We are fast approaching the latter end of the UN’s Decade of Action on Nutrition - 2016–2025 (1). This Nutrition Decade, the implementation of which is being co-led by the UN FAO and the WHO, is intended to provide an enabling environment for all countries, regardless of their income, the nature of their malnutrition challenges or the characteristics of their food and health systems, to ensure that action is taken by governments and stakeholders to develop and implement inclusive policies aimed at ending all forms of malnutrition(1). Malnutrition, in all its forms, includes undernutrition (wasting, stunting and underweight), micronutrient (vitamins and/or minerals) deficiencies, overweight/obesity and resulting diet-related noncommunicable diseases. In adopting the draft resolution on the UN Decade of Action on Nutrition, the General Assembly expressed concern that more than 2 billion people were suffering from micronutrient deficiencies, as well as the other forms of malnutrition being evident around the globe(1). Deficiencies of micronutrients, such as iron, zinc, iodine, folate, vitamins A, B12 and D, amongst others, compromise the immune system, disrupt childhood growth and brain development and accelerate multi-system aging and non-communicable diseases(Reference Stevens, Beal and Mbuya2). At a societal level, micronutrient deficiencies can adversely affect the development potential of individuals, reducing educational attainment, as well as work capacity and productivity, ultimately hindering the development of societies and nations(Reference Stevens, Beal and Mbuya2). The staggering numbers affected by micronutrient deficiencies globally stem from the fact that a high proportion of the population is at risk of these deficiencies. For example, children, adolescents, women of reproductive age and pregnant and lactating women are at greater risk of micronutrient deficiencies due to their higher nutritional requirements during these stages of life(Reference Stevens, Beal and Mbuya2), whereas micronutrient deficiencies can also disproportionately affect older adults, because of biological and environmental challenges and often coexist with and can be exacerbated by obesity(Reference Eggersdorfer, Akobundu and Bailey3). Collectively, these population subgroups represent more than two-thirds of the European population, a statistic that does not include immigrant and ethnic minority groups, who are also at risk of micronutrient deficiencies(Reference Ngo, Roman-Viñas and Ribas-Barba4).
The UN Sustainable Development Goal 2 has a highly ambitious, but laudable, target of ending micronutrient deficiencies, as well as other all forms of malnutrition, by 2030(5). This requires that inclusive policies need to be urgently built around sound data on aspects of micronutrient status and intake, prevalence of deficiencies, contributory factors, as well as evidence-based strategies for improving micronutrient status in the affected populations. Data is key to decision making. The mathematician Clive Humby said that ‘like oil, data is valuable, but if unrefined it cannot really be used’(Reference Blackwell6). The present review seeks to illustrate the critical importance of data, including its careful interrogation and strategic use, in the generation of the evidence-base that can inform guidelines and policies for addressing micronutrient deficiency. It will do this firstly by presenting the case for data from the prism of tackling vitamin D deficiency, as an exemplar micronutrient from among the two dozen or more currently considered essential. It will then consider how lessons learned from the vitamin D experience, may benefit our attempts at tackling deficiencies of other vitamins as well as minerals, also known as hidden hunger. The review will also stress the importance of the uptake and use of such data by key expert groups, agencies, policy decision-makers and other relevant stakeholders in terms of tackling micronutrient deficiencies. Finally, it will also acknowledge the role funding agencies have had, and continue to have, in terms of the generation of data that is much needed to inform inclusive policies for tackling micronutrient deficiencies.
Tackling vitamin D deficiency at a national, regional and global level: the role of and need for data
The following is not intended in any way as a review of the means of tackling vitamin D deficiency, but rather, as aligned with the overall data-related theme of the inaugural Nutrition Society Congress, more a demonstration of the role of, and need for, data in the development of the evidence-base, guidelines and, in some cases, policies for addressing vitamin D deficiency and inadequacy of vitamin D intake. The following subsections will highlight how data in its many guises, ranging from small to very large datasets, new data as well as existing data, data from studies of human subjects, animal trials and indeed other sources and from study designs ranging from randomised controlled trials (RCT) to observational studies, all contribute to the collective evidence-base that underpins decision-making in relation to tackling vitamin D deficiency. Also, it is important to stress that the following subsections draw on our own experience of how research in the field of vitamin D nutrition generates data that can underpin the development of such an evidence-base. In this regard, they represent exemplars and personal reflections on how data is key to decision-making in relation to guidelines and policies for addressing vitamin D deficiency.
National data on vitamin D status and intake
In 1998, the European Commission (EC) set up an expert working group to produce a report for the European Parliament on the prevention of osteoporosis(7). The report contained 8 clear recommendations targeted at improving the prevention of osteoporosis. The 4th recommendation related to the need to develop, integrate and implement policies to advise the general public and health professionals about calcium and vitamin D nutrition at all stages of life(7). Importantly, the report highlighted that data on dietary intake and status of vitamin D and calcium were not available in many European countries at that time. This was our first exposure to an expert body clearly identifying the priority need for such data at European member state level, which would then also inform the situation for the overall region. There was also the suggestion that there would be an audit of recommendations at member state level as a follow-on activity to the publication of the report. At around that time, there were a very limited number of publications (∼4) reporting on vitamin D status in Ireland (see Fig. 1), and the studies contributing this limited data were of generally non-representative, relatively small (n in the range 29–196) samples of elderly subjects, and in some cases institutionalised individuals(Reference Freaney, McBrinn and McKenna8–Reference Vir and Love11). Nevertheless, these studies were hugely important in highlighting vitamin D deficiency as a potential public health problem in Ireland. It was clear to us that additional data on vitamin D intake and status for the Irish population was urgently needed, and accordingly, the research programme of our then Vitamin D Research Group (now the Cork Centre for Vitamin D and Nutrition Research) at University College Cork, aspects of which were funded by two EC Framework Programme 5 projects (OSTEODIET and OPTIFORD), and by national funding from the Irish Department of Agriculture, Food and the Marine, under their Food Institutional Research Measure programme, amongst other funding sources, sought to address this key data gap.

Figure 1. The number of studies/papers of vitamin D status (and intake) in Ireland by year within the PUBMED database.
1998 EC Report = Report for the European Parliament on the prevention of osteoporosis; VDSP, Vitamin D Standardization Program.
From around 2000 onwards, a significant component of vitamin D research effort of the Centre focused on the generation of this new vitamin D status and intake data in different population subgroups in Ireland (Fig. 1); however, many of these studies were also, by necessity, relatively small, non-representative studies. Notwithstanding this, these new data, when taken with the previous data, again highlighted vitamin D status as a potential public health nutrition concern for Ireland. Importantly, the 2008–2010 nationally representative nutrition survey of adults in Ireland was resourced to include blood sampling, which allowed for the generation of much needed representative vitamin D status data for Irish adults for the first time. As part of the National Adult Nutrition Survey, the Centre generated this new 25-hydroxyvitamin D (25(OH)D) data based on analysis of survey serum samples by enzyme-linked immunoassay(Reference Cashman, Muldowney and McNulty12). Fortuitously, around that time, the Centre was invited to become a member of the US National Institutes of Health-led Vitamin D Standardization Program (VDSP) which had as its key goal to improve the detection, evaluation and treatment of vitamin D malnutrition by making serum total 25(OH)D measurements accurate and comparable over time, location and laboratory procedure(Reference Sempos, Vesper and Phinney13). The VDSP, coordinated by the Office of Dietary Supplements at the National Institutes of Health, was in response to the well-recognised variability in serum 25(OH)D estimates arising from method-related differences in its measurement(Reference Binkley, Krueger and Cowgill14). Accordingly, the VDSP developed protocols for standardising existing serum 25(OH)D data from national surveys(Reference Durazo-Arvizu, Tian and Brooks15), and thus minimising the impact of method-related differences in their estimates. As part of the VDSP, the Centre applied these protocols to the National Adult Nutrition Survey serum 25(OH)D data, and based on these standardised data it was estimated that the prevalence of serum 25(OH)D < 30 nmol/l and < 50 nmol/l, reflective of risk of vitamin D deficiency and risk of inadequacy of vitamin D status, respectively(16), was 12·3 % and 45·9 % of Irish adults, respectively(Reference Cashman, Kiely and Kinsella17). Importantly, these estimates, based on standardised data, allowed for comparison with standardised data from nationally representative surveys in the US (National Health and Nutrition Examination Survey 2011–2014) and Canada (Canadian Health Measures Surveys Cycle 1 and 2) which had lower prevalence estimates (e.g. 5·0 % and 8·8 % with serum 25(OH)D < 30 nmol/l, respectively), and when limiting data to white only survey participants, making for a more valid comparison with the Irish survey data, estimates were even lower again (2·1 % and 5·9 % with serum 25(OH)D < 30 nmol/l, respectively)(Reference Herrick, Storandt and Afful18,Reference Brooks, Greene-Finestone and Whiting19) . In recent times, the Centre has generated equivalent prevalence data for Irish teenagers aged 13–18 years, based on analysis of bloods from the 2019–2020 National Teens Survey II (NTS II) – the second national survey to have included blood sampling in its design(Reference Cashman, Kehoe and Kearney20). The standardised serum 25(OH)D data from this work suggests that about 21·7 % and 54·8 % of Irish teenagers, are at risk of vitamin D deficiency and inadequacy, respectively. Both the National Adult Nutrition Survey and NTS II also reported vitamin D intake data which allowed for benchmarking of the degree of inadequate intakes in both population subgroups. Using the US Estimated Average Requirement (EAR) value for vitamin D of 10 µg/d(16), it was estimated that 90 % and 94 % of adults and teenagers, respectively, in Ireland had inadequate intakes of vitamin D(Reference Cashman, Kehoe and Kearney20,Reference Black, Walton and Flynn21) , and this low intake likely contributed to the risk of vitamin D deficiency, particularly in the absence of dermal synthesis of vitamin D on exposure of skin to sunlight with sufficient ultraviolet B (UVB) radiation. Other nationally representative surveys in other population subgroups in Ireland have also reported vitamin D intake data, even where blood sampling wasn’t included in their survey designs (https://www.iuna.net/surveyreports).
Overall, as can be seen in Fig. 1, since the 1998 EC report, there has been a noticeable increase in the number of studies of Irish population subgroups conducted by the vitamin D research community on the island of Ireland, ranging from relatively small and non-representative samples, up to regionally and, in some cases, nationally representative samples. Thus, the important data-gap around estimates of vitamin D intake and status, as highlighted by the EC report, has been largely addressed for Ireland. However, it should be stressed that as such data needs to be iterative to best capture the current situation in relation to vitamin D status and intake, this strategic data requirement needs to be addressed on an ongoing basis. It is also encouraging to see evidence of the uptake and use of such data in terms of informing national guidelines and policies. For example, three recent reports by the Scientific Committee of the Food Safety Authority of Ireland (FSAI) focused on vitamin D recommendations, as part of their healthy eating guidelines, used the data from the various studies since ∼2000 to inform on current status of vitamin D status and/or intakes in Irish children, teenagers, adults (including pregnancy) and older adults(22–24).
European data on vitamin D status and intake
Unlike the situation in the US and Canada, where, as mentioned already, very good estimates exist for the prevalence of vitamin D deficiency and inadequacy based on data from their representative surveys(Reference Herrick, Storandt and Afful18,Reference Brooks, Greene-Finestone and Whiting19) , the equivalent estimates did not exist for Europe as a whole, only for some of its member state countries individually. In 2011, the Centre had highlighted that data on the distribution of serum 25(OH)D concentrations and vitamin D intakes, including food sources, in nationally representative populations, together with sustainable food-based strategies to bridge the gap between current and recommended intakes of vitamin D to minimise the prevalence of serum 25(OHD concentrations < 30 nmol/l, were three critical and prioritised research requirements for Europe(Reference Cashman and Kiely25). Addressing these, and other, priority data gaps around vitamin D nutrition and health in Europe was the focus of a EC Framework Programme 7-funded ODIN project on vitamin D, coordinated by the Cork Centre for Vitamin D and Nutrition Research at University College Cork (Professor Mairead Kiely and Professor Kevin Cashman, Joint Coordinators)(Reference Kiely and Cashman26). In 2016, the ODIN project delivered the first internationally standardised dataset on vitamin D status and reported the prevalence of vitamin D deficiency across Europe for the first time(Reference Cashman, Dowling and Škrabáková27). This European estimate was based on a collection of 14 nationally or regionally representative studies gathered as part of the project and their existing serum 25(OH)D data (based on a wide collection of analytical methods) were standardised as per the VDSP protocol. While the project included nationally representative nutrition surveys from Ireland, the UK and Germany, some member states in Europe did not have such nationally representative surveys. Thus, in the absence of such data, well-curated samples from regionally representative health surveys were used, as they can also achieve some degree of population coverage(Reference Cashman, Dowling and Škrabáková27). While there was considerable variation across study populations, the prevalence of vitamin D deficiency (standardised serum 25(OH)D concentrations < 30 nmol/l), when these representative population samples were pooled (n 55 844), was 13 %, which would broadly correspond to one in eight Europeans(Reference Cashman, Dowling and Škrabáková27). Even a crude estimation based on the magnitude of population in Europe coupled with this population-wide prevalence estimate, suggest something in the region of 66 million individuals deficient(Reference Cashman28). This European estimate was also considerably greater than that for the US or Canada(Reference Herrick, Storandt and Afful18,Reference Brooks, Greene-Finestone and Whiting19) , as mentioned above. In terms of vitamin D inadequacy, the average yearly population prevalence of standardised serum 25(OH)D < 50 nmol/l in Europe, the US and Canada is 40·0 %, 24·0 % and 36·8 %, respectively(Reference Herrick, Storandt and Afful18,Reference Brooks, Greene-Finestone and Whiting19,Reference Cashman, Dowling and Škrabáková27) .
These are whole-population estimates and the prevalence can vary by age-grouping with a tendency for it to be lowest in childhood and possibly later life(Reference Kiely and Cashman26). It should also be stressed that the population-wide estimates, do not capture the differences by ethnicity in these regions, which can be significant. For example, the ODIN project showed that dark-skinned ethnic groups within Europe are worryingly at much increased risk of vitamin D deficiency compared to their white counterparts (prevalence < 30 nmol/l in the range 28–65 %, depending on the country and the ethnic group)(Reference Cashman, Dowling and Škrabáková27,Reference Cashman, Dowling and Škrabáková29) , which aligns with higher risk of vitamin D deficiency reported among the non-white participants in the North American surveys(Reference Herrick, Storandt and Afful18,Reference Brooks, Greene-Finestone and Whiting19) . This was only a partial picture, however, as the proportion of participants from ethnic minorities was low in most studies within the ODIN collection. The project highlighted a lack of well-curated and characterised biobanks of ethnic minorities in Europe, representing a major research gap that should be prioritised.
Finally, while these important first of their kind estimates for Europe provided firm evidence that vitamin D deficiency is widespread across Europe, that analysis is fast approaching being 10 years old, which again underscores why this strategic data requirement needs to be addressed on an ongoing basis to maintain currency.
There is no one single underlying reason for vitamin D deficiency, but the combination of low UVB availability and/or exposure coupled with a low dietary vitamin D supply are of key importance(Reference Cashman30). The availability of UVB of sufficient intensity to stimulate the conversion of 7-dehydrocholesterol in the skin to pre-vitamin D3 and then vitamin D3 is impacted by several environmental factors, such as season, latitude and prevailing weather conditions. To get a better understanding of vitamin D-effective UVB availability across Europe, the ODIN project modelled such availability data for nine European countries/regions(Reference O’Neill, Kazantzidis and Ryan31). It did this using a validated UV irradiance model which used data on atmospheric and geophysical parameters, such as local cloud, ozone and aerosol, plus topography, accessed from a number of orbiting satellites. The results showed that UVB availability decreased with increasing latitude within Europe (from 35°N to 69°N), while all locations exhibited significant seasonal variation in UVB. The number of months in which UVB availability was too low to allow for skin synthesis of vitamin D, referred to as the ‘vitamin D winter’, was estimated to range from being largely absent in the very south of Europe to lasting for as long as 7 or 8 months in northern Europe(Reference O’Neill, Kazantzidis and Ryan31). Beyond vitamin D-effective UVB availability, personal characteristics, such as skin pigmentation, age, attire, sunscreen usage, working environment, outdoor physical activity and sun exposure behaviour, can also prevent or impede vitamin D synthesis(Reference Cashman30).
In the absence of sufficient UVB availability/exposure to enable synthesis in the skin, dietary supply of vitamin D is critical to meeting population requirements and prevention of vitamin D deficiency. However, vitamin D intake data from national surveys of adults and children in 21 and 13 European countries, respectively, suggest habitual intakes are relatively low on average (mean intakes in the range 2·2–3·3 μg/d; depending on sex and age group)(Reference Rippin, Hutchinson and Jewell32,Reference Rippin, Hutchinson and Jewell33) . There was variation in the estimated mean daily vitamin D intakes across countries and most notable among adults from countries in Northern Europe compared to those in Western, Southern and Central and Eastern Europe (mean daily intakes of 7·8, 4·0, 3·5 and 1·5 µg/d, respectively, for males; and 6·1, 3·3, 2·9 and 1·1 µg/d, respectively, for females)(Reference Rippin, Hutchinson and Jewell32).
Estimates of the vitamin D dietary requirement for Irish, UK, European and global populations
Since its derivation in 2011, the US Institute of Medicine’s EAR for vitamin D of 10 μg/d(16) has become a touchstone target intake with which to benchmark typical intakes of vitamin D within a population. The percentage of the population with a habitual daily nutrient intake lower than the EAR is taken as an estimate of the percentage of the population with probable inadequate intakes(34). While the prevalence of vitamin D intakes less than the EAR of 10 μg/d were not provided in many of the above mentioned European surveys(Reference Rippin, Hutchinson and Jewell32,Reference Rippin, Hutchinson and Jewell33) , it is clear from their mean daily intake data that many of the countries have a high majority of their respective populations with an inadequate intake of vitamin D. Roman Viñas et al. in 2011 projected that among European national surveys reporting vitamin D intake data, 77–100 % and 55–100 % of adults (18–64 years) and older adults (> 64 years), respectively, had intakes below 10 μg/d, which was the 2004 Nordic recommended EAR for vitamin D available at the time(Reference Roman Viñas, Ribas Barba and Ngo35).
Estimates of dietary requirements for vitamin D as dietary targets are crucial from a public health perspective in providing a framework for prevention of vitamin D deficiency and optimising vitamin D status of individuals. The Food Standards Agency (FSA) in the UK issued a research funding call in late 2004 with the aim of adding to the evidence-base around dietary requirements for vitamin D and the significance of both dietary sources and sunlight to vitamin D status and/or functional markers. University College Cork and the University of Ulster jointly competed for and won a contract within this call to undertake research on dietary requirements for vitamin D. As a key part of this work, the two institutions conducted two winter-based, dose-related vitamin D RCT during the period 2006–2008 specifically designed to establish the distribution of dietary vitamin D required to maintain serum 25(OH)D concentrations above contemporary cut-offs (> 25, > 37·5, > 50 > 80 nmol/l) during wintertime, accounting for the impact of summer sunshine exposure and diet, in adults and in older adults, separately(Reference Cashman, Hill and Lucey36,Reference Cashman, Wallace and Horigan37) . Both RCT were two-site trials (Cork; 51oN and Coleraine; 55oN) to provide the longitudinal coverage for much of the UK. While estimated dietary requirements for vitamin D at a number of selected percentiles were provided, major focus was on the vitamin D intake estimates to maintain 97·5 % of a population life-stage group above 25 nmol/l, as well as the other contemporary cut-offs, during winter, as this corresponds to a Reference Nutrition Intake (RNI) value in the UK (a Recommended Daily Allowance or Population Reference Intake in the US and EU, respectively). With the publication of the Institute of Medicine report in 2011, which highlighted an increased risk of vitamin D deficiency at serum concentrations below 30 nmol/l, we have subsequently added this threshold to the four other previously suggested ones, and derived the corresponding intake requirement estimates(Reference Cashman38). The vitamin D intakes that would maintain serum 25(OH)D concentrations ≥ 30 nmol/l during wintertime in 97·5 % of adults (20–40 years olds) and older adults (64+ years olds) were around 14 and 12 µg/d, respectively (Table 1).
Table 1. Estimated dietary requirements for vitamin D at the 97·5th percentile in different population subgroups to maintain wintertime serum 25(OH)D above two selected thresholds associated with increased risk of vitamin D deficiency *
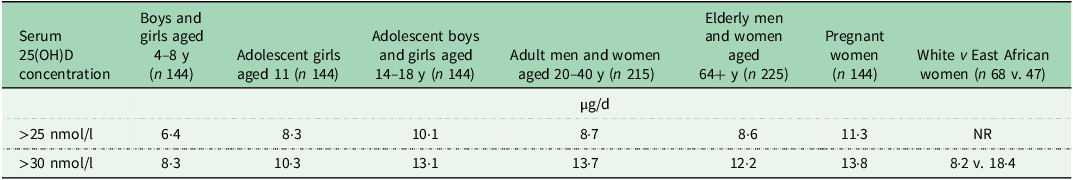
y, year; NR, not reported.
* Estimates from(Reference Cashman, Hill and Lucey36–Reference Cashman, Ritz and Adebayo43).
In relation to the uptake and use of such data in terms of informing national guidelines and policies, in 2009, a UK FSA workshop was convened to review this new vitamin D data together with that from two other FSA-funded vitamin D projects in the 2004 call(Reference Ashwell, Stone and Stolte44). The discussion and debate during this workshop helped inform the decision by the FSA and Department of Health in 2010 to request that the UK Scientific Advisory Committee on Nutrition (SACN) would review the existing Dietary Reference Values (DRV) for vitamin D, established in 1991 and re-iterated by a SACN update in 2007, and also make recommendations. The data from above-mentioned two vitamin D requirement RCT(Reference Cashman, Hill and Lucey36,Reference Cashman, Wallace and Horigan37) , together with data from an additional analysis of vitamin D RCT data in girls (see below), were used as a basis to establish the current RNI of 10 µg/d for all those aged 4 years and older(45). This RNI was based on a population protective serum 25(OH)D concentration of 25 nmol/l.
Data on dietary requirements for vitamin D during childhood were extremely limited. The strategic secondary use of wintertime only data from a wider 12-month vitamin D trial in adolescent Finnish and Danish girls, which examined the impact of vitamin D supplementation on bone mass acquisition (as part of the OPTIFORD EU project(Reference Mølgaard, Larnkjaer and Cashman46)), allowed us to generate equivalent estimates for this population subgroup in 2011(Reference Cashman, FitzGerald and Viljakainen39). The estimates for the girls were relatively similar to those of the adults and older adults (Table 1). Since then, and as part of the ODIN project, four additional bespoke vitamin D requirement RCT were conducted in young children aged 4–8 years (Copenhagen, Denmark; 55oN), teenagers aged 14–18 years (Surrey, UK; 51oN), pregnant women (Cork, Ireland; 51oN) and in persons of ethnic minority (Helsinki, Finland; 60oN)(Reference Mortensen, Damsgaard and Hauger40–Reference Cashman, Ritz and Adebayo43), as under-represented groups among the healthy population for whom data to base estimates of vitamin D requirements were scarce or absent. These vitamin D dose-related RCT estimated the dietary requirements for vitamin D to meet serum 25(OH)D thresholds of 25 and 30 nmol/l, as well as 50 nmol/l, in 97·5 % of individuals in these subgroups largely under conditions of minimal UVB exposure. The first two RCT showed that for white 4–8 year-old children and 14–18 year-old adolescents, 8 and 13 µg/d of vitamin D, respectively, prevented serum 25(OH)D concentrations falling below 30 nmol/l during wintertime (Table 1)(Reference Mortensen, Damsgaard and Hauger40,Reference Smith, Tripkovic and Damsgaard41) . Interestingly the following year, Ohlund et al. in a study in Northern and Southern Sweden, at 63 and 55°N, respectively(Reference Öhlund, Lind and Hernell47) showed that white and dark-skinned children (aged 5–7 years) had varying requirements for vitamin D intakes, needing 6 and 14 μg/d, respectively, to maintain serum 25(OH)D concentrations > 30 nmol/l during winter. In relation to possible ethnic differences in vitamin D requirements, the third ODIN vitamin D dose-response RCT, with a similar design to those mentioned above, compared vitamin D requirements of women of Finnish descent (n 69) with East African women (n 47) in Helsinki, Finland (60°N), during wintertime(Reference Cashman, Ritz and Adebayo43). Models adjusted for baseline differences in age, weight, vitamin D intake and serum 25(OH)D found that dietary requirements for vitamin D to maintain wintertime 25(OH)D above 30 nmol/l were significantly higher in women of East African descent (18 µg/d) than in white women of Finnish descent (8 µg/d) (Table 1)(Reference Cashman, Ritz and Adebayo43).
The last ODIN vitamin D dose-related RCT (conducted in Cork, Ireland; 51oN) among 144 white pregnant women estimated the vitamin D intake required to maintain maternal serum 25(OH)D in late gestation above 25, 30 and 50 nmol/l, but also at concentrations sufficient to prevent neonatal deficiency (i.e. keep concentrations in infants at or above about 25/30 nmol/l)(Reference O’Callaghan, Hennessy and Hull42). The study showed that an intake of about 14 µg/d of vitamin D prevented maternal serum 25(OH)D concentrations falling below 30 nmol/l during wintertime (Table 1). However, this would still not protect serum concentrations in the infants from falling below this threshold. The study showed that when maternal serum 25(OH)D concentration was ≥ 50 nmol/l, 95 % of cord sera had concentrations ≥ 30 nmol/l. A total vitamin D intake of 30 μg/d maintained serum 25(OH)D concentrations ≥ 50 nmol/l during pregnancy, which ensured that serum 25(OH)D concentration was ≥ 30 nmol/l in 95 % of umbilical cord sera(Reference O’Callaghan, Hennessy and Hull42).
Again in terms of uptake of these data, a number of agencies/authorities charged with the development of DRV for vitamin D have used data from these RCT either directly as is, such as the UK SACN(45), the German Nutrition Society(48) and the Irish FSAI(22,23) , or in combination with that from other relevant vitamin D RCT to provide an overall collection of aggregate data points for use in the agencies’ respective meta-regression modelling to derive their dietary recommended intake values, such as the Institute of Medicine(16), the Nordic Nutrient Recommendations(49) and the European Food Safety Authority (EFSA)(50).
In terms of establishing the vitamin D intake – serum 25(OH)D dose response, which is integral to deriving the estimates of vitamin D intake requirements, beyond the meta-regression of aggregate (or statistical summary) RCT data as used by Institute of Medicine, Nordic Nutrient Recommendations and EFSA(16,49,50) , a meta-regression based on analysis of individual participant data (IPD), in which the raw data for each RCT are used for synthesis, offers many potential advantages, is increasingly recognised as best practice and is likely to provide the most appropriate DRV estimates(Reference Cashman51). Again as part of the ODIN project, the Centre completed the first-ever IPD-level meta-regression from seven suitable winter-based RCT (with data from 882 participants ranging in age from 4 to 90 years) of the vitamin D intake-serum 25(OH)D dose–response(Reference Cashman, Ritz and Kiely52). Notably, vitamin D intakes and serum 25(OH)D concentrations had been analysed using the same methods in these studies, ensuring compatibility across the dose-response relationship. The IPD analysis confirmed that 10 and 13 µg/d would be required to maintain wintertime serum 25(OH)D concentrations > 25 and 30 nmol/l, respectively, in 97·5 % of individuals(Reference Cashman, Ritz and Kiely52). These also represent composite estimates across the white, non-pregnant/lactating population over 5 years of age.
In the last few years, the Centre has also completed two additional vitamin D requirement IPD-level meta-analyses, one based on 11 RCT (n 1429) with vitamin D-fortified foods(Reference Cashman, Kiely and Andersen53) and the second based on 10 RCT (n 677) to estimate the vitamin D dietary requirements in dark-skinned individuals (of black or South Asian descent) resident at high latitude(Reference Cashman, Kiely and Andersen54). The IPD based on fortified food RCT in light-skinned individuals provided estimates of the vitamin D intake required to maintain 97·5 % of winter 25(OH)D concentrations ≥ 30 nmol/l as 12 µg/d(Reference Cashman, Kiely and Andersen53). The IPD-derived estimate of the vitamin D intakes required by dark-skinned individuals to maintain 97·5 % of winter 25(OH)D concentrations ≥ 30 nmol/l was 27·3 μg/d and 33·2 μg/d, in South Asians and black individuals, respectively(Reference Cashman, Kiely and Andersen54). These estimates are radically different from the equivalent estimates for white individuals and highlight the need to consider, and possibly adapt, the approach for setting vitamin D RDAs in such cases. The IPD estimates are also higher than that currently recommended internationally by several agencies, which are based predominantly on data from white individuals and derived from standard meta-regression based on aggregate data(16,49,50) . It is important to highlight and acknowledge that such extensive data-rich IPD analyses would not be possible without the generous collaboration of large numbers of willing principal investigators that see the merit of this secondary, but nonetheless strategic, use of their RCT data. The requirement intake estimates from these IPD analyses have been used as supportive evidence by the FSAI in their vitamin D recommendations(22).
The approach of using data from available vitamin D studies, including IPD, to derive a vitamin D intake requirement estimate for young children (0–3·9 years) has been implemented by the WHO together with the FAO in an update of their 2004 Nutrient Requirements. A large body of studies (117 in total, and including both observational studies and RCT) were used to define a serum 25(OH)D threshold of 28 nmol/l, based on its association with minimised risk of rickets in young children with adequate calcium intake(Reference Rios-Leyvraz, Thacher and Dabas55). The ability to stratify on the basis of adequacy/inadequacy of calcium intake within the analysis was novel, since most exercises up to that could not distinguish, and vitamin D dietary requirements are set on the basis of assumed adequacy of calcium intake. This threshold was then used in multi-level and multivariable dose-response modelling of serum 25(OH)D to total vitamin D intake, based on RCT data from 31 trials from North America, Europe, Asia and Australasia/Oceania, with latitudes ranging from 38°S to 61°N, reflecting the global nature of the WHO-FAO recommendations. This modelling allowed for the derivation of an updated vitamin D requirement estimate of 10 µg/d for young children below 4 years of age(Reference Rios-Leyvraz, Martino and Cashman56). Interestingly, while the meta-regression was based on aggregate data (as acquisition of IPD from 31 RCT was not feasible within the timelines and resources available in the WHO-FAO update exercise), an individual variability component, as would be derived from an IPD-level meta-regression, was imputed within the modelling to allow the best estimate of the vitamin D intake requirement covering the needs of 98 % of young children possible(Reference Rios-Leyvraz, Martino and Cashman56).
Data around possible food-based strategies for improving vitamin D intakes
The Centre’s work on vitamin D intake requirements over the last 2 decades suggests that intakes of between 8 to 14 µg/d are needed to prevent risk of vitamin D deficiency (reflected as serum 25(OH)D concentrations below 30 nmol/l(16,50) ) in most population subgroups and maybe higher in the case of dark-skinned ethnic subgroups. The above-mentioned representative survey data for Ireland, the UK and Europe show that habitual vitamin D intakes frequently fall well below these requirement intakes. This underscores the fact that we had previously suggested that sustainable food-based strategies to bridge the gap between current and recommended intakes of vitamin D to minimise the prevalence of serum 25(OHD concentrations < 30 nmol/l, was one of the three critical and prioritised research requirements for Europe(Reference Cashman and Kiely25). The WHO-FAO suggest that in terms of strategies for tackling micronutrient malnutrition, while food fortification tends to have a less immediate impact compared to micronutrient supplementation, it nevertheless has a much wider and more sustained impact(Reference Allen, de Benoist and Dary57). Food-based strategies for improving vitamin D intakes, particularly food fortification and biofortification, have also been a focus within the Centre’s wider vitamin D research programme. The Centre’s collaborative studies over the last decade, funded by national agencies as well as the EC, have ranged from exploration of the potential of UV radiation of bakers’ yeast and edible mushrooms in terms of enhancing their vitamin D2 content, to addition of increased levels of vitamin D3 and/or 25-hydroxyvitamin D3 into the feed of aqua-cultured fish, chickens, pigs and beef cattle, so as enhance the total vitamin D content of fillets, eggs and meats, respectively, for human consumption (a process known as ‘biofortification’(Reference Hayes and Cashman58)), to more traditional addition of vitamin D to foods, such as low-fat cheese, amongst other vehicles(Reference Itkonen, Skaffari and Saaristo59–Reference Manios, Moschonis and Mavrogianni66) (see Fig. 2). These studies have provided good evidence of the technological feasibility of the enhancement of their vitamin D content, and, in some cases where assessed, of the consumer acceptability of these fortified/biofortified food products. A number of the studies have included RCT with the vitamin D-fortified/biofortified foods which highlight the effectiveness of food fortification in terms of improving vitamin D status(Reference Cashman, Kiely and Seamans60,Reference Hayes, Duffy and O’Grady62,Reference Manios, Moschonis and Mavrogianni66,Reference Grønborg, Tetens and Christensen67) , or not, as in the case of bread made from UV-irradiated yeast(Reference Itkonen, Skaffari and Saaristo59). Finally, the evidence from these RCT have been combined with that of other available RCT of vitamin D fortified/biofortified foods in the form of a number of systematic reviews and meta-analyses which have also highlighted their effectiveness in terms of improving vitamin D status and adding to the overall convincing evidence-base(Reference Cashman, Kiely and Seamans60,Reference Dunlop, Kiely and James68–Reference Nyakundi, Némethné Kontár and Kovács70) .

Figure 2. Vitamin D-fortified and vitamin D-biofortified foods investigated over the last decade as part of our vitamin D research programme.
*Vitamin D3 and/or 25-hydroxyvitamin D3 as the fortificant in foods or animal feeds; RCT, randomised controlled trials.
Finally, the data on the vitamin D content that can be feasibly achieved in these fortified/biofortified foods have also been used in in silico dietary modelling experiments based on national nutrition survey data to predict their impact, as part of current consumption practises, on the vitamin D intake in a population subgroup(s) of interest. For example, as part of the ODIN project, such dietary modelling has been performed using data from three relevant Irish national nutrition surveys to estimate the impact of hypothetical addition of vitamin D to the food chain using some of the biofortified food strategies mentioned above, such as eggs, beef and pork, either alone or in combination with fortification of milk and cheese, on the intake of vitamin D by adults, as well as separately by children (aged 5–12 years) and adolescents in Ireland. While the impact of single biofortified foods on the distributions of vitamin D intakes were relatively small, when all three foods were combined together with fortification of milk and cheese, projected mean and median intakes close to the EAR of 10 µg/d were achieved, without increasing the risk of excessive intakes(22,Reference Kiely and Cashman26,Reference Buttriss, Lanham-New and Steenson71) . This dietary modelling was also conducted across nationally representative surveys in three other EU countries and demonstrated the feasibility of achieving average intakes of ∼10 µg/d vitamin D, without increasing the risk of excessive intakes(Reference Kiely and Cashman26). These modelling data complement that from other dietary modelling exercises in Ireland and the UK which were based on more traditional fortification of milk and/or wheat flour/bread, and some other foods(Reference Black, Walton and Flynn21,Reference Allen, Dangour and Tedstone72,Reference McCourt, McNulty and Walton73) . The out-turn of this dietary modelling work has been used by the FSAI in their vitamin D recommendation reports in relation to strategies for addressing low vitamin D intakes within the population(22). Data on food fortification/biofortification, including the dietary modelling, together with other aspects of the Centre’s vitamin D research, such as status and intake data as well as dietary requirement estimates, have been shared at specifically organised meetings among vitamin D scientists and relevant policy decision-makers together with other stakeholders in Canada (during 2015) and Chile (during 2018), which helped inform their fortification strategies. The uptake and translation of data/knowledge around food-based approaches for tackling vitamin D deficiency to policy and action has been more variable within Europe. To further encourage the conversion of the such data/knowledge to action in Ireland, a nationally funded project called ‘Vitamin D-Deficiency Prevention IRELAND’ (acronym, VitD-DPI; https://www.craft.do/s/o7HuSea30GNWvt) aims to use a multi-actor approach to develop, validate and present a policy-ready national framework for vitamin D deficiency prevention in Ireland, that is endorsed by all stakeholders. The project started on the 1st of September 2022 and will be completed by the end of August 2026.
Tackling micronutrient deficiencies or hidden hunger in Europe: again, the need for data
The term ‘micronutrient deficiencies’ has been used in recent times as a synonym of ‘hidden hunger’ and/or ‘micronutrient malnutrition’(Reference Das and Padhani74), all of which, in many cases, are used interchangeably. This section of the review will again attempt to highlight how data is critically needed to address persistent data/knowledge gaps in relation to hidden hunger and, in so doing, can help to contribute to the development of strategies for their prevention. It will also point to how some of the above-mentioned data-driven approaches in relation to tackling vitamin D deficiency, can be put to use for tackling hidden hunger. However, before that it might be useful to define what is meant by hidden hunger.
Hidden hunger, micronutrient malnutrition and micronutrient deficiencies
While not new, the terms ‘hidden hunger’ and ‘micronutrient malnutrition’ have been increasingly used in recent years, with 229 and 123 articles in PUBMED having used the respective terms in the last 5 years alone. However, over 3 decades ago, the WHO together with UNICEF convened a conference in Montreal called ‘Ending hidden hunger: a policy conference on micronutrient malnutrition’(75). This helped bring both terms to international prominence. The purpose of the WHO-UNICEF conference in 1991 was to reinforce the commitment and collaboration to accelerate progress towards the goals of elimination or control of micronutrient malnutrition by the year 2000, endorsed at the World Summit for Children the year before(75). At that time, the WHO explained that ‘micronutrient malnutrition’ is the term used when referring to the main vitamin or mineral nutritional deficiencies of public health significant, referencing iodine deficiency disorders, vitamin A deficiency and iron deficiency anaemias(75). The term ‘hidden hunger’, as a form of malnutrition that occurs when the intake and/or absorption of minerals and vitamins are too low to sustain good health and development as well as normal physical and mental function(Reference Das and Padhani74–77), and which can exist unaccompanied by obvious clinical signs, was helpful to distinguish it from hunger and calorie deficit representing a more widely recognised form of malnutrition. A former deputy executive of UNICEF has suggested that ‘hidden hunger due to micronutrient deficiency does not produce hunger as we know it. You might not feel it in the belly, but it strikes at the core of your health and vitality’(77).
The prevalence of hidden hunger
Over three decades ago, a global prevalence of hidden hunger was estimated at a staggering two billion people, more than double the 805 million people who had insufficient energy intake at that point in time(76). This still widely quoted estimate has its origins in the WHO’s data from 1991 that 1 billion people were at risk of iodine deficiency disorders, 190 million children of pre-school age are at risk of vitamin A deficiency, and over 2 billion people are at risk or iron deficiency anaemia or are affected by some form of anaemia. As many of the affected people are the same people in the at-risk groups, this led to the estimate that the total number of subjects is around 2 billion(75).
Recently, an analysis of pooled global individual-level data on micronutrient status of two key at-risk groups (pre-school-aged children and non-pregnant women of reproductive age) from nationally representative, population-based surveys of 22 countries was based on iron and zinc as well as folate or vitamin A (in the case of non-pregnant women and young children, respectively)(Reference Stevens, Beal and Mbuya2). The work showed that on a global basis, approximately half and two-thirds of the young children and women, respectively, had a deficiency of at least one of these micronutrients (representing ∼1·6 billion individuals). Of note, the analysis showed that no world region, including high-income countries, was spared from the high burden of micronutrient deficiency. For Europe, prevalence estimates of 48 % and 43 % for young children and non-pregnant women, respectively, were reported for these nutrients (representing 82 million individuals)(Reference Stevens, Beal and Mbuya2). However, this is an incomplete picture of the true burden of prevalence of micronutrient deficiencies for Europe, and elsewhere. For example, the estimates for Europe were based on data from just one survey, the UK National Diet and Nutrition Survey. In addition, as the exercise had a global focus, it only focussed on those micronutrient deficiencies of world-wide significance and just in the two at-risk groups’ population subgroups. Thus, the prevalence of hidden hunger for Europe should ideally be based on data from as many representative surveys of the various at-risk population subgroups, such as children and adolescents, women of reproductive age, pregnant women, older adults and immigrant and ethnic minority groups, as possible, and include micronutrients of public health relevance to the region.
Zero Hidden Hunger EU project will generate new data on prevalence and other aspects of hidden hunger in Europe
Thus, while addressing the public health problem of hidden hunger is a priority, it is not possible until data on the true prevalence of micronutrient deficiencies across the EU population and the causes of these deficiencies is available to predict and identify those most at risk. Without this information, discussions on how to meet dietary requirements for the priority micronutrients of public health concern, and improve their status, take place in a vacuum. This information is the key starting point towards defining food-based strategies for micronutrient deficiency prevention and health promotion throughout the life-course. In recognition of this, in 2023, the EC issued a research funding call for a large-scale (€10 M) project on eradication of micronutrient deficiencies in the EU, as part of its Horizon Europe progamme. The Cork Centre for Vitamin D and Nutrition Research at University College Cork (Professor Kevin Cashman and Professor Mairead Kiely, Joint Coordinators), together with 18 collaborative partners across Europe, led a successful bid for this call. The four-year ‘Tackling micronutrient malnutrition and hidden hunger to improve health in the EU’ project, with Zero Hidden Hunger EU as its acronym (http://www.zerohiddenhunger.eu), commenced in early 2024. One of the key initial aims of Zero Hidden Hunger EU is to assess the true prevalence of micronutrient deficiencies, based on priority biomarker and micronutrient intake data in European populations, especially in high-risk population subgroups. It will do this by using existing high-quality data resources on population micronutrient status and intake for various European countries. As with the ODIN vitamin D project, again national nutrition surveys and health surveys, as well as quality representative population cohorts, will be used in Zero Hidden Hunger EU to supply the needed data on micronutrient status, based on validated status markers, and intakes of the prioritised micronutrients of public health concern among the selected at risk groups within the EU population, namely, children and teenagers, women of reproductive age, pregnant women, older adults and ethnic minority groups. To complement this existing micronutrient status data, some new analysis in bio-banked blood samples will provide some key missing micronutrient status data for incorporation and use in the overall data collection. In this way, the Zero Hidden Hunger EU project will maximise previous EC and member state investment.
This new data on the true prevalence of micronutrient deficiencies across the EU, and information on their dietary causes, will then be utilised in the development of proposals for context-specific, foods-first, sustainable and cost-effective approaches for prevention of micronutrient deficiencies in Europe, using standardised approaches to data collection and novel dietary modelling to generate multiple predictions of the effect of food-based strategies to ensure adequate micronutrient intake, while optimising environmental outcomes. The project will also evaluate the cost effectiveness of these proposed mitigation strategies using the new prevalence data and dietary proposals. Greater detail of these planned activities will be provided elsewhere in due course.
Conclusions
There is a clear and pressing need to develop and implement inclusive policies aimed at ending hidden hunger, as well as other forms of malnutrition. In terms of tackling deficiency of vitamin D and other micronutrients – be it in relation to the quantification of the magnitude of the public health problem, establishing micronutrient intake requirement values, or proposing food-based strategies for the deficiency prevention, amongst other aspects, data is the life-blood. The present review provides additional insight into the critical importance of data, in its many forms, in the generation of the evidence-base that can inform recommendations, guidelines and other policy instruments for addressing micronutrient deficiency. Priority data can be translated into meaning and knowledge, which can inform policy decision-making and formulation, and these policies, to be effective, need to then be acted upon, if there is any chance of achieving the elimination of micronutrient deficiencies in the near future.
Acknowledgements
The oral presentation given at the inaugural Nutrition Society Congress, and this associated review article, overviewed research that has been conducted at the Cork Centre for Vitamin D and Nutrition Research at University College Cork together with many important collaborators nationally (including Irish Universities Nutrition Alliance; UCD; NICHE at UU, Coleraine; amongst others) and internationally (Vitamin D Standardization Program; our EU project collaborators, especially the ODIN and Zero Hidden Hunger EU projects; the Principal Investigators involved in our Individual Participant Data activities; as well as colleagues at the WHO and FAO in relation to vitamin D requirements; amongst others), all of whom have been key in terms of generation of data and knowledge on aspects of vitamin D and/or hidden hunger. Locally, Professor Mairead Kiely, as joint director of the Cork centre, has been at the forefront of much of the research activities which were included in the presentation/review. There is also a long list of key scientists and researchers, past and present, from the Cork centre that have been instrumental in our research activities and the generation of the key data that was presented.
Author contributions
Kevin D. Cashman is the sole author of this review.
Financial support
No specific grant from any funding agency, commercial or not-for-profit sectors has been received for the preparation of this review article. The research referenced within the review has been on foot of many research grants and each is acknowledged in the associated citations used in the review.
Competing interests
The author does not have conflicts of interest to declare. In terms of disclosures, the author has previously served as a member of the FAO-WHO expert group on nutrient requirements for infants and young children aged 0–36 months and the UK Scientific Advisory Committee on Nutrition working group on vitamin D. He is currently a member of the Scientific Committee of the Food Safety Authority of Ireland (FSAI) and chairs its Public Health Nutrition sub-committee.
Ethical statement
None applicable.





