conducted investigating the effect of nutritional supplements, foods, drinks and whole diets in chronic constipation(Reference van der Schoot, Creedon and Whelan11–Reference Van Der Schoot, Katsirma and Whelan14). Indeed, many clinical guidelines currently include dietary recommendations as a first-line management strategy, although they primarily focus on increasing fibre intake(Reference Chang, Chey and Imdad15,Reference Serra, Pohl and Azpiroz16) . Therefore, it is unclear whether the clinical guidelines represent the current evidence base. This review comprehensively examines the current evidence on the dietary management of chronic constipation, and the dietary recommendations presented in clinical guidelines for chronic primary constipation.
Dietary management of chronic constipation: research evidence
Dietary supplements
Fibre supplements
Fibre is a very common over-the-counter treatment strategy people with chronic constipation choose to use(Reference Johanson and Kralstein9). Fibre includes all carbohydrates that are neither digested nor absorbed in the small intestine and have a degree of polymerization of 3 or more monomeric units, plus lignin(17). The physicochemical properties of fibre, such as solubility, viscosity and fermentability, determine its mechanisms of action in the bowel and, thus, its ability to modify gut motility and improve constipation(Reference Gill, Rossi and Bajka18). Soluble, viscous fibres result to a stool softening effect by retaining water and creating a viscoelastic substance in the colon, thereby increasing stool bulk. Insoluble, non-viscous fibres result in a mechanical stimulation of the gut mucosa, which may result in a faster gut transit time. Fermentable fibres can lead to the production of fermentation byproducts, such as SCFA, that may stimulate gut motility, and may also increase stool weight by increasing microbial mass(Reference Gill, Rossi and Bajka18). Therefore, different types of fibre are expected to exert different effect on constipation.
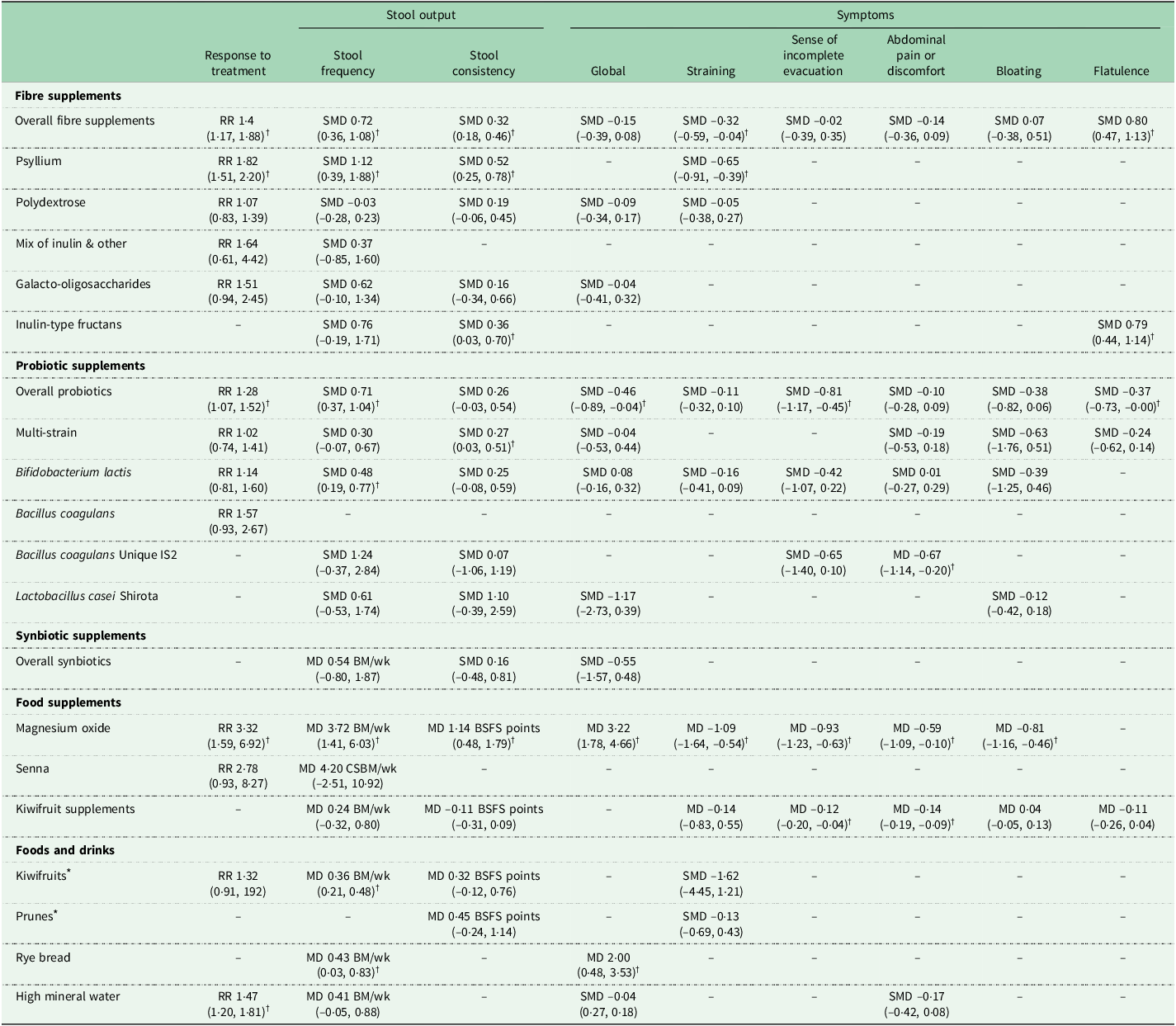
A systematic review and meta-analysis of 16 RCTs involving 1251 people with chronic constipation found that fibre supplements resulted in a significantly higher response to treatment (RR 1·48, 95 % CI 1·17, 1·88; P = 0·001) and stool frequency (SMD 0·72, 95 % CI 0·36, 10·8; P = 0·0001), compared to control(Reference van der Schoot, Drysdale and Whelan12). However, subgroup analyses showed that the effects differed based on the type of fibre used. Psyllium supplements, a soluble and viscous fibre, was consistently shown to lead to significantly higher response to treatment and stool frequency, softer stool consistency and lower straining, compared to control (Table 1)(Reference van der Schoot, Drysdale and Whelan12). Similarly, inulin-type fructan supplements, a soluble and fermentable fibre, softened stool consistency, but had no impact on stool frequency. However, polydextrose and galacto-oligosaccharide supplements did not improve any constipation outcomes; such contrary results are likely due to differences in the fibres’ physicochemical properties, but also mode of administration. For example, a higher stool frequency was shown for fibre doses of > 10 g/d (MD + 1·6 bowel movements/week, 95 % CI 0·7, 2·5, P = 0·0004) and treatment durations of ≥ 4 weeks (MD + 1·3 bowel movements/week, 95 % CI 0·6, 2·0, P = 0·0002)(Reference van der Schoot, Drysdale and Whelan12).
Table 1 The effect of dietary interventions on constipation outcomes as demonstrated in systematic reviews and meta-analyses of RCTs in people with chronic constipation(Reference van der Schoot, Creedon and Whelan11–Reference Van Der Schoot, Katsirma and Whelan14). Data provided as meta-analysis overall estimate (95 % CI)

RR, risk ratio; MD, mean difference; SMD, standardised mean difference; BM, bowel movements; CSBM, complete spontaneous bowel movements; wk, week; BSFS, Bristol Stool Form Scale.
* All interventions were compared to a negative control (e.g. placebo), except for those marked with *, which were compared with psyllium supplements.
† Outcome significantly different to placebo or comparator.
Importantly, while some fibres do improve some symptoms, they may also exacerbate others. For instance, although inulin-type fructans did soften stool consistency, they also increased severity of flatulence, compared to control (SMD 0·79, 95 % CI 0·44, 1·44, P < 0·001)(Reference van der Schoot, Drysdale and Whelan12). This is an expected outcome, given that inulin-type fructans are highly fermentable fibres, resulting in colonic gas production by the gut microbiota(Reference Gunn, Murthy and Major19).
Taken together, the evidence supports the use of psyllium supplements, at doses exceeding 10 g/d for at least 4 weeks, for the management of chronic constipation. A gradual dose increase is often recommended to minimise and closely monitor potential side effects, such as flatulence.
Magnesium oxide supplements
Magnesium oxide is converted to magnesium chloride in the stomach due to its acidic environment. It is further converted into magnesium bicarbonate by sodium bicarbonate in the duodenum, subsequently forming magnesium carbonate(Reference Mori, Tack and Suzuki20). Both magnesium bicarbonate and magnesium carbonate act as osmotic agents, promoting water retention in the gut lumen. This may result in softer stools and increased stool bulk, with the latter leading to mechanical stimulation of the gut wall and increased gut motility(Reference Mori, Tack and Suzuki20). Indeed, a RCT showed that magnesium oxide supplements significantly reduced gut transit time from baseline (mean 75·5, sd 37·3 h) to week 4 (mean 41·6, sd 30·5 h; P < 0·001), whereas no difference was found in the control group; however, no direct comparison between the two groups was reported at week 4(Reference Mori, Tomita and Fujimura21).
A systematic review and meta-analysis of two RCTs involving 94 healthy participants with chronic constipation showed that 68 % of those receiving magnesium oxide supplements responded to the treatment, compared to only 19 % in the control group (RR 3·32, 95 % CI 1·59, 6·92, P = 0·001). Magnesium oxide supplements significantly increased stool frequency by 3·72 complete spontaneous bowel movements (CSBM) per week (95 % CI 1·41, 6·03 CSBM/week, P = 0·0002) and softened stool consistency, compared to control. They also reduced global gut symptoms, as well as straining and incomplete evacuation (Table 1). The dose used in both RCTs was 1·5 g/d (0·5 g/d, three times a day) for four weeks. However, in one study, 16/30 (53 %) of participants in the magnesium oxide groups had a dose reduction, with 56 % of them ultimately receiving 0·5 g/d and 44 % receiving 1 g/d, due to treatment-related side effects of mild abdominal pain and diarrhoea(Reference Morishita, Tomita and Mori22). Therefore, magnesium oxide supplements should be initiated, when clinically appropriate, at a lower dose of 0·5 g/d and increased gradually based on symptom response and tolerance. However, dose should not surpass 1·5 g/d to avoid hypermagnesemia(Reference Mori, Tack and Suzuki20).
Probiotic and synbiotic supplements
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit to the host(Reference Hill, Guarner and Reid23). Some probiotics modulate the composition of the gut microbiota and their metabolite production, and also interact with the immune and enteric nervous system, the latter being the primary regulator of gut motility(Reference Dimidi, Christodoulides and Scott24). Therefore, there is potential that probiotics may improve constipation symptoms(Reference Dimidi, Mark Scott and Whelan25).
A survey of 934 people with self-reported constipation showed that 37 % have previously or are currently using probiotics for their gut health (OR 4·7, 95 % CI 3·8, 5·7, P < 0·001)(Reference Dimidi, Cox and Scott26). This suggests that people with chronic constipation commonly choose probiotics as a potential management option for their symptoms. However, the majority of GPs and gastrointestinal specialists do not recommend probiotics for constipation and, importantly, do not believe they have been tested in research studies in constipation(Reference Dimidi, Cox and Scott26). In fact, believing that probiotics have been tested in research for their effect in constipation was a significant predictor for probiotic use by people with constipation (OR 2·06, 95 % CI 1·56, 2·72, P < 0·001) and for doctors recommending them to patients for constipation (OR 1·91, 95 % CI 1·20, 3·03, P = 0·006)(Reference Dimidi, Cox and Scott26). This highlights not only the influence of perceived research evidence in choosing probiotics as a treatment for constipation but also the fact that it is imperative to appropriately communicate and educate the public and clinicians on the current evidence and its strength in this area.
Several systematic reviews have investigated the effect of probiotics in chronic constipation. The first systematic review and meta-analysis of 14 RCTs was published in 2014 and showed that overall, probiotics reduced whole gut transit time by –12·4 h (95 % CI –22·3, –2·5 h) and improved key symptoms of constipation, compared to placebo, although a high risk of bias and significant statistical heterogeneity was found across several outcomes(Reference Dimidi, Christodoulides and Fragkos27). Recently, an updated systematic review and meta-analysis of 30 RCTs also showed that overall, probiotics significantly increased response to treatment, compared to placebo (RR 1·28, 95 % CI 1·07, 1·52, P = 0·007) (Table 1).
Importantly, species- and strain-specific effects were observed for most constipation outcomes. Outcome-specific effects were also evident, with some probiotics improving certain constipation outcomes, but not others(Reference van der Schoot, Helander and Whelan13,Reference Dimidi, Christodoulides and Fragkos27) . Bacillus coagulans Unique IS2 significantly reduced frequency of defecation pain and abdominal pain, compared to placebo, but had no impact on stool frequency and consistency and sense of incomplete evacuation(Reference van der Schoot, Helander and Whelan13) (Table 1). Multi-strain probiotics significantly softened stool consistency (SMD 0·27, 95 % CI 0·03, 0·51, P = 0·03), but had no impact on response to treatment, stool frequency or global gut symptoms. Lactobacillus casei Shirota did not improve any of the outcomes evaluated, including stool output, global gut symptoms and severity of bloating and is thus not effective in relieving constipation(Reference van der Schoot, Helander and Whelan13).
B. lactis significantly increased stool frequency (SMD 0·48, 95 % CI 0·19, 0·77, P = 0·001), but not other outcomes, such as global gut symptoms or straining or severity(Reference van der Schoot, Helander and Whelan13,Reference Dimidi, Christodoulides and Fragkos27) . However, even within the same species, not all B. lactis strains were effective in increasing stool frequency. For example, a RCT involving 75 people with chronic constipation showed that the novel B. lactis NCC2818 strain had no impact on any gut-specific symptomatic, physiological or microbiological outcomes assessed, compared to placebo(Reference Dimidi, Zdanaviciene and Christodoulides28). In contrast, RCTs evaluating other strains, B. lactis DN-173 010, B. lactis HN019 and B. lactis LMG P-2138, each significantly increased stool frequency, highlighting the fact the effect on stool frequency is strain-specific(Reference Del Piano, Carmagnola and Anderloni29–Reference Ibarra, Latreille-Barbier and Donazzolo31).
Synbiotics are a mixture comprising live microorganisms and substrate(s) selectively utilised by host microorganisms that confers a health benefit on the host(Reference Swanson, Gibson and Hutkins32). A systematic review and meta-analysis identified four RCTs investigating synbiotic supplements made of various probiotics (multi-strain, B. lactis) and prebiotics (inulin and oligofructose, fructo-oligosaccharides) in chronic constipation(Reference van der Schoot, Helander and Whelan13). Synbiotic supplements had no impact on stool frequency and consistency and global symptoms, compared to placebo (Table 1), and are therefore not considered to be effective in constipation(Reference van der Schoot, Helander and Whelan13).
Other supplements
Senna supplements are stimulant anthraquinone laxatives made from extracts of the leaves and fruit of the plant species Cassia acutifolia and Cassia angustifolio, with the active components being the sennosides. They act on the myenteric plexus of the colon increasing peristalsis and reducing gut transit time(Reference Wilkins and Hardcastle33,Reference Ewe, Ueberschaer and Press34) . Until very recently, no placebo-controlled RCTs existed on the effectiveness of Senna supplements in chronic constipation. Instead, evidence for senna’s efficacy was drawn from RCTs conducted over 30 years ago, which compared senna supplements with another active comparator (psyllium; lactulose), and showed senna supplements to be as or even more effective than the active comparators in improving constipation outcomes(Reference Passmore, Davies and Flanagan35–Reference Marlett, Li and Patrow37). However, in all these RCTs, the senna supplements were combined with fibre and, therefore, any potential effects cannot be solely attributed to senna. Recently, however, two placebo-controlled RCTs in people with chronic constipation have been published and both showed that senna supplements led to higher stool frequency, softer stool consistency, compared to placebo(Reference Morishita, Tomita and Mori22,Reference Zhong, Cheng and Kun38) . However, when these two trials were meta-analysed together, response to treatment, stool frequency and stool consistency were no longer significant (Table 1)(Reference van der Schoot, Creedon and Whelan11). This may be attributed to the very different effect sizes for these outcomes, contributing to a large CI in the meta-analysis.
Kiwifruit supplements, administered as freeze-dried powders, contain kiwifruit-derived bioactive components, such as actinidin, polyphenols and fibre. A systematic review and meta-analysis of three RCTs involving 135 people with chronic constipation showed that kiwifruit supplements had no impact on stool frequency, stool consistency, straining, use of manual manoeuvres to facilitate defecation, or bloating, but did improve the sense of incomplete evacuation and abdominal pain, compared to placebo (Table 1)(Reference van der Schoot, Creedon and Whelan11).
Foods and drinks
Fruits
Several fruits have been investigated in RCTs for their effectiveness in chronic constipation(Reference Van Der Schoot, Katsirma and Whelan14), including kiwifruits, prunes, mangos and figs. A systematic review and meta-analysis of RCTs that compared whole fruits (kiwifruits, prunes, mangos; studied separately) to psyllium supplements (i.e. active comparator) showed that fruits resulted in a similar overall response to treatment (RR 1·18, 95 % CI 0·29, 1·58, P = 0·25) and gut symptom response, compared to psyllium supplements(Reference Van Der Schoot, Katsirma and Whelan14). They were, however, even more effective that psyllium in increasing stool frequency (MD + 0·36 bowel movements/week, 95 % CI 0·24, 0·48, P < 0·0001) and softening stool consistency (MD + 0·48 points in Bristol Stool Form Scale (BSFS), 95 % CI 0·11, 0·86, P = 0·01), although the clinical meaningfulness of these effect sizes is limited. The fact that none of the outcomes assessed favoured psyllium over these fruits, potentially indicates that certain fruits may be as effective as psyllium in improving constipation outcomes.
Kiwifruit is the most extensively studied fruit in RCTs on chronic constipation. Kiwifruits are high in soluble and insoluble fibre, and have been shown to increase butyrate-producing bacteria (e.g. Feacalibacterium prausnitzii) and Bifidobacterium ssp in a human gastrointestinal model simulating mild constipation(Reference Goya-Jorge, Bondue and Gonza39). A cross-over RCT in healthy people using MRI techniques to identify kiwifruits’ mechanisms of action found that the consumption of four kiwifruits a day led to higher small bowel water retention and increased total colonic volume, compared to an isocaloric control(Reference Wilkinson-Smith, Dellschaft and Ansell40). Kiwifruits also contain actinidin, a proteolytic enzyme, with preliminary evidence suggesting a potential impact on gut motility(Reference Bayer, Blair and Drummond41). A systematic review and meta-analysis identified 4 RCTs in people with chronic constipation that compared 2–3 kiwifruits, consumed without the skin, per day to a psyllium supplement (i.e. active control)(Reference Van Der Schoot, Katsirma and Whelan14). Kiwifruits were as effective as psyllium supplements in softening stool consistency and reducing frequency of straining, and were more effective than psyllium in increasing stool frequency (MD + 0·36, 95 % CI 0·24, 0·48, P < 0·0001), suggesting kiwifruits may be an effective management option for constipation(Reference Van Der Schoot, Katsirma and Whelan14).
Prunes (dried plums) are high in fibre, including hemicellulose, pectin and cellulose, as well as sorbitol. They increase stool weight, but not stool water, indicating that prunes increase stool bulk, rather than stool water per se (Reference Lever, Scott and Louis42,Reference Katsirma, Dimidi and Rodriguez-Mateos43) . A trend in increasing bifidobacteria has also been shown when consuming prunes for 4 weeks in a RCT, compared to control (P = 0·057)(Reference Lever, Scott and Louis42). A systematic review and meta-analysis identified two RCTs in people with chronic constipation that compared whole prune consumption to a psyllium supplement (active control)(Reference Van Der Schoot, Katsirma and Whelan14). Prunes were as effective as psyllium supplements in softening stool consistency (MD: +0·45 BSFS points, 95 % CI –0·24, 1·14, P = 0·20) and reducing severity of straining (Table 1)(Reference Van Der Schoot, Katsirma and Whelan14). One RCT examined the effect of 54 g/d prune juice, compared to a control juice, in 84 people with chronic constipation and showed that prune juice also significantly softened stool consistency, compared with control (3·57 ± 0·81 v. 3·03 ± 1·10 BSFS points, P = 0·012), but had no impact on stool frequency, flatulence or sense of incomplete evacuation(Reference Koyama, Nagata and Nishiura44).
Adverse events were reported in a partially RCT that investigated whole kiwifruits, whole prunes and psyllium supplements(Reference Chey, Chey and Jackson45). Kiwifruit led to significantly fewer adverse events in terms of abdominal pain, compared to both the prunes and psyllium groups (P = 0·02). Both kiwifruit and psyllium led to fewer adverse events of bloating, compared to prunes (P = 0·03). Therefore, kiwifruits may be better tolerated in people with chronic constipation, compared to prunes and psyllium, and may be a more suitable treatment option to those who experience abdominal pain and bloating symptoms.
Mango has been investigated in one RCT only that compared 300 g/d mango to psyllium supplement (i.e. active control) in 48 people with chronic constipation and showed a significant improvement in softening stool consistency (MD + 1·41 BSFS points, 95 % CI 0·58, 2·24, P = 0·0008), but no difference was shown for global gut symptoms, compared to psyllium(Reference van der Schoot, Creedon and Whelan11).
Fig paste has been investigated in a double-blind RCT that compared 300 g/d fig paste to a control paste containing sugar and modified starch in 80 people with chronic constipation. Fig paste resulted in significantly softer stool consistency and shorter gut transit time (38·7 ± 20·3 h v. 46·7 ± 16·3 h, P = 0·045), though no impact was found on stool frequency, abdominal pain, sense of incomplete evacuation or effort for evacuation(Reference Baek, Ha and Kim46).
Taken together, the evidence suggests certain fruits, particularly kiwifruit and prunes which have been each studied in at least two RCTs, may be effective in improving specific constipation outcomes.
Flaxseeds
Flaxseeds, a rich source of fibre, have been investigated in chronic primary constipation in only one RCT that compared 50 g/d flaxseed flour with meals to a lactulose solution (i.e. active control). Flaxseeds led to higher stool frequency and lower global gut symptoms, compared to control, although the authors acknowledged the effect size being small and, thus, likely not clinically meaningful(Reference Sun, Bai and Ma47). Therefore, there is currently lack of evidence supporting the use of flaxseeds in chronic constipation.
Cereal-based foods
Rye bread, a rich source of fibre, has been investigated in two RCTs that compared 6–8 slices of rye bread with white bread (control) in 48 people with chronic constipation(Reference Van Der Schoot, Katsirma and Whelan14). Rye bread has been shown to significantly increase stool weight and decrease gut transit time (MD –20·94 h, 95 % CI –29·35, –0·53, P < 0·001), compared to white bread. Although it significantly increased stool frequency, it also worsened global gut symptoms (P = 0·01) (Table 1)(Reference Van Der Schoot, Katsirma and Whelan14). This is likely due to the high dose of rye bread (6–8 slices daily) and, therefore, large increase in fructan and fructose intake, which may had deteriorated symptoms related to fermentation-induced colonic gas production (e.g. flatulence)(Reference Van Der Schoot, Katsirma and Whelan14).
There is a lack of RCTs on the effect of other cereal-based foods in chronic constipation, such as oats. One uncontrolled trial in 50 people with chronic constipation showed that 2 oat biscuits per day increased stool frequency and softened stool consistency, compared to baseline(Reference Valle-Jones48). Another uncontrolled trial on a high-fibre cereal in 41 women with disorders of the pelvic floor also showed increased stool frequency and improved global symptom scores, straining and sense of incomplete evacuation, compared to baseline(Reference Shariati, Maceda and Hale49). However, these findings have yet to be confirmed in RCTs.
Dairy foods
Fermented dairy foods have been shown to modulate the gut microbiome and the production of fermentation-derived metabolites (e.g. SCFA), and to modulate immune responses(Reference Dimidi, Cox and Rossi50,Reference Mukherjee, Breselge and Dimidi51) ; therefore, there is a potential that certain fermented dairy foods could impact constipation outcomes, though this remains to be confirmed in robust clinical trials. Pasteurised yoghurt has been investigated in one RCT that compared 220 ml/d pasteurised yoghurt to pasteurised milk in 120 people with chronic constipation. Pasteurised yoghurt led to significantly higher stool frequency, compared to control(Reference Liu, Xu and Han52). Although findings on gut symptoms were numerically described, no inferential statistics were reported and, thus, no conclusions could be made on the impact on gut symptoms. The RCT was rated to be at high risk of bias, as assessed using the Cochrane Risk of Bias 2.0 tool(Reference Van Der Schoot, Katsirma and Whelan14,Reference Sterne, Savovic and Page53) .
Kefir, a fermented food, has only been investigated in uncontrolled studies in chronic constipation(Reference Dimidi, Cox and Rossi50). One uncontrolled trial administered 500 ml/d kefir for four weeks in 20 people with chronic constipation and showed an increase in stool frequency and improvement in bowel satisfaction scores, compared to baseline(Reference Turan, Dedeli and Bor54). However, these findings have yet to be confirmed in RCTs.
Water
Water has been studied in RCTs both in terms of its mineral content, and also quantity consumed. High mineral-content water is rich in magnesium sulphate, which has an osmotic effect in the gut lumen, potentially leading to softer stools(Reference Van Der Schoot, Katsirma and Whelan14). A systematic review and meta-analysis of four RCTs in 755 people with chronic constipation showed that a significantly higher proportion of people responded to high mineral-content water, compared to low mineral-content water (RR 1·47, 95 % CI 1·20, 1·81, P = 0·0002)(Reference Van Der Schoot, Katsirma and Whelan14). However, no difference was shown for stool frequency, global gut symptoms and abdominal pain/discomfort (Table 1).
Water supplementation (2 l/d) was investigated in one RCT in 141 people with chronic constipation, compared to ad libitum water consumption (1·1 l/d); both groups also followed a diet containing 25 g/d fibre. Water supplementation resulted in higher stool frequency (mean 4·2, sd 1·3 v. mean 3·3, sd 1·8 bowel movements/week, P < 0·001) and lower laxative use, compared to control.
Taken together, high mineral-content water may be beneficial for the management of chronic constipation. There is however no evidence suggesting that increasing total water intake beyond public health recommendations improves constipation outcomes.
Whole diets
Despite studies suggesting that specific fibre supplements (psyllium) and foods high in fibre (e.g. kiwifruits) may improve constipation outcomes, there is limited evidence on the effect of high-fibre diet, in which fibre comes from a wide and varied range of foods and drinks(Reference Van Der Schoot, Katsirma and Whelan14). One RCT investigated a high-fibre diet (25–30 g/d) compared to a low-fibre diet (control, 15–20 g/d) for 9 weeks in 44 people with chronic constipation(Reference Mego, Huaman and Videla55). There was no difference in response to treatment, stool frequency, stool consistency or gut transit time between the two groups. In fact, the high-fibre diet led to worse flatulence (P = 0·03), abdominal distension (P = 0·04) and digestive well-being (P = 0·04), compared to the low-fibre diet. This RCT was rated to be at high risk of bias, as assessed using the Cochrane Risk of Bias 2.0 tool(Reference Van Der Schoot, Katsirma and Whelan14,Reference Sterne, Savovic and Page53) .
Several uncontrolled trials have also investigated the effect of a high- or low-fibre diet in chronic constipation. One uncontrolled trial investigated stopping or lowering dietary fibre intake for six months in 63 people with chronic constipation. According to their fibre intake after six months, participants were categorised as ‘no fibre diet’, ‘reduced fibre diet’ and ‘high fibre diet’. Unexpectedly, authors reported that participants in the ‘no fibre diet’ and ‘reduced fibre diet’ groups experienced higher stool frequency and fewer gut symptoms, compared to baseline, whereas those in the ‘high fibre diet’ did not experience any improvement(Reference Ho, Tan and Mohd Daud56). Another uncontrolled diet investigated the effect of a digital tool administering personalised high-fibre dietary advice, that resulted in an increase in fibre intake of 5·1 g/d (sd 6·4 g). This resulted in reduced constipation severity, softer stool consistency and improved abdominal pain at week 8, compared to baseline. However, although the change in fibre intake from baseline to week 8 was reported, the absolute fibre intake at week 8 remains unclear(Reference Rijnaarts, de Roos and Wang57).
Taken together, there is currently lack of evidence to suggest that a high-fibre diet, in which fibre comes from a wide and varied range of foods and drinks, is effective in managing symptoms of chronic constipation, and further RCTs are warranted in this area.
Dietary management of chronic constipation: clinical guidelines
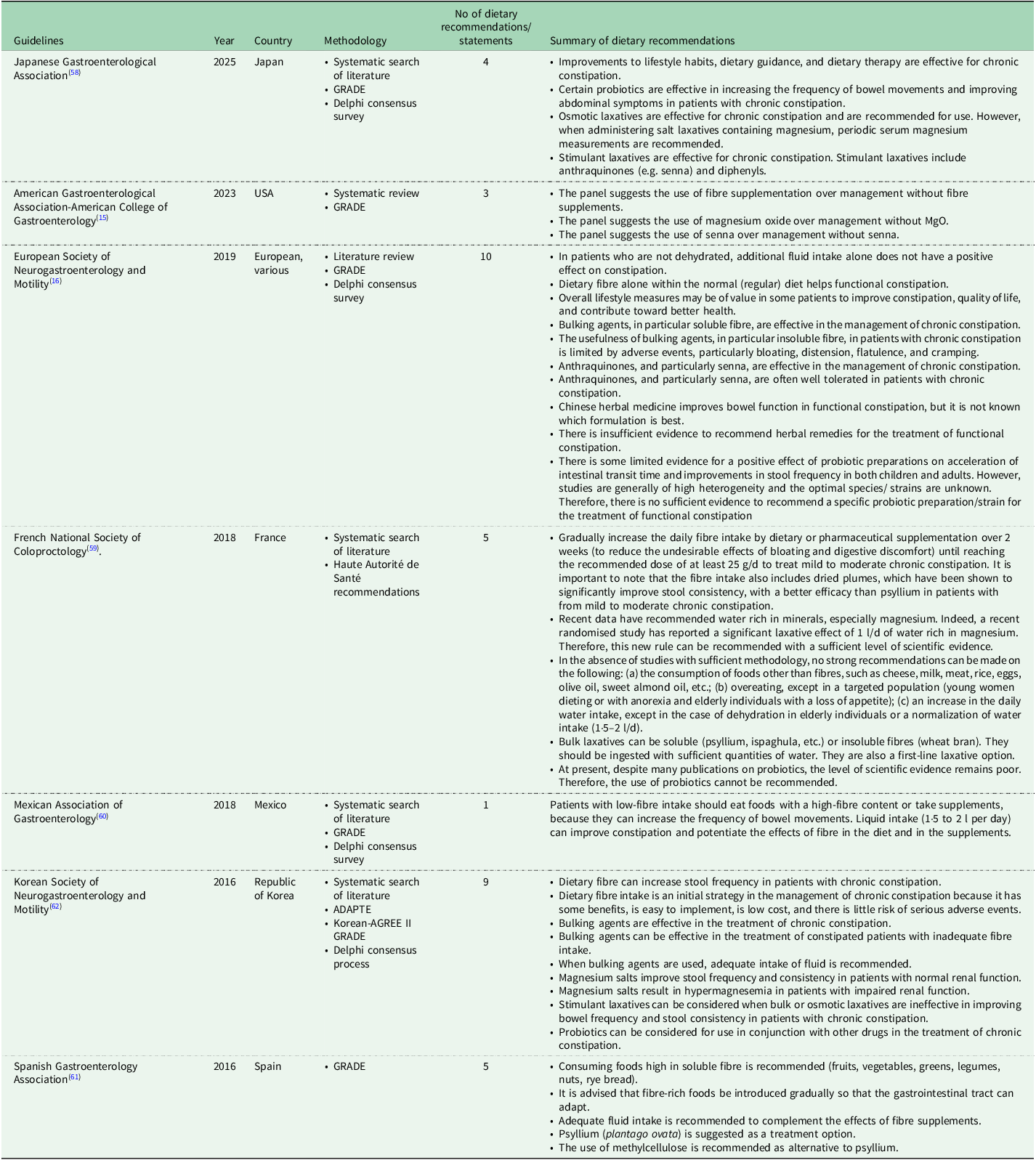
Several national and international clinical guidelines have been published in the past decade for the management of chronic constipation, including those developed by the Japanese Gastroenterological Association (Japan, 2025)(Reference Ihara, Manabe and Ohkubo58), American Gastroenterological Association-American College of Gastroenterology (US, 2023)(Reference Chang, Chey and Imdad15), European Society of Neurogastroenterology and Motility (Europe, 2020)(Reference Serra, Pohl and Azpiroz16), French National Society of Coloproctology (France, 2018)(Reference Vitton, Damon and Benezech59), Mexican Association of Gastroenterology (Mexico, 2018)(Reference Remes-Troche, Coss-Adame and Lopéz-Colombo60), Spanish Gastroenterology Association (Spain, 2017)(Reference Serra, Mascort-Roca and Marzo-Castillejo61) and Korean Society of Neurogastroenterology and Motility (Korea, 2016)(Reference Shin, Jung and Lee62) (Table 2). The methodology followed to develop these seven clinical guidelines varied. To identify relevant evidence, five of the seven guidelines performed a systematic search of the literature (Japan, US, France, Mexico, Korea)(Reference Chang, Chey and Imdad15,Reference Ihara, Manabe and Ohkubo58–Reference Remes-Troche, Coss-Adame and Lopéz-Colombo60,Reference Shin, Jung and Lee62) , one performed a literature review (Europe)(Reference Serra, Pohl and Azpiroz16) and one did not state a method of identifying evidence (Spain)(Reference Serra, Mascort-Roca and Marzo-Castillejo61). Six of seven clinical guidelines used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to rate the level of evidence and strength of recommendations (Japan, US, Europe, Mexico, Korea and Spain)(Reference Chang, Chey and Imdad15,Reference Serra, Pohl and Azpiroz16,Reference Ihara, Manabe and Ohkubo58,Reference Remes-Troche, Coss-Adame and Lopéz-Colombo60–Reference Shin, Jung and Lee62) , and one used the Haute Autorité de Santé recommendations to rate the level of evidence (France)(Reference Vitton, Damon and Benezech59). Four clinical guidelines also performed a Delphi expert consensus survey to establish the final recommendations (Japan, Europe, Mexico and Korea)(Reference Serra, Pohl and Azpiroz16,Reference Shin, Jung and Lee62) .
Table 2 Dietary recommendations in clinical guidelines for the management of chronic constipation

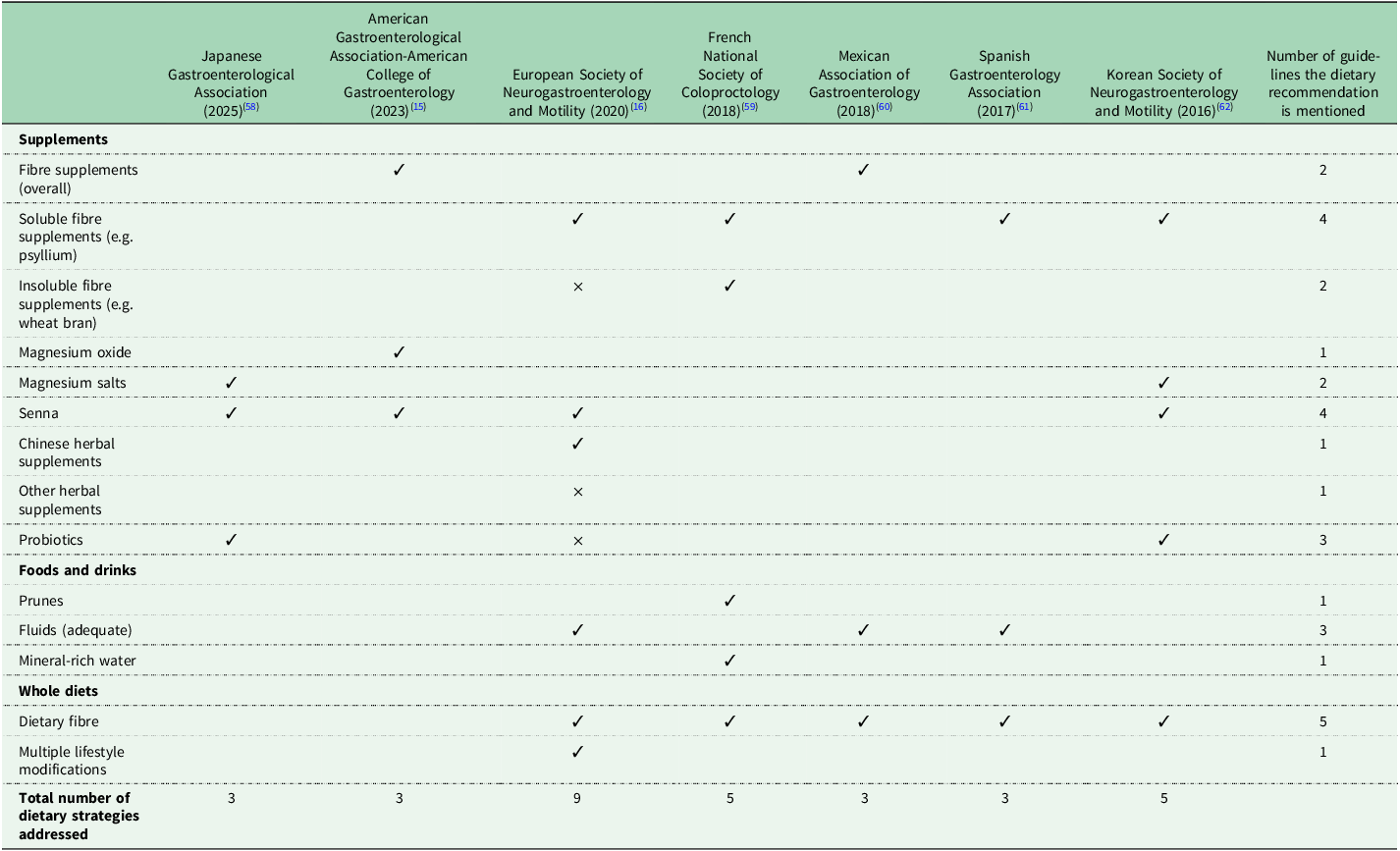
All clinical guidelines included dietary, as well as medical treatment recommendations. The guidelines varied not only in the number of dietary recommendations included (ranging from 1 to 9), but also in the types of dietary strategies they addressed. The dietary recommendations in each clinical guideline are detailed in Table 2. Overall, the most commonly recommended dietary strategy was increasing or having an adequate dietary fibre intake and was reported in five of seven clinical guidelines (Europe, France, Mexico, Korea and Spain) (Table 3). In terms of dietary fibre, the French guidelines mention prunes as a source of dietary fibre, the Mexican ones mention prunes and kiwifruits as sources, and the Spanish guidelines recommend foods high in soluble fibre, such as fruits, vegetables, greens, legumes, nuts and rye bread; however, this food group list is too broad and would also include many foods that are low in soluble fibre(Reference Serra, Mascort-Roca and Marzo-Castillejo61). The least commonly recommended dietary strategies were magnesium oxide (US), Chinese herbal supplements (Europe), prunes (French), mineral-rich water (French) and multiple lifestyle modifications (diet, water, and so on, Europe); these were each reported by one clinical guideline only.
Table 3 Summary of the dietary approaches mentioned in international clinical guidelines(Reference Chang, Chey and Imdad15,Reference Serra, Pohl and Azpiroz16,Reference Ihara, Manabe and Ohkubo58–Reference Shin, Jung and Lee62)

✓ indicates support for dietary strategy; × indicates insufficient evidence or limited effectiveness.
Several dietary recommendations in current clinical guidelines are evidence-based. Psyllium supplements, magnesium oxide supplements, (certain) probiotic supplements, prunes and mineral-rich water have indeed been shown to be effective in chronic constipation, compared to control, in systematic reviews and meta-analyses(Reference van der Schoot, Creedon and Whelan11–Reference Van Der Schoot, Katsirma and Whelan14).
Interestingly, clinical guidelines also report dietary recommendations that are not always supported by current evidence. Dietary fibre is recommended in five guidelines (Europe, France, Mexico, Korea and Spain); however, there is currently lack of evidence to support an increase in dietary fibre, where fibre comes from a wide and varied range of foods and drinks, in constipation(Reference Van Der Schoot, Katsirma and Whelan14). Insoluble fibre supplements were also mentioned in two guidelines, with conflicting statements; the French guidelines supported their use, while the European ones stated there is ‘limited usefulness’(Reference Serra, Pohl and Azpiroz16,Reference Vitton, Damon and Benezech59) . Indeed, a systematic review and meta-analysis did not identify any insoluble fibre supplements that were effective in chronic constipation(Reference van der Schoot, Drysdale and Whelan12). Senna supplements are recommended by four clinical guidelines (Japan, US, Europe, Korea) (Table 3). Although individual placebo-controlled RCTs indicated a potential benefit of senna in chronic constipation, when these were meta-analysed, the effect was no longer significant (see ‘Other supplements’ above)(Reference van der Schoot, Creedon and Whelan11).
There is also ambiguity in certain dietary recommendations. The US and Mexican clinical guidelines broadly recommend the use of fibre supplements. While systematic reviews and meta-analyses have shown that fibre supplements are effective in constipation, the effect depends on the type of fibre used(Reference van der Schoot, Drysdale and Whelan12). Among various types of fibre assessed, psyllium supplements have consistently been shown to improve multiple constipation outcomes(Reference van der Schoot, Drysdale and Whelan12,Reference Christodoulides, Dimidi and Fragkos63) . While it is mentioned within the US and Mexican guidelines that psyllium may be effective, the formal dietary recommendation per se only refers to ‘fibre supplements’(Reference Chang, Chey and Imdad15). Probiotics were mentioned in three guidelines, with conflicting statements; the Korean guidelines support the use of ‘probiotics’ in conjunction with other medications, the Japanese guidelines recommend the use of ‘certain probiotics’, while the European ones state there is no sufficient evidence to recommend a specific probiotic (Table 2)(Reference Serra, Pohl and Azpiroz16,Reference Shin, Jung and Lee62) . Current evidence shows that, while not all probiotics are effective, certain strains, such as Bacillus coagulans Unique IS2 and specific Bifidobacterium lactis strains, may improve constipation outcomes and, thus, effects are strain-specific(Reference van der Schoot, Helander and Whelan13,Reference Dimidi, Christodoulides and Fragkos27) . Therefore, future recommendations could be refined to specifically name these evidence-base strains. Chinese herbal supplements are recommended by the European guidelines, however, this is based on a small number of RCTs investigating specific herbal formulations at specific dosages. The observed effects are unlikely to apply broadly to all Chinese herbal mixtures and, therefore, future recommendations should specify the formulations shown to be effective, including details on their constituent ingredients.
Notably, several evidence-based dietary strategies that have been shown to improve constipation outcomes are either entirely absent from clinical guidelines or mentioned in only one. Kiwifruits have been investigated in multiple RCTs and have been shown to be as effective as psyllium supplements in improving key constipation outcomes(Reference Van Der Schoot, Katsirma and Whelan14). Despite this, kiwifruits are not formally recommended in any clinical guideline as a dietary recommendation. Instead, they are only cited as an example of a fibre-rich food that has been studied in two clinical guidelines (Japan, Mexico), rather than being presented as a formal dietary recommendation. Rye bread has been found to increase stool frequency, but also worsen global gut symptoms in RCTs(Reference Van Der Schoot, Katsirma and Whelan14). However, no clinical guidelines address the use of rye bread in the management of constipation. Prunes are mentioned in only three clinical guidelines (Japan, France and Mexico), but only formally recommended in the form of a dietary recommendation in one (France), despite evidence from at least two RCTs demonstrating similar effectiveness in improving constipation to psyllium supplements(Reference Van Der Schoot, Katsirma and Whelan14). High mineral-content water is only recommended in one clinical guideline (France), even though it has been investigated in multiple RCTs and shown to increase response to treatment, compared to low mineral-content water(Reference Van Der Schoot, Katsirma and Whelan14,Reference Vitton, Damon and Benezech59) . Magnesium oxide supplements have been investigated in two RCTs and significantly improve several constipation symptoms, compared to placebo(Reference van der Schoot, Creedon and Whelan11); despite this, magnesium oxide supplements are only explicitly recommended by one clinical guideline (US), although two guidelines recommend ‘magnesium salts’ more broadly too (Japan, Korean)(Reference Chang, Chey and Imdad15,Reference Shin, Jung and Lee62) .
Five of seven clinical guidelines (US, Europe, France, Mexico and Spain) provide recommendations for constipation broadly, rather than for specific constipation outcomes (e.g. stool frequency, straining and bloating). The Japanese and Korean clinical guidelines do provide outcome-specific dietary recommendations, although this is not consistently applied across all their recommendations and, when applied, the recommendations are mainly focused on stool frequency or consistency only, potentially omitting other key constipation symptoms. Considering that (a) people with chronic constipation experience a wide and varied range of symptoms, which differ between individuals(Reference Dimidi, Cox and Grant4,Reference Dimidi, Dibley and Cotterill5) , and (b) that dietary treatments may improve certain, and not all, symptoms(Reference van der Schoot, Creedon and Whelan11–Reference Van Der Schoot, Katsirma and Whelan14), future clinical guidelines would benefit from offering more targeted, symptom-specific dietary recommendations.
New UK dietary guidelines for the management of chronic constipation, endorsed by the British Dietetic Association, have been recently released in the form of an abstract, with the guidelines expected to be published in full by the end of 2025(Reference Dimidi, van der Schoot and Barrett64). Dietary recommendations were produced via conducting four systematic reviews and meta-analyses of RCTs on the effect of diet in chronic constipation(Reference van der Schoot, Creedon and Whelan11–Reference Van Der Schoot, Katsirma and Whelan14), applying the GRADE approach and conducting a Delphi consensus survey. Overall, 59 evidence-based dietary recommendations were generated and addressed fibre supplements (overall; specific types), probiotics (overall; specific species and strains), synbiotics, magnesium oxide, senna supplements, kiwifruit supplements (extract), kiwifruits (whole), prunes, rye bread and high mineral-content water. These are outcome-specific recommendations addressing key constipation outcomes (treatment response, stool output, gut symptoms, adverse events and quality of life) and provide positive (improvement) and null (no impact) and negative (worsening) statements. These will be the first evidence-based dietary guidelines for the management of chronic constipation, providing recommendations on various dietary interventions and for several constipation-related outcomes.
Conclusion
Diet plays an important role in the management of chronic constipation, with numerous RCTs demonstrating that specific dietary supplements, foods and drinks are effective in improving key constipation outcomes. Current medical guidelines include dietary recommendations as a first-line management strategy, although several limitations have been identified. Firstly, many guidelines mostly focus on a small number of dietary recommendations, omitting several other evidence-based dietary strategies; such dietary strategies include magnesium oxide supplements, specific probiotic strains, kiwifruits, prunes and high mineral-content water. Secondly, some clinical guidelines state dietary recommendations for which limited evidence exist to support their use in constipation (e.g. insoluble fibre supplements). Thirdly, dietary recommendations can often be ambiguous (e.g. probiotics), lacking information essential for appropriate implementation in clinical care. Future clinical guidelines would benefit from rigorous and systematic assessment of the evidence prior to generating dietary recommendations and also from generating recommendations that are outcome-specific, recognising the heterogeneity of the disorder. Further RCTs are warranted to investigate the effect of dietary approaches that are commonly recommended, but there is currently lack of evidence to support their use (e.g. high-fibre diet).
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
E.D. has received an education grant from Alpro, research grants from the Almond Board of California, the International Nut and Dried Fruit Council and Nestec Ltd, and has served as a consultant for Puratos and Danone.