The intake levels of phytosterols in Japan, North America and Spain are approximately 370(Reference Hirai, Shimadzu and Takezoe1), 360(Reference Jaceldo, Lütjohann and Sirirat2) and 280(Reference Jiménez, Santos and Saura3) mg/d, respectively, which are similar to that of cholesterol(Reference Hirai, Shimadzu and Takezoe1). Because dietary phytosterols exert hypocholesterolemic effects(Reference Lees, Mok and Lees4), functional foods and supplements enriched with phytosterols are expected to increase in the future.
Phytosterols, similar to cholesterol, can be oxidised to form various derivatives. Because oxidised phytosterols are generated during the heating of vegetable oils, significant amounts of oxidised phytosterols are particularly found in fried foods such as potato chips(Reference Dutta and Appeleqvist5) and French fries(Reference Dutta6). Moreover, oxidised phytosterol levels in food have been reported to increase upon microwave heating(Reference Menéndez, Ansorena and Astiasarán7). Scholz et al. (Reference Scholz, Guth and Engel8) reported that oxidised phytosterols comprise 1·9 % of the daily phytosterols intake and estimated that the daily intake of oxidised phytosterols ranges from 1·2 to 2·9 mg/d for nonheated foods, 3·5 to 4·2 mg/d for heated milk and 29·6 mg/d for liquid spread for cooking and baking.
Oxidised phytosterols exert various harmful effects, such as cytotoxicity(Reference Ryan, Chopra and McCarthy9), apoptosis induction(Reference Ryan, Chopra and McCarthy9), inflammation(Reference Alemany, Laparra and Barberá10) and promotion of atherosclerosis(Reference Plat, Theuwissen and Husche11), similar to oxidised cholesterol(Reference Schroepfer12). Liang et al. (Reference Liang, Wong and Guan13) found that dietary oxidised phytosterols inhibit cholesterol metabolism in vivo. Laparra et al. (Reference Laparra, Alfonso and Alegría14) revealed that oxidised phytosterol affects cholesterol metabolism by downregulating the mRNA expression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CR) and sterol regulatory element-binding protein 2 (SREBP2), which are involved in cholesterol synthesis, and ATP-binding cassette subfamily G member 5 (ABCG5), which is involved in sterol excretion in vivo. Moreover, Koyama et al. (Reference Koyama and Osada15) reported that dietary oxidised phytosterols change fatty acid composition and increase the proportion of arachidonic acid by upregulating the mRNA expression of fatty acid desaturase in the liver. Thus, multiple studies have examined the effects of oxidised phytosterols on cholesterol metabolism; however, the specific effects of individual oxidised phytosterols remain unclear.
Stigmasterol is a major phytosterol, and its structure differs from cholesterol, campesterol and β-sitosterol by a double bond at the C22–23 position(Reference Foley, O’Callaghan and O’Brien16). The bioavailability of stigmasterol is significantly lower than that of campesterol or β-sitosterol(Reference Batta, Xu and Bollineni17). Some studies have shown that stigmasterol exerts useful pharmacological effects, including anti-inflammatory(Reference Jie, Yang and Yang18), anti-diabetic(Reference Wang, Huang and Yang19) and anti-cancer activities(Reference Wang, Chen and Lee20). Therefore, stigmasterol may be used in healthy foods and medicines in the future. Multiple studies have suggested that oxidised phytosterols, in contrast to unoxidised phytosterols, are readily absorbed by the small intestine(Reference Tomoyori, Kawata and Higuchi21). Thus, once absorbed, oxidised phytosterols may exert harmful effects similar to oxidised cholesterol.
Previously, we reported that dietary oxidised stigmasterol (OS) accumulates in the liver and then induces the production of reactive oxygen species and modulates the antioxidant system in mice(Reference Ohara and Osada22). In this study, we aimed to study the different effects of dietary stigmasterol or OS on cholesterol metabolism, particularly their potential harmful effects in mice. At present, the effect of oxidised phytosterols, particularly that of the individual oxidised phytosterols, on cholesterol metabolism has been scarcely studied in vivo. Therefore, this study constitutes one of the first in mice to examine the effects of dietary OS on cholesterol metabolism.
Methods
Preparation of purified oxidised stigmasterol
Stigmasterol (Biosynth, Ltd.) was heated at 170℃ for 6 h. The heated stigmasterol was dissolved in diethyl ether and applied to a silica gel column (Silica Gel 60, Spherical; Nacalai Tesque, Inc.). The unoxidised and decomposed stigmasterol was eluted with diethyl ether, and the fraction containing OS was eluted with methanol. The fraction eluted with methanol was re-dissolved in hexane and diethyl ether (1:1, v/v) after the solvent was removed and then reapplied to a silica gel column. Subsequently, hexane and diethyl ether (1:1, v/v), followed by diethyl ether, were poured into the column to remove the unoxidised stigmasterol. Finally, purified OS was eluted with methanol and dried using a rotary evaporator.
Analysis of oxidised stigmasterol
A portion of the purified OS was converted to trimethylsilyl ether in a mixture of trimethylchlorosilane1,1,1,3,3,3-hexamethyldisilazane, and dried pyridine (1:3:9, v/v/v) for 30 min at 60℃ after 5α-cholestane was added to an aliquot as an internal standard. OS was identified using GC–MS. GC–MS analyses were performed on Shimadzu GC-2010 and Shimadzu PARVUM2 instruments (Shimadzu Co.) with a DB-5MS column (0·25 mm, 60·0 m × 0·25 μm I.D.; Agilent Technologies Ltd.). Helium was used as the carrier gas at a flow rate of 1·08 ml/min and a split ratio of 1:10. The column oven, evaporation chamber, ion source and interface temperatures were set at 270, 300, 200 and 300℃, respectively. The electron ionisation mode mass spectrum was obtained at 70 eV and measured within a mass range of 35–1000 m/z.
Major OS derivatives were identified based on retention time and characteristic m/z, as reported by Menéndez-C et al. (Reference Menéndez, García and Astiasarán23), Conchill et al. (Reference Conchillo, Cercaci and Ansorena24), Leal-C et al. (Reference Leal, Inchingolo and Cardenia25) and Foley et al. (Reference Foley, O’Callaghan and O’Brien16). The prepared OS consisted of the following components: 0·2 % unoxidised stigmasterol; 5·8 % stigmasta-5,22-dien-3β,4β-diol (4β-hydroxystigmasterol); 4·8 % stigmasta-5,22-diene-3β,7α-diol (7α-hydroxystigmasterol); 11·0 % stigmasta-5,22-diene-3β,7β-diol (7β-hydroxystigmasterol); 10·2 % 5α,6α-epoxystigmast-22-en-3β-ol (5α,6α-epoxystigmasterol); 10·1 % 5β,6β-epoxystigmast-22-en-3β-ol (5β,6β-epoxystigmasterol); 6·2 % stigmastanetriol (stigmatriol); 22·3 % stigmasta-5,22-dien-7-on-3β-ol (7-ketostigmasterol) and 29·4 % unidentified OS. Unidentified OS was composed of seventeen unknown peaks.
Animals and diet
The animal experiments were conducted according to the guidelines of the Ethics Committee of Experimental Animal Care at Meiji University (Kanagawa, Japan) (approval code: MUIACUC2020-01).
ICR mice (6-week-old healthy males with no genetic modifications and previous procedures; Japan SLC, Inc.) were housed individually in a temperature (24℃) and light-controlled (07.00–19.00) windowless room, and animal cage locations were not rotated during the experiment. All treatments were performed by the same experimenter. After eight days of acclimation, 22 ICR mice were divided randomly into four groups to avoid differences in the initial body weight: the standard (St) group (five mice) was fed an AIN standard diet, the control (C) group (five mice) was fed a standard diet supplemented with 0·25 % cholesterol, the stigmasterol (S) group (six mice) was fed a standard diet supplemented with 0·25 % cholesterol and 0·25 % stigmasterol and OS group (six mice) fed a standard diet supplemented with 0·25 % cholesterol and 0·25 % OS. The diets were prepared according to the AIN76 recommendations(26), and the detailed composition of the diets is shown in Table 1. The mice in each group were fed the same quantity (in grams) of their respective diets to avoid differences in intake levels among the four groups. The feeding period was set at 14 d to ensure consistency with our previous study(Reference Ohara and Osada22). After 14 d, blood was collected from the mice via cardiac puncture after anaesthetisation with isoflurane using a syringe containing heparin Na (Mochida Pharmaceutical Co., Ltd.) as an anticoagulant by alternating sequence among groups (St1→C1→S1→OS1→St2→…). The liver and mucosa of the small intestine were immediately excised, and the plasma was separated by centrifugation at 3000 rpm for 25 min. These tissues were kept at –80℃ until further analyses. Some liver and mucosa of the small intestine samples were used for RNA analysis after immersion in RNAlater solution (Thermo Fisher Scientific Inc.) at 4℃ for 1 d. Faecal samples were collected for 4 days (beginning four days before euthanasia) and lyophilised. Blinding was not performed. All stages of the experiment, including group allocation, conduct, outcome assessment and data analysis, were carried out by a single experimenter who was aware of group assignments.
Table 1. Diet composition

St: standard diet fed group; C: 0·25 % cholesterol fed group; S: 0·25 % cholesterol and 0·25 % stigmasterol fed group; OS: 0·25 % cholesterol and 0·25 % oxidised stigmasterol fed group.
* Nihon Shokuhin Kako Co., Ltd.
† Feed One Co., Ltd.
‡ Mitsui. DM Sugar Co., Ltd.
§ Nisshin OilliO Group, Ltd.
|| Oriental Yeast Co., Ltd.
¶ Nacalai Tesque, Inc.
** Biosynth Ltd.
Analyses of plasma and hepatic total cholesterol levels
Plasma total cholesterol (TC) levels were measured using commercial kits (Fujifilm Wako Pure Chemical Industries Ltd.). Hepatic lipids were extracted as described by Folch et al. (Reference Folch, Ascoli and Lees27). Hepatic TC levels were measured as described by Sperry et al. (Reference Sperry and Webb28).
Analyses of the faecal steroid levels
Neutral and acidic faecal steroids were extracted as described by Isaksson et al. (Reference Isaksson, Asp and Ihse29). Neutral steroids were analysed using a gas chromatograph GC-2025 (Shimadzu Co.) equipped with an FID and a ZB-5MS column (0·25 mm, 60·0 m × 0·25 um I.D.; Phenomenex), with 5α-cholestane as the internal standard, as described by Sugano et al. (Reference Sugano, Yamada and Yoshida30). Nitrogen was used as the carrier gas at a flow rate of 1·0 ml/min. The column oven and injector temperatures were set at 240 and 300°C, respectively.
Acidic steroids were analysed using a gas chromatograph GC-2014 (Shimadzu Co.) equipped with an FID and a TC-5 column (0·25 mm, 30·0 m × 0·25 um I.D.; GL Sciences), with 23-nor-deoxycholic acid as the internal standard, as described by Sugano et al. (Reference Sugano, Yamada and Yoshida30). Nitrogen was used as a carrier gas at a flow rate of 1·5 ml/min. The column oven and injector temperatures were set at 230–270°C (2°C/min temperature elevation) and 310°C, respectively.
RNA extraction from liver and mucosa of the small intestine
Total RNA was extracted from liver and small intestinal mucosal tissues using Sepasol-RNA I Super G (Nacalai Tesque, Inc.). It was then mixed with a 6 M lithium chloride solution for RNA purification. The RNA concentration was measured using a NanoDrop Lite spectrophotometer (Thermo Fisher Scientific Inc.).
Oligonucleotide primer sequences
The primers used to detect mouse β-actin (Gene ID: 11461), sterol regulatory element-binding protein 2 (SREBP2; Gene ID: 20788), 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CR; Gene ID: 15357), liver X receptor α (LXRα: NR1H3; Gene ID: 22259), pregnane X receptor (PXR: NR1I2; Gene ID: 18171), Farnesoid X receptor (FXR: NR1H4; Gene ID: 20186), retinoid X receptor β (RXRβ: NR2B2; Gene ID: 20182), cytochrome P450 family 7 subfamily A member 1 (CYP7A1: cholesterol 7α-hydroxylase; Gene ID: 13122), cytochrome P450 family 27 subfamily A member 1 (CYP27A1: sterol 27-hydroxylase; Gene ID: 104086), Niemann-Pick C1-like 1 protein (NPC1L1; Gene ID: 237636), ATP binding cassette subfamily G member 5 (ABCG5; Gene ID: 27409) and ATP binding cassette subfamily G member 8 (ABCG8; Gene ID: 67470) via reverse transcription PCR were designed using Primer3Plus software (https://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi). Primers synthesised by Eurofins Genomics (Tokyo, Japan) were designed to flank known or putative introns of the target gene, thereby preventing amplification of contaminating genomic DNA. The primer sequences were as follows: β-actin, 5′-TGTCGAGTCGCGTCCACC-3′ (sense) and 5′-TATCGTCATCCATGGCGAACTGG-3′ (antisense); SREBP2, 5′-CAGGGAACTCTCCCACTTGA-3′ (sense) and 5′-GAGACCATGGAGACCCTCAC-3′ (antisense); HMG-CR, 5′-CCAGGATGCAGCACAGAATGT-3′ (sense) and 5′-CCAATTC GGGCAAGCTGCCG-3′ (antisense); LXRα, 5′-TGCCATCAGCAT CTTCTCTG-3′ (sense) and 5′-GGCTCACCAGCTTCATTAGC-3′ (antisense); PXR, 5′-GACCTGCCTATTGAGGACCA-3′ (sense) and 5′-TTCTGGAAGCCACCATTAGG-3′ (antisense); FXR, 5′-TA CCACTACAACGCGCTCAC-3′ (sense) and 5′-ACATCCCCAT CTCTCTGCAC-3′ (antisense); CYP7A1, 5′-GAGCCCTGAAGC AATGAAAG-3′ (sense) and 5′-GCTGTCCGGATATTCAAGGA-3′ (antisense); RXRβ, 5′-CTTGGTCTCCAAGTCGAAGG-3′ (sense) and 5′-GCCAAATGAGAAGGAAGCAG-3′ (antisense); CYP27A1, 5′-CACTTTCCTTCCCAAATGGA-3′ (sense) and 5′-AGGAAGT GCAGGTAGCCAGA-3′ (antisense); NPC1L1, 5′-GGAAATGCA ATCCTTCCAGA-3′ (sense) and 5′-GCCAGGGAGATGTACAG GAA-3′ (antisense); ABCG5, 5′-TCACTTGCATTTGCTTCCTG-3′ (sense) and 5′-TTGCTGACGCTGTAGGACAC-3′ (antisense) and ABCG8, 5′-TCTCCAGGTCCTGATTGGTC-3′ (sense) and 5′-GGGTGCTTTTGACTCTGCTC-3′ (antisense).
Real-time quantitative PCR
One microgram of RNA was incubated at 65℃ for 5 min and then quickly cooled on ice. Reverse transcription of RNA was performed using a ReverTra Ace qPCR RT Master Mix (Toyobo Co., Ltd.) with the temperature increased to 37℃ for 15 min, followed by 98℃ for 5 min. An aliquot of the generated cDNA samples was mixed with 9 µl THUNDERBIRD NEXT SYBR qPCR Mix (Toyobo Co., Ltd.) in the presence of 12 nmol each of the sense and antisense primers for β-actin and the target gene. This reaction mix was then subjected to the following cycling conditions in a Thermal Cycler Dice Real Time System III (Takara Bio Inc.): one cycle at 95℃ for 1 min. The results (fold changes) are expressed as relative fold changes based on comparisons with the amount of RNA of the target gene to that of β-actin (the internal control) using the equation, 2−Cq(target)+Cq(β-actin).
Effect of stigmasterol or oxidised stigmasterol on the incorporation of cholesterol into micellar solutions
A mixed micellar solutions composed of 40 ml 15 mM sodium phosphate buffer (pH 7·4) containing 308·6 mg NaCl (Nacalai Tesque, Inc.), 16·4 mg phosphatidylcholine (from Egg Yolk, Nacalai Tesque, Inc.), 127·8 mg sodium taurocholate, 40 mg trilinolenin (Olbracht Serdary Research Laboratories), 35 mg cholesterol (Nacalai Tesque, Inc.) (C group) and 35 mg stigmasterol (S group) or OS group (four samples each) were prepared by sonication as described by Ogino et al. (Reference Ogino, Osada and Nakamura31) The prepared micellar solutions were incubated at 37°C for 1 h and then centrifuged at 1000 g for 20 min. The supernatant was filtered using a 220 nm filter (Membrane Solutions LLC). Lipids were extracted from the filtered solution as described by Folch et al. (Reference Folch, Ascoli and Lees27). Thereafter, sterol levels were analysed by GC–MS, as described in the previous section.
Statistical analyses and justification of sample size
Based on studies performed in rats and mice administered cholesterol, cholesterol + phytosterols or cholesterol + oxidised phytosterols, with group sizes of 7–12 rodents per group(Reference Wang, Chen and Lee20), this study was designed with five or six mice per experimental group, consistent with the group sizes used in our previous study(Reference Ohara and Osada22). No prior inclusion or exclusion criteria were specified. All animals and data points were included in the experiment and analysis. All data are expressed as the mean (standard error of the mean (sem)). Statistical analyses were performed using Excel Statistics version 4.08 (Social Survey Research Information Co., Ltd.). The Shapiro–Wilk test was used to evaluate normality, revealing that the majority of variables, except some growth parameters, acidic steroid levels and mRNA expression levels in the mucosa of the small intestine, were normally distributed. Therefore, if the data were normally distributed, the Student’s t test was used to evaluate significant differences between values of the St and C groups, and one-way ANOVA and the Tukey–Kramer test were used to evaluate significant differences among values of the C, S and OS groups. If the data were not normally distributed, the exact Mann–Whitney U test was used to evaluate significant differences between the St and C groups, and the Kruskal–Wallis and Steel-Dwass tests were used to evaluate significant differences among the C, S and OS groups. Statistical significance was set at P < 0·05. No data on the effect size were available for power analysis because this was an exploratory study comparing the effects of dietary stigmasterol or OS on cholesterol metabolism in mice. Instead of conducting a conventional power analysis, minimally detectable effect sizes were estimated using G * Power version 3.1.9.7 based on predetermined sample sizes. For the St and C group comparisons (n 5 each), the minimally detectable effect size (Cohen’s d) was approximately 2·02, assuming a two-sided significance level of 0·05, and a statistical power of 80 %. For the C, S and OS groups (n 5, 6 and 6, respectively) comparison, the minimally detectable effect size (Cohen’s f) for the one-way ANOVA was approximately 0·84 under the same assumptions.
Results
Effects of dietary stigmasterol or oxidised stigmasterol on growth parameters
Liver weight tended to be slightly higher in the C group than in the St group (P = 0·06) (Table 2). However, no significant effects were observed on the other growth parameters among the four groups.
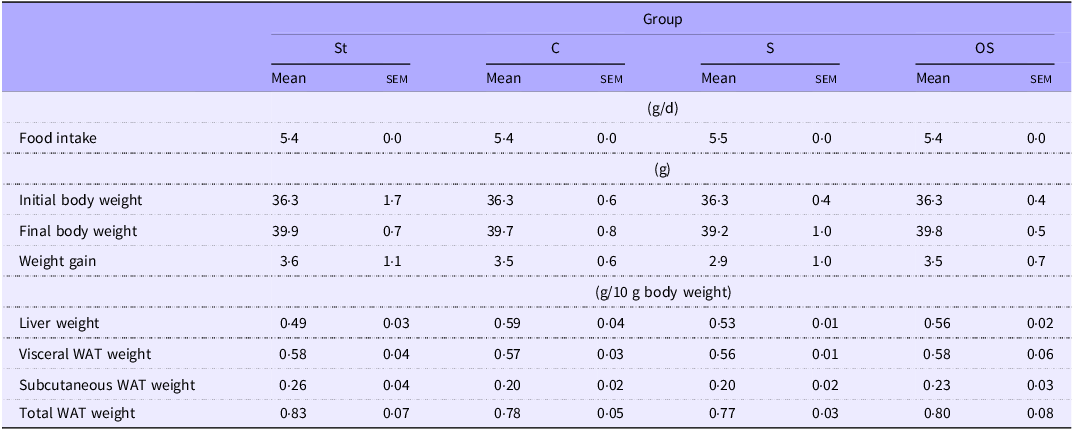
Table 2. Effect of dietary stigmasterol or oxidised stigmasterol on growth parameters
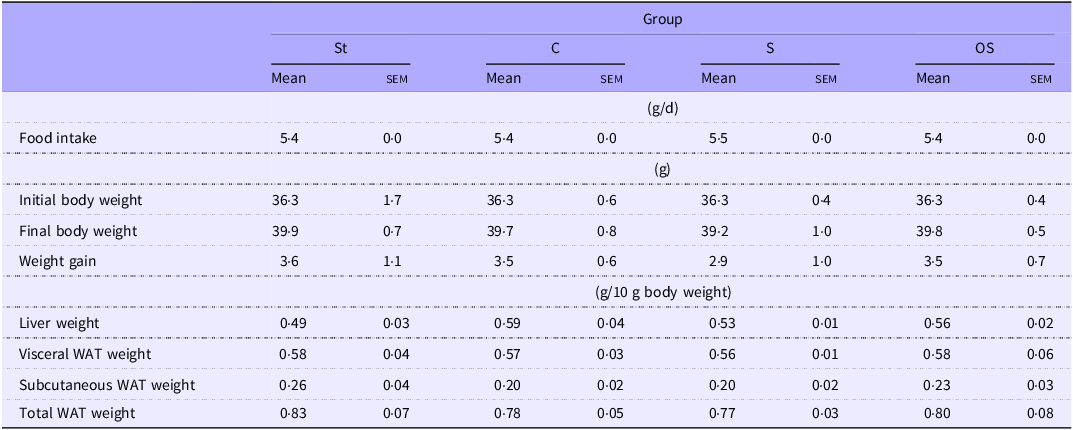
Data are presented as the mean and sem of 5–6 mice in each group. WAT: white adipose tissue. Other abbreviations are the same as shown in Table 1.
Effect of dietary stigmasterol or oxidised stigmasterol on plasma and hepatic cholesterol levels
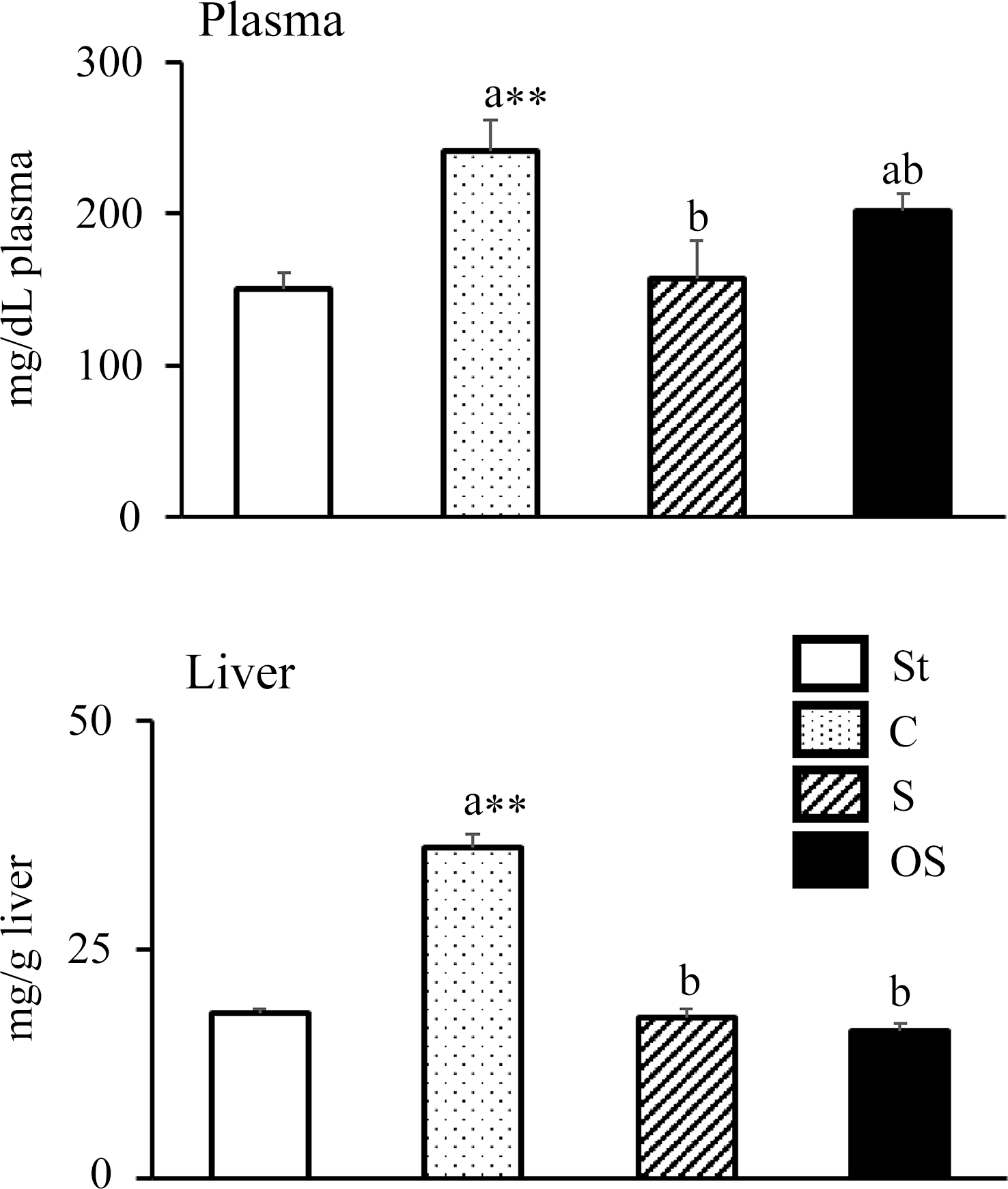
Plasma TC levels were significantly higher in the C group than in the St group, but were significantly lower in the S group than in the C group (Figure 1). However, no significant effect was observed in the OS group compared with the C and S groups. Hepatic TC levels were significantly higher in the C group than in the St group, but were significantly lower in the S and OS groups than in the C group.
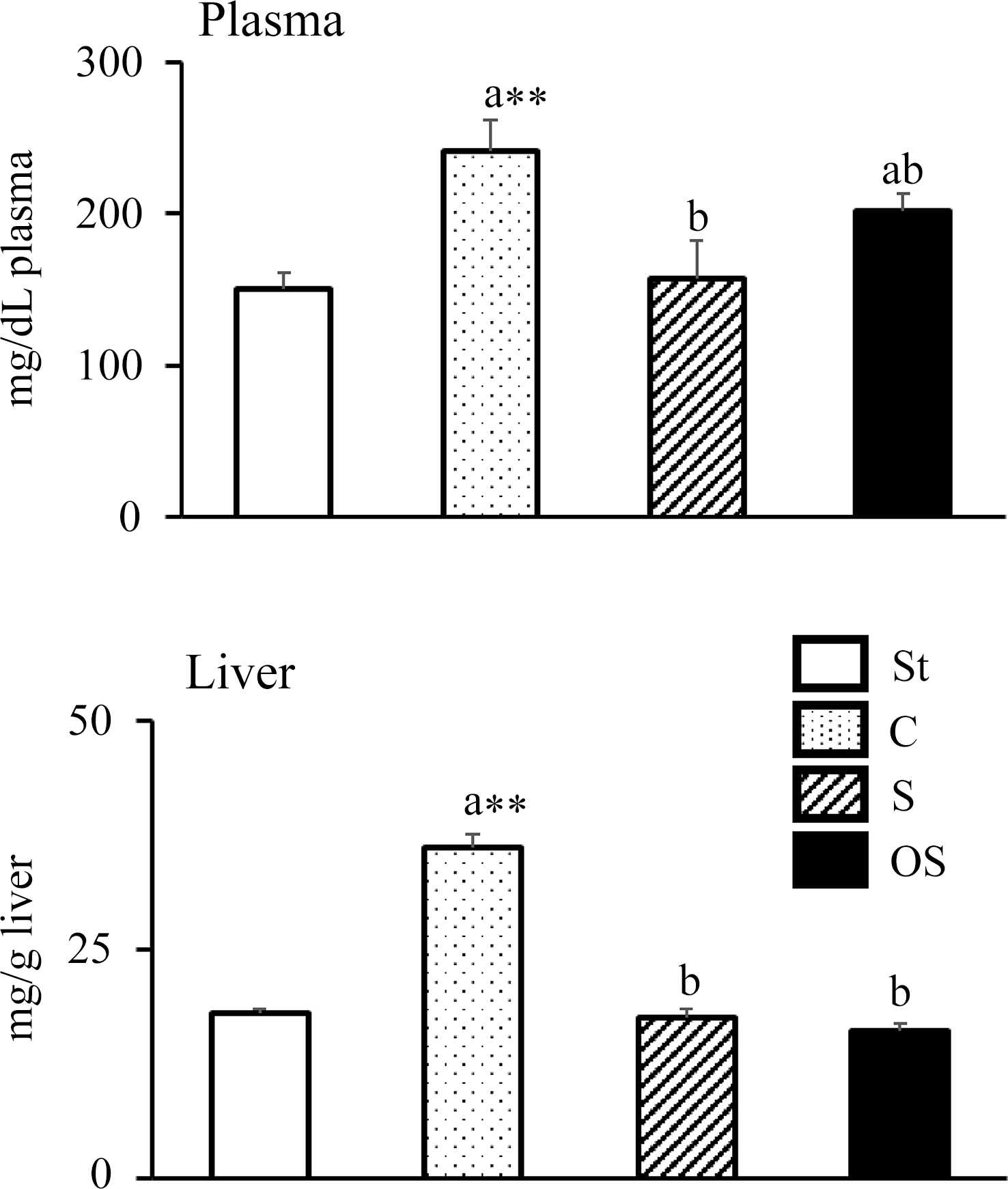
Figure 1. Effects of dietary stigmasterol or oxidised stigmasterol on plasma and hepatic cholesterol levels. Data are presented as the mean and sem of 5–6 mice in each group. Significant differences between values of the St and C groups at P < 0·01**. abValues without a common superscript letter are significantly different among values of the C, S and OS groups at P < 0·05. Other abbreviations are the same as shown in Table 1. Plasma cholesterol level was the level per dl of plasma, and hepatic cholesterol level was the level per gram of tissue.
Effect of dietary stigmasterol or oxidised stigmasterol on the faecal steroid levels
The faecal cholesterol and neutral steroid levels were significantly higher in the C group than in the St group and significantly higher in the S and OS groups than in the C group (Table 3). The coprostanol levels were significantly higher in the C group than in the St group and significantly higher in the OS group than in the C and S groups.
Table 3. Effects of dietary stigmasterol or oxidised stigmasterol on faecal neutral steroid levels

Data are presented as the mean and sem of 5–6 mice in each group. Significant differences between values of the St and C groups at P < 0·05*. abValues without a common superscript letter are significantly different among values of the C, S and OS groups at P < 0·05. Other abbreviations are the same as shown in Table 1. Total neutral sterolids represent the sum of cholesterol and coprostanol.
The faecal cholic acid levels did not differ between the St and C groups, the C and S groups and the C and OS groups, but were significantly lower in the OS group than in the S group (Table 4). The total levels of α-muricholic acid and chenodeoxycholic acid were significantly higher in the C group than in the St group, but did not differ among the C, S and OS groups. The β-muricholic acid level was significantly higher in the C group than in the St group and significantly higher in the OS group than in the C and S groups. Total primary bile acid levels were significantly higher in the C group than in the St group and significantly higher in the OS group than in the C and S groups. The total levels of ω-muricholic acid and deoxycholic were significantly higher in the C group than in the St group, but significantly lower in the S group than in the C group. The lithocholic acid level tended to be slightly higher in the C group than in the St group (P = 0·10), but was significantly lower in the S group than in the C group. The hyodeoxycholic acid level did not differ between the St and C groups, the C and S groups or the C and OS groups, but tended to be slightly lower in the OS group than in the S group (P = 0·06). The ursodeoxycholic acid levels tended to be slightly higher in the C group than in the St group (P = 0·05) and were significantly higher in the S and OS groups than in the C group. The total secondary bile acids and total bile acids levels were significantly higher in the C group than in the St group, but did not differ among the C, S and OS groups.
Table 4. Effects of dietary stigmasterol or oxidised stigmasterol on faecal acidic steroid levels
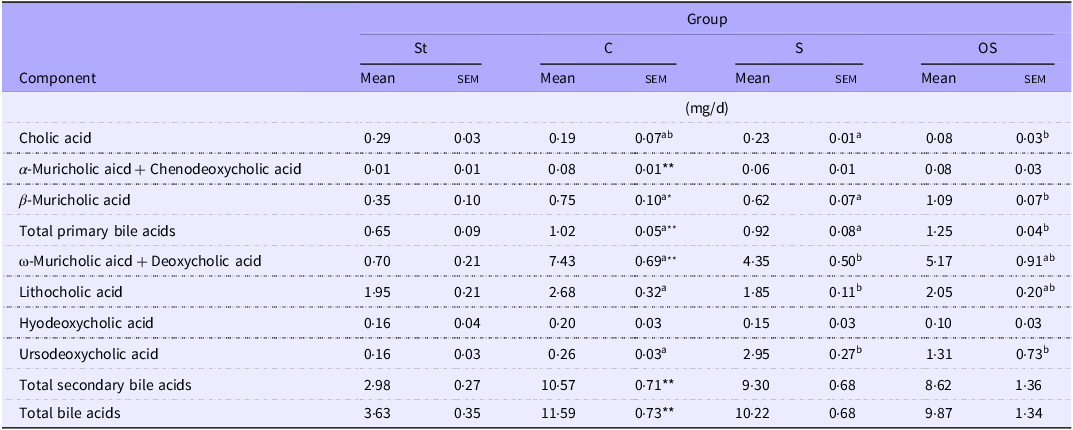
Data are presented as the mean and sem of 5–6 mice in each group. Significant differences between values of the St and C groups at P < 0·01** and P < 0·05*. abValues without a common superscript letter are significantly different among values of the C, S and OS groups at P < 0·05. Other abbreviations are the same as shown in Table 1. Total primary bile acids represent the sum of cholic acid, a-muricholic acid + chenodeoxycholic acid and b-muricholic acid. Total secondary bile acids represent the sum of w-muricholic acid + deoxycholic acid, lithocholic acid, hyodeoxycholic acid and ursodeoxycholic acid.
Effects of dietary stigmasterol or oxidised stigmasterol on the mRNA expression of enzymes and nuclear receptors involved in cholesterol synthesis and catabolism in the liver
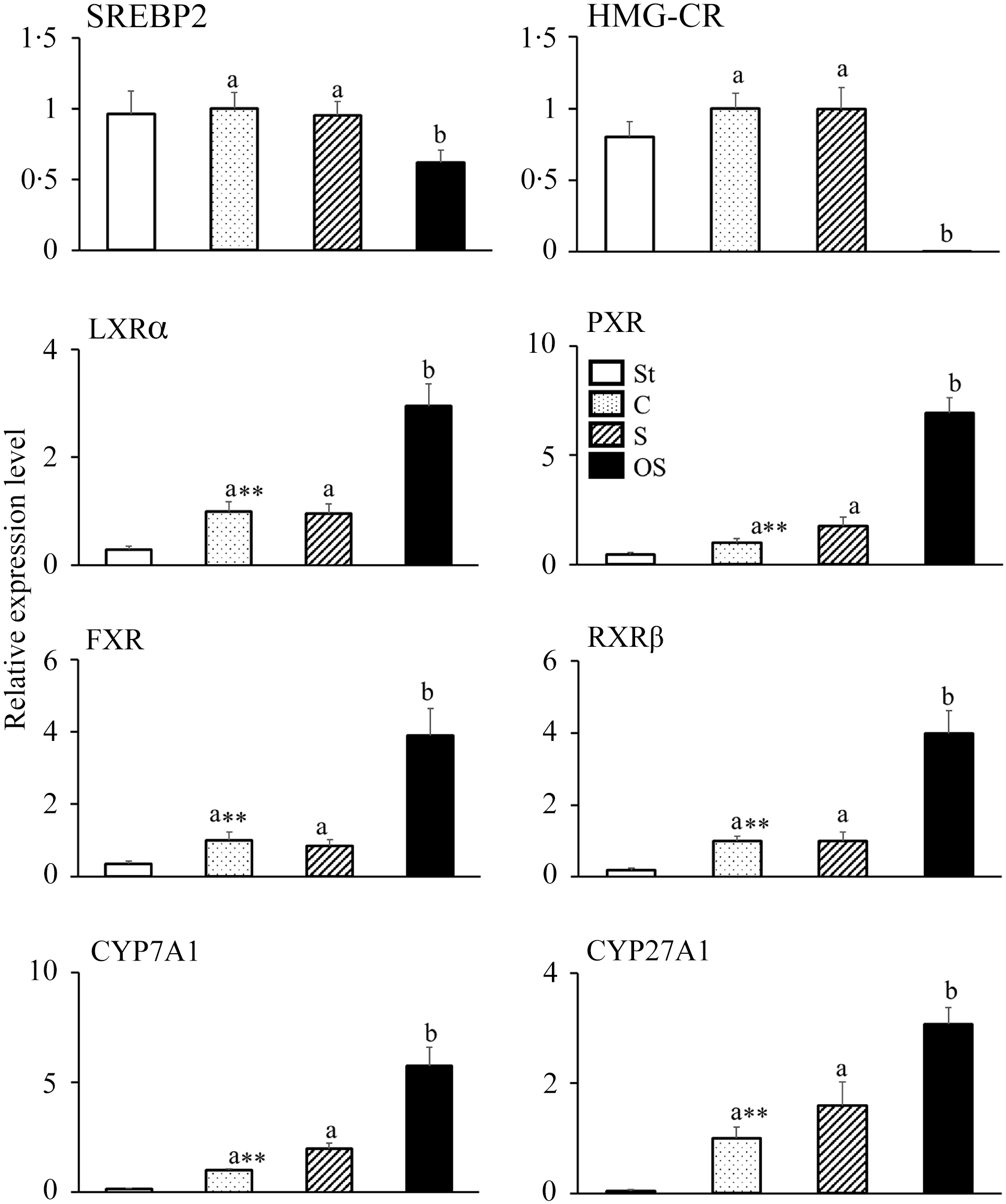
The mRNA expression levels of following nuclear receptors and enzymes involved in cholesterol synthesis did not differ between the St and C groups but were significantly lower in the OS group than in the C and S groups: SREBP2, a nuclear receptor that upregulates mRNA expression in cholesterol synthesis; HMG-CR, a rate-limiting enzyme of the cholesterol synthesis pathway in the liver (Figure 2).
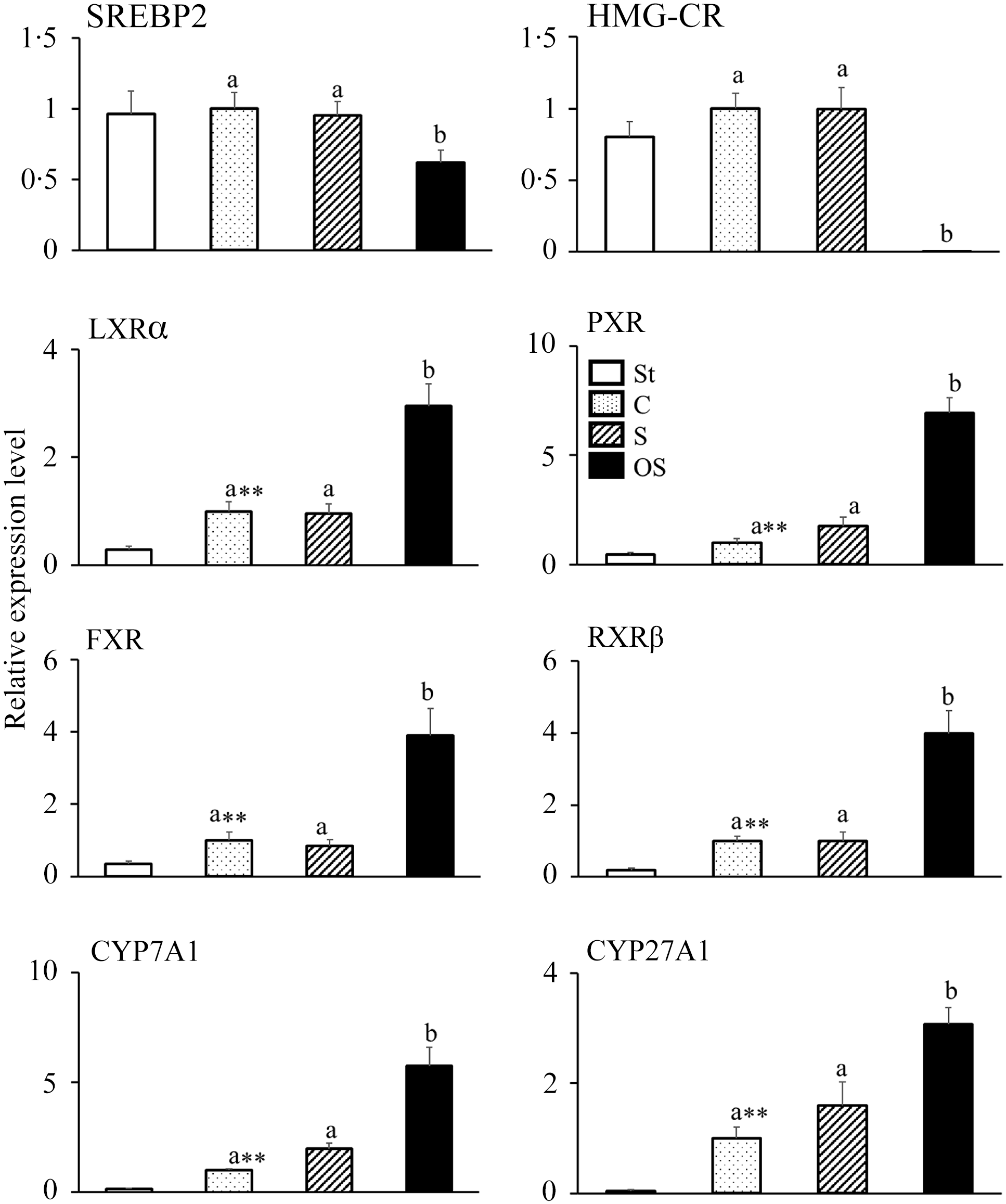
Figure 2. Effects of dietary stigmasterol or oxidised stigmasterol on the mRNA expression of enzymes and nuclear receptors involved in cholesterol synthesis and catabolism in the liver. Data are presented as the mean and sem of 5–6 mice in each group. Significant differences between values of the St and C groups at P < 0·01**. abValues without a common superscript letter are significantly different among values of the C, S and OS groups at P < 0·05. Other abbreviations are the same as shown in Table 1. Each relative expression level was calculated by comparing the expression level of the target gene to that of the reference β-actin, with the C group set to 1.
In contrast, the mRNA expression levels of following nuclear receptors and enzymes involved in cholesterol catabolism were significantly higher in the C group than in the St group and significantly higher in the OS group than in the C and S groups: LXRα, a nuclear receptor that upregulates the mRNA expression of CYP7A1; PXR, a nuclear receptor that upregulates the mRNA expression of CYP27A1; FXR, a nuclear receptor that is activated by bile acids; RXRβ, a nuclear receptor that forms heterodimers with LXRα, PXR and FXR; CYP7A1, a rate-limiting enzyme of the classic pathway of cholesterol catabolism in the liver and CYP27A1, a rate-limiting enzyme of the alternative pathway of cholesterol catabolism in the liver.
Effects of dietary stigmasterol or oxidised stigmasterol on the mRNA expression of transporters involved in sterol absorption in the mucosa of the small intestine
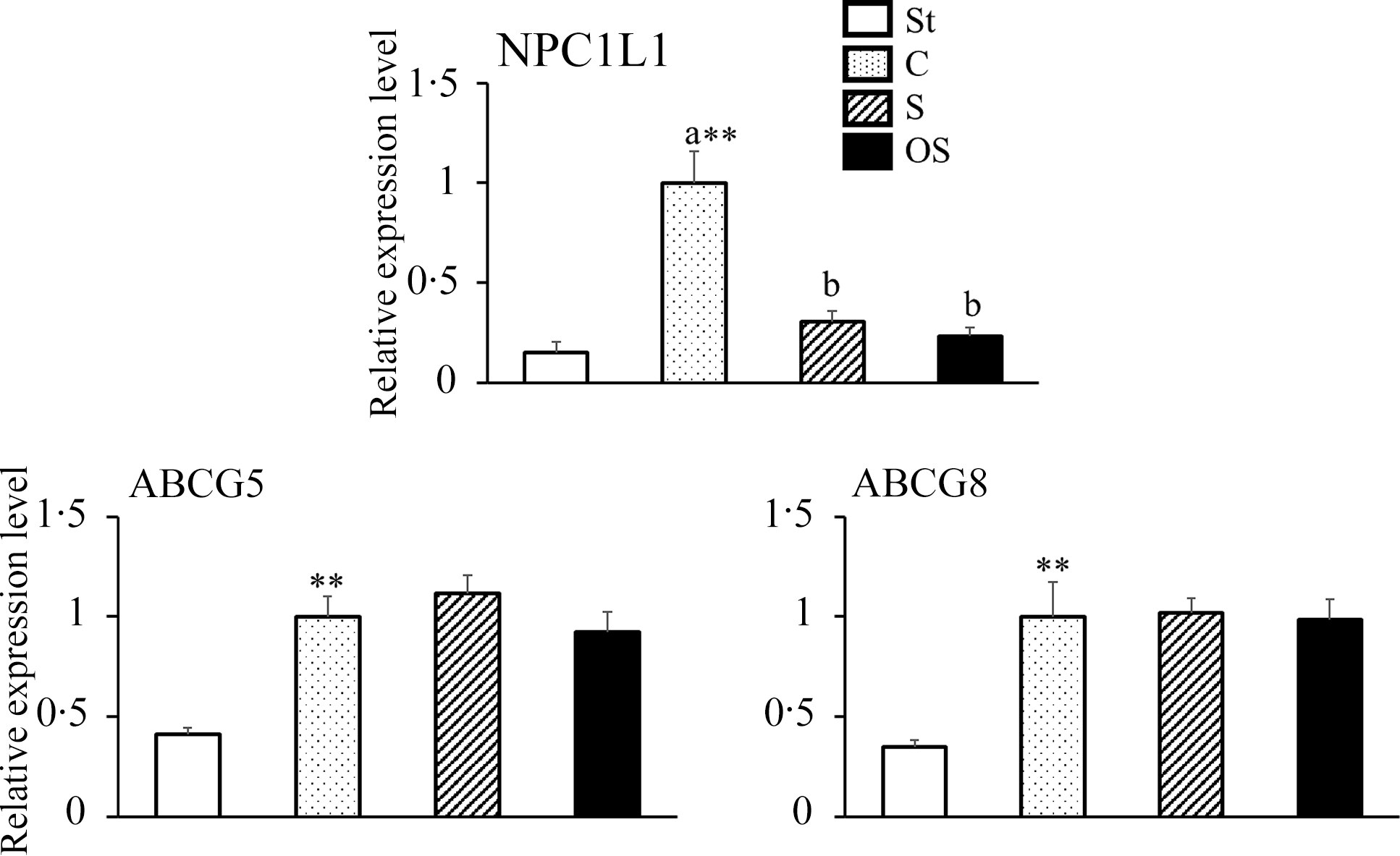
The mRNA expression level of NPC1L1, a transporter involved in cholesterol absorption in the small intestine, was significantly higher in the C group than in the St group, but significantly lower in the S and OS groups than in the C group (Figure 3). The mRNA expression levels of ABCG5 and ABCG8, transporters involved in cholesterol excretion in the small intestine, were significantly higher in the C group than in the St group; however, no significant differences were observed in the S and OS groups compared with the C group.
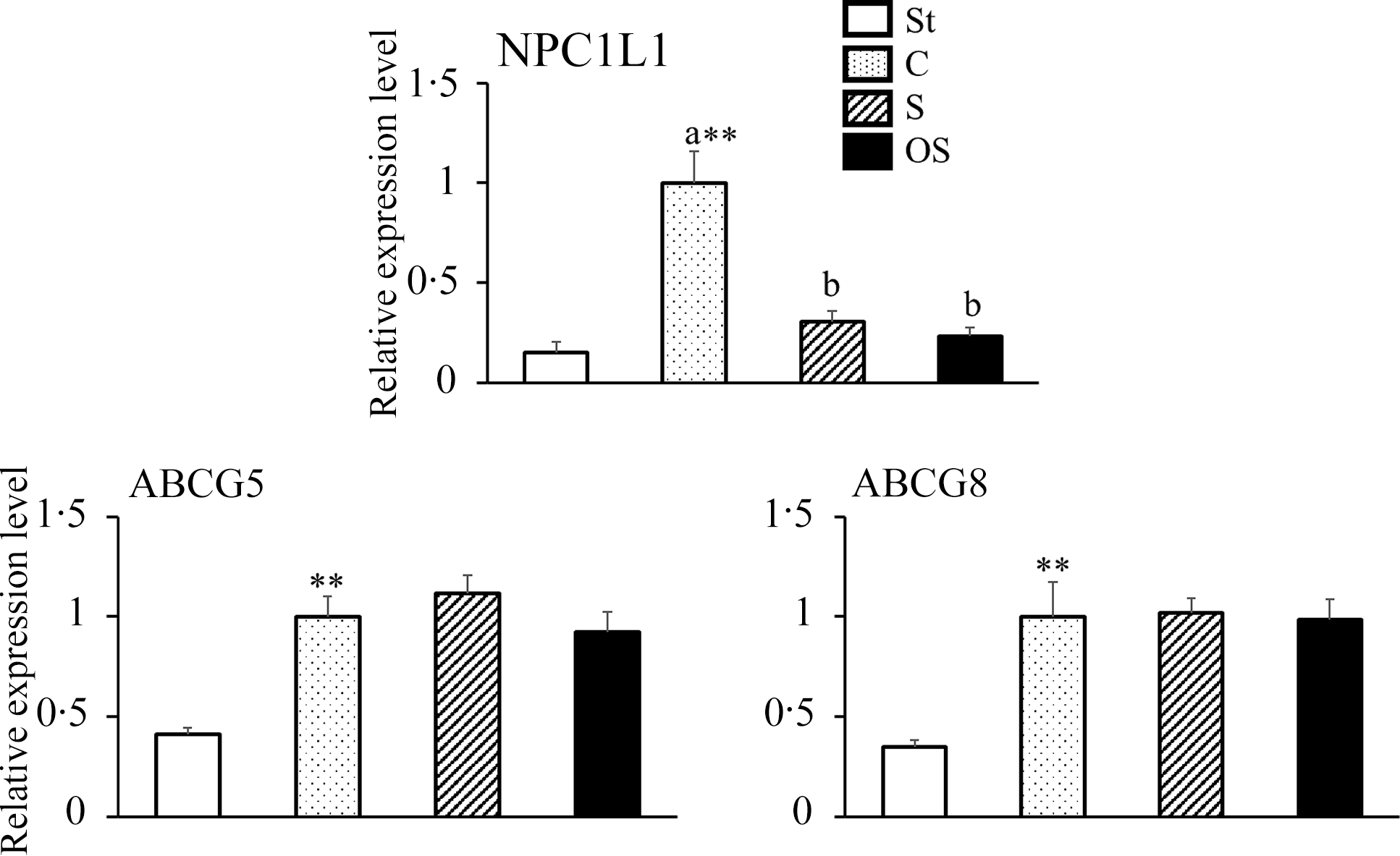
Figure 3. Effects of dietary stigmasterol or oxidised stigmasterol on the mRNA expression of transporters involved in sterol absorption in the mucosa of the small intestines. Data are presented as the mean and sem of 5–6 mice in each group. Significant differences between values of the St and C groups at P < 0·01**. abValues without a common superscript letter are significantly different among values of the C, S and OS groups at P < 0·05. Other abbreviations are the same as shown in Table 1. Each relative expression level was presented using the same calculation method as described in the footnote of Figure 2.
Effect of stigmasterol or oxidised stigmasterol on the incorporation of cholesterol into micellar solutions
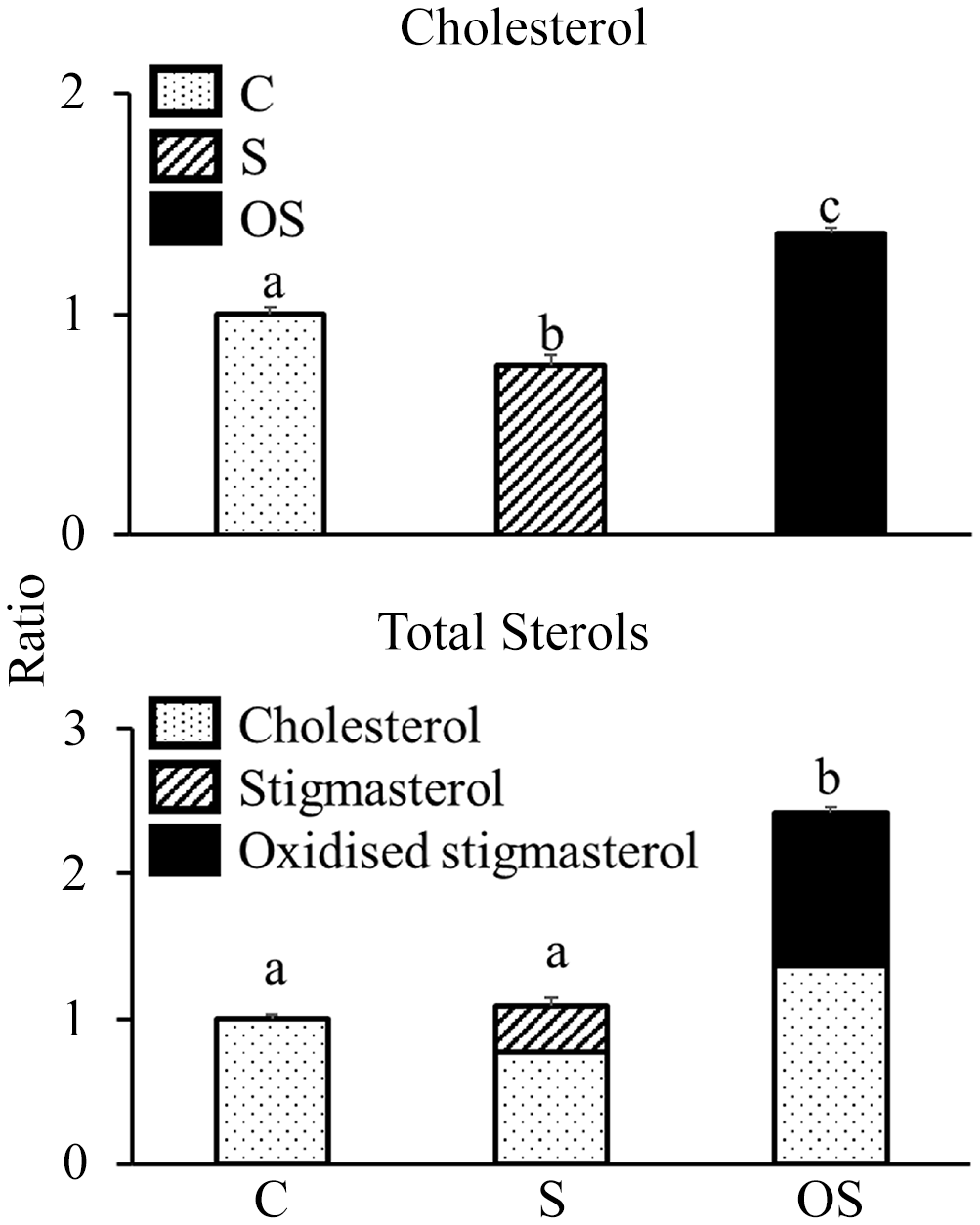
Cholesterol levels in the micellar solutions were significantly lower in the S group than in the C group, but were significantly higher in the OS group than in the C and S groups (Figure 4). Total sterol (cholesterol + stigmasterol + OS) levels did not differ between the C and S groups but were significantly higher in the OS group than in the C and S groups. Moreover, the OS in micellar solutions of the OS group consisted of the following components: 5·5 % 4β-hydroxystigmasterol, 6·1 % 7α-hydroxystigmasterol, 12·2 % 7β-hydroxystigmasterol, 15·7 % 5α,6α-epoxystigmasterol, 8·7 % 5β,6β-epoxystigmasterol, 2·3 % stigmastanetriol, 0·0 % 7-ketostigmasterol and 49·5 % unidentified OS.

Figure 4. Effect of stigmasterol or oxidised stigmasterol on the incorporation of cholesterol into micellar solutions. Data are presented as the mean and sem of 4 samples in each group. abValues without a common superscript letter are significantly different among values of the C, S and OS groups at P < 0·05. Other abbreviations are the same as shown in Table 1.
Discussion
Dietary phytosterols exert hypocholesterolemic effects(Reference Lees, Mok and Lees4) by inhibiting cholesterol absorption from the small intestine. Moreover, functional foods enriched with phytosterols may have increased levels of oxidised phytosterols during storage. Therefore, it is expected that oxidised phytosterols will be consumed at a substantial level in the daily diet. Unlike cholesterol, phytosterols are comprised of multiple compounds. Hence, the specific effects of individual oxidised phytosterols remain unclear. In this study, we examined the effects of dietary OS on cholesterol metabolism in mice, focusing on its potential harmful effects, because the effects of individual oxidised phytosterols remain unexplored in vivo.
We observed that dietary stigmasterol, but not OS, decreased plasma TC levels in mice. Moreover, we observed that dietary OS, unlike stigmasterol, increased cholesterol levels in micellar solutions in vitro. Thus, OS did not displace the cholesterol in micellar solutions. Therefore, it was expected that dietary OS would not exert hypocholesterolemic effects. Dietary stigmasterol inhibits cholesterol absorption in the small intestine by competitively inhibiting cholesterol incorporation into micelles in the intestinal lumen(Reference Katan, Grundy and Jones32). However, Liang et al. (Reference Liang, Wong and Guan13) and Wang et al. (Reference Wang, Yang and Shao33) reported that dietary oxidised phytosterols do not decrease plasma cholesterol levels. Therefore, upon oxidation, dietary stigmasterol may lose its inhibitory effects on cholesterol absorption in the small intestine.
We observed that dietary OS decreased hepatic TC levels in mice, although it did not exert hypocholesterolemic effects. This may be because of the inhibitory effect on cholesterol synthesis and promotion of cholesterol catabolism in the liver. Dietary OS decreased the mRNA expression of SREBP2 and HMG-CR in the liver. Liang et al. (Reference Liang, Wong and Guan13) also reported that dietary OS decreases the mRNA expression of HMG-CR in hamster livers. Moreover, Koyama et al. (Reference Koyama, Fukuoka and Osada34) found that dietary oxidised phytosterols decreased the mRNA expression of HMG-CR and TC in the liver. Similarly, Osada et al. (Reference Osada, Kodama and Cui35,Reference Osada, Kodama and Noda36) observed that dietary oxidised cholesterol decreased HMG-CR activity and TC levels in the liver. Thus, dietary OS may decrease hepatic TC levels by inhibiting cholesterol synthesis. In contrast, we observed that dietary OS increased faecal total primary bile acid levels and the mRNA expression levels of LXRα, PXR, FXR, RXRβ, CYP7A1 and CYP27A in the liver. Future studies should clarify the relationship between the changes in individual bile acid levels and nuclear receptors such as LXR and FXR. Oxidised cholesterol acts as an LXRα agonist(Reference Schwartz, Lawn and Wade37). Plat et al. (Reference Plat, Nichols and Mensink38) and Kaneko et al. (Reference Kaneko, Matsuda and Yamada39) showed that phytosterols act as an LXR agonist in vitro. Yang et al. (Reference Yang, Yu and Li40) have reported that dietary stigmasterol acts as an LXR agonist in vivo. Previously, we found that dietary OS accumulates in the liver(Reference Ohara and Osada22); therefore, accumulated OS in the liver may also act as an LXR agonist, which may decrease hepatic TC levels by promoting cholesterol catabolism. Future studies should examine the effects of dietary OS on each nuclear receptor.
We observed that dietary stigmasterol and OS increased faecal cholesterol, coprostanol and neutral steroid levels. This may have occurred because dietary stigmasterol and OS suppressed the mRNA expression of NPC1L1 in the small intestine. Alrefai et al. (Reference Alrefai, Annaba and Sarwar41) found that the mRNA overexpression of SREBP2 significantly increased the mRNA expression of NPC1L1; therefore, SREBP2 may upregulate the mRNA expression of NPC1L1 in Caco-2 cells. Meng et al. (Reference Meng, Zhou and Huang42) reported that chlorogenic acid administration in mice suppressed the nuclear translocation of SREBP2 in the liver and downregulated the mRNA expression of NPC1L1 in the mucosa of the small intestine. Duval et al. (Reference Duval, Touche and Tailleux43) reported that LXRα is involved in the regulation of the mRNA expression of NPC1L1, and an LXRα agonist may downregulate the mRNA expression of NPC1L1 in mice and Caco-2/TC7 cells. Therefore, dietary stigmasterol may directly regulate the mRNA expression of NPC1L1, whereas dietary OS may reduce the mRNA expression of SREBP2 and act as an LXRα agonist, thus reducing the mRNA expression of NPC1L1. This study examined only mRNA expression levels. Therefore, further study is required to examine the protein levels to clarify the specific effects of OS.
In summary, dietary stigmasterol decreased hepatic TC level by inhibiting cholesterol absorption from the small intestine, whereas dietary OS may decrease hepatic TC level by decreasing cholesterol absorption from the small intestine by acting as an LXR agonist in addition to suppressing hepatic cholesterol synthesis and promoting cholesterol catabolism. Although OS has been reported to exhibit cytotoxicity(Reference O’Callaghan, Foley and O’Connell44), it may be of interest as a hepatic cholesterol-lowering agent via LXR activation. Future studies should investigate the effects of OS on LXRα in detail.
Interestingly, we observed that the composition of the OS in micellar solutions differed from that of the prepared OS. Therefore, the incorporation of OS into micellar solutions may depend on the molecular species. In fact, 7-hydroxystigmasterols were easily incorporated into micellar solutions; however, 7-ketostigmasterol is barely incorporated into micellar solutions. This difference may be related to the differences in the composition of accumulated OS in the liver. In our previous study, we observed the accumulation of oxidised derivatives other than 7-ketostigmasterol in the liver of mice fed with OS(Reference Ohara and Osada22). Liang et al. (Reference Liang, Wong and Guan13) reported that 7-hydroxystigmasterol and 5,6-epoxystigmasterol were detected in the livers of hamsters fed 0·1 % OS, whereas 7-ketostigmasterol was not detected, despite its level being the highest among the OSs. Therefore, it is necessary to examine the relationship between the incorporation of OS into micellar solutions and its bioaccumulation.
Thus, we found that dietary OS, unlike stigmasterol, does not have an inhibitory effect on cholesterol absorption from the small intestine and modulates cholesterol metabolism in mice. In this study, a high level of OS was administered to clarify its effects over a short period, corresponding to approximately 0·34 g/kg/d in humans. However, future studies should examine the long-term feeding of each sterol at lower levels using a large number of mice to consider the effect on human health because high levels of each sterol were fed to the mice in this study. A mixture of OS derivatives was administered in this study. Therefore, it is necessary to examine the effects of individual oxidised derivatives on cholesterol metabolism in vivo because many of these derivatives remain unidentified.
Conclusion
In this study, we revealed that dietary OS, unlike stigmasterol, may lose its inhibitory effect on cholesterol absorption from the small intestine because it cannot displace cholesterol in micellar solutions. However, it may decrease hepatic TC levels by inhibiting cholesterol synthesis and promoting cholesterol catabolism. Furthermore, it may inhibit cholesterol absorption from the small intestine by downregulating the mRNA expression of NPC1L1 in the small intestine.
These findings provide new insights into the biological effects of dietary oxidised phytosterols, particularly OS. While these results were obtained in mice, the mechanisms may also be relevant to cholesterol absorption and metabolism in humans. They underscore the need for further studies on the underlying molecular mechanisms. Research focusing on individual oxidised phytosterols may contribute to the development of safer functional foods and supplements enriched with phytosterols.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
This study was supported by the JSPS KAKENHI (Grant Number: 21K05475).
Conceptualisation: Y. O. and K. O.; methodology: Y. O. and K. O.; formal analysis and investigation: Y. O.; writing – original draft preparation: Y. O.; writing – review and editing: K. O.; funding acquisition: K. O; resources: K. O.; supervision: K. O.
The first draft of the manuscript was written by Y. O., and all authors commented on previous versions of the manuscript. All the authors have read and approved the final manuscript.
The authors declare no competing interests relevant to the contents of this article.
The manuscript does not contain clinical studies or patient data.