Psoriasis is a chronic inflammatory skin condition presenting as red or pink (grey in skin of colour) scaly plaques on the extensor aspects of elbows and knees, scalp and lumbosacral areas of the body(Reference Raharja, Mahil and Barker1). The prevalence of psoriasis increases with age, affects both sexes equally, and is more pronounced in the Nordic regions of the world, with a 1·92 % prevalence (95 % CI 1·07 %, 3·46 %) in western Europe and a 1·83 % prevalence (95 % CI 0·62 %, 5·32 %) in central Europe(Reference Parisi, Iskandar and Kontopantelis2). There is no cure for psoriasis, and people affected fluctuate between phases of relapse and remission, which are managed with topical creams, and in more severe cases with photo-, systemic or injection therapies(Reference Armstrong and Read3). Whilst psoriasis onset is influenced by a genetic predisposition(Reference Ogawa and Okada4), environmental exposures inclusive of infections, stress, smoking, alcohol and raised body mass index (BMI) have emerged as contributing candidates for psoriasis severity and incidence(Reference Zeng, Luo and Huang5). The role of lifestyle in psoriasis was emphasised in recent findings from the UK Biobank cohort where a ‘poor’ lifestyle, defined as a BMI > 30 kg/m2, an active smoking status, low physical activity levels, and low adherence to the American Heart Association dietary guidelines, was associated with an increased risk of incident psoriasis; Hazards Ratio 2·32 (95 % CI 1·30, 4·17), in participants within the lowest category of genetic susceptibility(Reference Shen, Xiao and Jing6).
Diet is a pivotal modifiable risk factor for the prevention and management of chronic inflammatory diseases. The National Institute of Health and Care Excellence (NICE) has embedded dietary advice in clinical guidelines for people living with chronic inflammatory diseases(7,8) . Where the evidence is convincing, a dietary recommendation is present e.g. reducing the intake of salt to reduce blood pressure for people with hypertension(9), and where the evidence is not as strong, a dietary principle is encouraged e.g. following a Mediterranean diet (MD) for joint health in rheumatoid arthritis(9). The role of diet has not yet been determined in the management of psoriasis and the proximate to a dietary recommendation in the NICE guidelines is monitoring alcohol intake to reduce the risk of hepatotoxicity for individuals receiving methotrexate(10).
Psoriasis is driven by pro-inflammatory cytokine circuits of the IL-17/23 axis coupled to the activity of TNF alpha (TNF-α); a cytokine whose activity is overexpressed in systemic inflammation(Reference Baliwag, Barnes and Johnston11,Reference Potestio, Martora and Lauletta12) . Furthermore, observational evidence has shown the prevalence of dyslipidaemia(Reference Miao, Li and Li13), high blood pressure(Reference Armstrong, Harskamp and Armstrong14) and raised leptin and resistin concentrations(Reference Kyriakou, Patsatsi and Sotiriadis15) in people with psoriasis. Research is gradually recognising the role of nutrition as a therapeutic tool to suppress inflammatory burdens in psoriasis(Reference Katsimbri, Korakas and Kountouri16). More importantly, as shown in Table 1. reporting the results of meta-analyses in psoriasis populations, given the multimorbid nature of psoriasis, introducing dietary modifications may serve to mitigate the ramifications of an underlying multisystemic pathology.
Table 1. Common morbidities associated with psoriasis
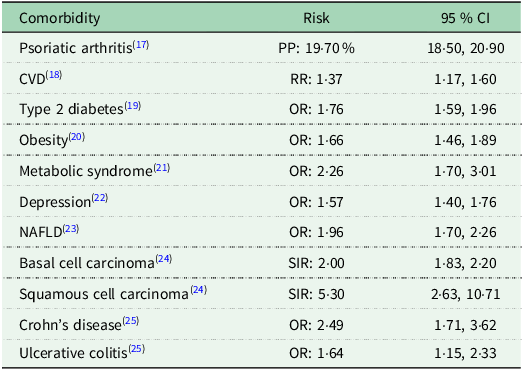
PP = Pooled Proportion; OR = Odds Ratio; SIR = Standardised Incidence Ratio; RR = Relative Risk.
Understanding the role of diet has been identified as the top research priority of the James Lind Alliance Psoriasis Priority Setting Partnership(Reference Majeed-Ariss, McPhee and McAteer26). This review will critically discuss the research examining dietary patterns in psoriasis populations, highlight the gaps in the evidence-base, and present directions for future research.
Low energy diets
Research examining the effect of diet on psoriasis has predominantly focused on low-energy diets, consistently showing improvements in psoriasis severity(Reference Gisondi, Del Giglio and Di Francesco27–Reference Jensen, Christensen and Zachariae31). Low-energy diets are currently recommended by the Medical Board of the National Psoriasis Foundation (NPF USA) for individuals who are overweight or living with obesity(Reference Ford, Siegel and Bagel32). This recommendation stems from the recognition of the role of excess adiposity in aggravating psoriasis and inflammation(Reference Rodríguez-Cerdeira, Cordeiro-Rodríguez and Carnero-Gregorio33), where aligned with the negative-energy balance hypothesis, low-energy diets downregulate diet and weight-induced inflammatory burdens, promote weight loss(Reference Hill, Wyatt and Peters34), consequentially improving the severity of psoriasis. Disease severity in psoriasis is clinically evaluated with the Psoriasis Area Severity Index (PASI) generating a score (out of 72) on the area, redness, thickness and scaliness of the skin lesions(Reference Ashcroft, Wan and William35). PASI scores below 5 are considered mild psoriasis, scores between 5 and 10 are representative of moderate psoriasis, whilst PASI scores above 10 are indicative of severe psoriasis(Reference Finlay36). A meta-analysis of randomised controlled trials (RCTs) has shown that weight-loss interventions improved psoriasis severity with a PASI reduction of –2·49 (95 % CI –3·90, –1·08)(Reference Upala and Sanguankeo37). As highlighted in a Cochrane review on lifestyle interventions, diets with strict energy restriction may improve psoriasis severity (based on low-quality evidence), and favour BMI reductions (based on moderate-quality evidence)(Reference Ko, Chi and Yeh38). The limitations underpinning the research investigating low-energy diets for psoriasis include the (i) lack of adequate comparators in RCTs; where control groups are exempt from dietary advice but continue or receive new prescriptions of anti-psoriatic therapies, masking the true effect of diet on psoriasis improvements and (ii) recruiting participants living with obesity; impeding the generalisability of the findings to individuals with a BMI in the normal healthy range(Reference Ko, Chi and Yeh38).
Gluten-free diets
A second recommendation of the NPF USA is a gluten-free diet (GFD), for adults with psoriasis with a biopsy-confirmed diagnosis of coeliac disease (CD), or for individuals who test positive for non-coeliac gluten-sensitivity serology(Reference Ford, Siegel and Bagel32). The GFD for psoriasis fostered interest following the identification of a shared location for psoriasis and CD susceptibility alleles, the mutual activation of helper T-cells (Th)-1 triggering pro-inflammatory responses(Reference Bhatia, Millsop and Debbaneh39), and the increased risk of CD with a OR of 2·16 (95 % CI 1·74, 2·69) in people with psoriasis(Reference Acharya and Mathur40). Three interventional trials have prescribed a GFD to people with psoriasis with raised antibodies to gliadin (AGA) titres(Reference Michaëlsson, Åhs and Hammarström41–Reference Kolchak, Tetarnikova and Theodoropoulou43), and one trial was conducted in people with confirmed CD(Reference De Bastiani, Gabrielli and Lora44). The primary outcomes of these studies have prioritised examining histological changes in the duodenum and dermis(Reference Michaëlsson, Åhs and Hammarström41,Reference Michaëlsson, Kristjánsson and Pihl Lundin42,Reference Michaëlsson, Wolfgang and Hagforsen45) , evaluated changes in antibody titres(Reference Kolchak, Tetarnikova and Theodoropoulou43) and were designed to estimate CD prevalence in psoriasis(Reference De Bastiani, Gabrielli and Lora44). Although the results consistently illustrated improvements in psoriasis severity, these findings, do not emanate from rigorous RCTs. Whilst this research is limited to psoriasis populations susceptible to CD and raised AGA titres, the evidence presents the importance of introducing CD screening in the care pathway of psoriasis for people reporting gastrointestinal symptoms.
Proinflammatory diets
Observational studies have examined associations between adherence to anti-inflammatory dietary patterns and psoriasis severity using a priori diet quality indices (DQIs). The inflammatory potential of a diet is assessed with the Diet Inflammatory Index (DII); a literature-derived score based on 45 components including whole foods, nutrients and bioactives(Reference Shivappa, Steck and Hurley46), originally validated with high-sensitivity C-reactive protein (CRP)(Reference Cavicchia, Steck and Hurley47). Results from a case-control study in participants with psoriasis and matched controls (n 149) revealed associations between higher energy-adjusted DII scores with increased disease severity (OR = 3·64, 95 % CI 1·74, 7·57) and incident psoriasis (OR = 3·64, 95 % CI 1·51, 8·79)(Reference Kashani, Moludi and Fateh48). The latter finding was not reproduced in a retrospective cohort study using data from the National Health and Nutrition Survey (NHANES) (n 13 284) where the DII was not associated with incident psoriasis (OR = 1·00, 95 % CI 0·89, 1·11) irrespective of confounder adjustments for demographic variables(Reference Liu, Zhang and Hua49). Methodological limitations may contribute to the conflict in the evidence examining the DII and psoriasis severity. This includes a small sample size in the case-control analysis(Reference Kashani, Moludi and Fateh48), requiring larger and more representative sample, and the different DII score computation in the NHANES analysis(Reference Liu, Zhang and Hua49), where the DII score was calculated using 26 out of the 45 components, potentially underestimating dietary inflammatory potential.
Mediterranean diets
When examining adherence to a MD in people living with psoriasis, cross-sectional analyses have reported inverse associations between adherence and psoriasis severity. MD adherence examined in a Spanish cohort study of middle-aged adult males and females with psoriasis (n 92) revealed that participants in the lowest adherence category (assessed with the 14-item PREvención con DIeta MEDiterránea (PREDIMED) questionnaire), reported a greater psoriasis severity (median PASI of 7·0, 95 % CI 3·60, 8·20), compared to participants in the highest adherence category (median PASI of 0·8, 95 % CI 0·00, 2·57)(Reference Molina-Leyva, Cuenca-Barrales and Vega-Castillo50). Similarly, in a case-control study of 138 participants (with equal male-to-female ratio, and a mean age 48 years), mean (± sd) adherence to a MD (evaluated using the 12-item MedDietScore) was lower in the psoriasis group (28·7 ± 4·4 v. 33·9 ± 5·6), was negatively correlated with the severity of psoriasis (r = –0·390), and was identified as a negative predictor for disease severity(Reference Korovesi, Dalamaga and Kotopouli51). Results from the French NutriNet Santé cohort (n 3557), primarily comprising of middle-aged females (76 %) revealed a lower risk of severe psoriasis with higher adherence to a MD (OR = 0·78, 95 % CI 0·59, 1·01), as measured per the 9-component MEDI-LITE Score(Reference Phan, Touvier and Kesse-Guyot52).
Methodological limitations in the currently available evidence for the MD in people living with psoriasis have interfered with the extrapolation of a dietary recommendation. Other than the NutriNet Santé cohort(Reference Phan, Touvier and Kesse-Guyot52), most of the studies evaluating the MD are relatively underpowered(Reference Molina-Leyva, Cuenca-Barrales and Vega-Castillo50,Reference Korovesi, Dalamaga and Kotopouli51,Reference Barrea, Balato and Di Somma53) . Furthermore, the lack of RCTs and prospective cross-sectional studies hinder inference on the directionality between adherence to the MD and the severity of psoriasis. Inconsistent scoring systems are adopted to determine MD adherence, and the lack of a single agreed definition
make comparisons difficult e.g. the MEDI-LITE score does not distinguish between poultry and red and processed meat(Reference Sofi, Dinu and Pagliai54), unlike the PREDIMED or MedDietScore(Reference Panagiotakos, Pitsavos and Arvaniti55,Reference Martínez-González, García-Arellano and Toledo56) . Finally, the Mediterranean ethnicities of the study populations evaluating associations between MD adherence and psoriasis severity limit the applicability of the findings outside of the Mediterranean basin whose traditional dietary pattern is not a MD.
To address the lack of observational evidence in populations outside the Mediterranean region, the APPLE study (NCT05448352) aimed to evaluate associations between DQIs and psoriasis severity in UK-based adults with psoriasis responding to an online survey. Three a priori DQIs were derived from a 147-item food frequency questionnaire and cross-compared; the Mediterranean Diet Score (MDS), the Dietary Approaches to Stop Hypertension (DASH) score and the Plant-based Diet Index (PDI) including the original (oPDI), the healthy (hPDI) and the unhealthy (uPDI) subtypes. Psoriasis severity was determined using the participant responses to the self-assessed Simplified Psoriasis Index (sa-SPI) a 3-domain score out of 70 points. The sa-SPI accounts for a self-rating of lesion severity across 10 body areas, a Likert-scale rating of psychosocial impact, and an intervention history recall scoring points for each systemic and biologic treatment received(Reference Chularojanamontri, Griffiths and Chalmers57). Whilst the sa-SPI was self-reported and is not as objective as the PASI, it is advantageous in that it also considers the impact of psoriasis on day-to-day tasks and examines 10 specific areas (avoiding estimates of body surface area coverage) as seen with the PASI(Reference Ashcroft, Wan and William35).
Inverse associations were revealed between adherence to the DASH, hPDI, MDS and oPDI and psoriasis severity(Reference Zanesco, Maruthappu and Griffiths58). After adjusting for BMI, these associations were attenuated, emphasising the mediating role of adiposity in disease severity. Analyses of the individual MDS components revealed that meat and poultry (combined as a single category) was a predictor for psoriasis severity, whilst fruits and nuts (combined as a single category) and legumes were negative predictors for disease severity(Reference Zanesco, Maruthappu and Griffiths58). This contrasts with findings from Mediterranean populations where, unlike the APPLE study, extra virgin olive oil and fish components were negative predictors of psoriasis severity(Reference Barrea, Balato and Di Somma53). These discrepancies may be attributed to regional variation in consumption of key food items. For example, fish consumption in the UK is lower than in Mediterranean countries with the average fish intake in adults at 15 g/d(59), compared to the approximate 50 g/d reported by the French NutriNet Santé cohort and Italian National Food Consumption Survey(Reference Leclercq, Arcella and Piccinelli60,Reference Brunin, Pointereau and Allès61) . Furthermore, compromised accessibility and availability of MD foods in non-Mediterranean countries is an often-critiqued aspect of dietary recommendations based on the MD(Reference Tsofliou, Vlachos and Hughes62).
The MD is yet to be tested at RCT level in study populations with psoriasis. Rich in fruits, vegetables, fish and olive oil, the MD is a characteristic diet in regions surrounding the Mediterranean basin. The transferability of this dietary pattern to non-Mediterranean regions is often limited by geography; such as access to sea food in regions distant from the sea, climatic; seasonality and availability of fresh fruits and vegetables, and sociocultural barriers; including the acceptability of certain food groups and cooking practices(Reference Tsofliou, Vlachos and Hughes62–Reference Mattavelli, Olmastroni and Bonofiglio64), which is why understanding feasibility of the MD is of paramount importance prior to implementation.
Ketogenic diets
An 8-week randomised cross-over trial in 26 participants with psoriasis and psoriatic arthritis compared a MD to a ketogenic diet (KeD) for improvements in anthropometric, disease severity and serological measures(Reference Lambadiari, Katsimbri and Kountouri65). In this study, both diets comprised of approximately 1550 (± 50) kcal/d with the KeD at 34 % energy from protein, 55 % from fat and 11 % from carbohydrates, whilst the MD intervention consisted of protein, fat and carbohydrate ratios at 20, 40 and 40 % of energy respectively(Reference Lambadiari, Katsimbri and Kountouri65). During the MD phase, improvements (reported as % differences from baseline Δ%) in weight (–7·48 %), waist circumference (–3·65 %) and fat mass (–12·45 %) were reported. These measures were relatively lower to the KeD improvements on weight (–10·63 %), waist circumference (–6·32 %) and fat mass (–17·47 %), although there was no control group that did not receive dietary advice for true effect estimates. Greater PASI (–61·58 %) and inflammatory marker (–55·60 %, for IL-6; –145·59 %, for IL-17; and –32·08 %, for IL-23) reductions were reported in favour of the KeD(Reference Lambadiari, Katsimbri and Kountouri65). This is congruent with previous literature examining a very-low energy KeD for psoriasis, where energy intake was restricted to <500 kcal/d, was composed of 10–20 g/d of carbohydrates, 20–30 g/d of fat and 1·4 g/kg per d of protein, and incorporated enteral nutrition strategies(Reference Castaldo, Pagano and Grimaldi66,Reference Castaldo, Rastrelli and Galdo67) .
Although promising, the long-term effectiveness of KeDs is uncertain. KeDs are aimed to create a state of diet-induced ketosis by minimising carbohydrate intake to less than 10 % of total energy(Reference Zhu, Bi and Zhang68). For the successful attainment of ketosis, the KeD follows regimented macronutrient requirements and proportions, restricts food choice and requires persistent medical supervision(Reference Muscogiuri, El Ghoch and Colao69). It is with no surprise that KeDs exhibit poor long-term tolerability, which for a chronic condition like psoriasis is not sustainable to introduce for long-term purposes, and there is a risk of adverse effects(Reference Muscogiuri, Barrea and Laudisio70). Furthermore, KeDs are suggested to increase LDL and triglyceride concentrations(Reference Wang, Chen and Wu71), which may be detrimental to people with psoriasis with existing elevated CVD risk(Reference Batch, Lamsal and Adkins72).
Time-restricted eating
Time-Restricted Eating (TRE) is a dietary pattern which through its prolonged fasting interval, activates metabolic pathways that mimic starvation. These include switching from carbohydrate-metabolism, the preferred energy source, to lipid metabolism (mediated by glucagon activity), for energetic reactions(Reference Longo and Mattson73). In scope of synchronising meal timings with circadian rhythms, TRE limits food intake to a diurnal ‘eating window’, which is coupled to an extended nocturnal ‘fasting window’(Reference Longo and Panda74). Prolonged fasting windows start from 14-hours (paired with 10-hours of eating), and range up to 20-hours of fasting (with 4-hours of eating) in resistant trained males(Reference Tinsley, Forsse and Butler75), the most studied being the 16-hours fasting (and 8-hours of eating) known as the 16:8(Reference Manoogian, Chow and Taub76). Extended fasting windows are suggested to activate stress-resistant pathways targeting inflammatory cascades(Reference Mattson, Longo and Harvie77). Meta-analyses of RCTs in study populations with obesity have reported weight-loss in participants subject to TRE protocols compared to control groups(Reference Pellegrini, Cioffi and Evangelista78–Reference Chen, Liu and Bao80) and recently, a meta-analysis of RCTs and non-RCTs (n 936) showcased significant reductions in inflammatory markers notably CRP, TNF-α and IL-6(Reference Turner, Charrouf and Martínez-Vizcaíno81). Considering the aberrant inflammatory milieu of psoriasis, the concomitance of obesity(Reference Armstrong, Harskamp and Armstrong20) and adiposity as a risk factor for pathogenesis(Reference Setty, Curhan and Choi82), people with psoriasis may benefit from the anti-inflammatory and weight-loss-inducing properties of TRE regimes, to mitigate disease onset and severity.
In psoriasis populations, TRE has only been examined under the lens of Ramadan fasting. This Islamic religious practice occurs during the Holy month of Ramadan where complete food and water abstinence takes place from dawn to sunset, reversing the chrono-nutritive principles of restricted diurnal eating and prolonged nocturnal fasting(Reference Johnston, Ordovás and Scheer83). Nevertheless, even though this temporal eating pattern may promote circadian misalignment of metabolic and inflammatory pathways(Reference BaHammam, Alrajeh and Albabtain84,Reference Bahijri, Borai and Ajabnoor85) , following a Ramadan TRE pattern was shown to improve psoriasis severity and inflammatory marker concentrations in people with psoriasis and psoriatic arthritis(Reference Damiani, Watad and Bridgewood86,Reference Adawi, Damiani and Bragazzi87) , suggesting that prolonged fasting periods between eating windows may be beneficial, irrespective of clock time.
A systematic scoping review by Termannsenn and colleagues presented the acceptability (high-retention rates) and safety of implementing TRE patterns in study populations living with obesity, pre-diabetes and type 2 diabetes(Reference Termannsen, Varming and van Elst88). This review showed that TRE adherence was influenced by the self-selection of eating windows, accessibility to caffeinated beverages and zero-energy drinks in fasting windows, and the disruption of social occasions, which should be taken into consideration when prescribing TRE interventions(Reference Termannsen, Varming and van Elst88). Compared to altering diet quality with a MD, modifying meal timing with TRE is potentially a more cost-effective and implementable behavioural intervention, requiring less time for meal preparation. The acceptability of TRE has been confirmed in fire-fighters of the Healthy Heroes RCT, where a 10-hour eating and 14-hour fasting TRE pattern (paired with a MD) is feasible to introduce and confers cardiometabolic and quality of life benefits to shift workers(Reference Manoogian, Zadourian and Lo89).
The MEditerranean diet and time-restricted eating dietary patterns for Psoriasis study
To understand the practicality of introducing dietary patterns to study populations with psoriasis, the MEditerranean diet and Time-Restricted Eating Dietary patterns for Psoriasis (METRED-P) (NCT05820698) study was designed to evaluate the feasibility of the MD, based on the PREDIMED recommendations(Reference Estruch, Ros and Salas-Salvadó90) and a 10-hour eating:14-hour fasting TRE pattern(Reference Manoogian, Zadourian and Lo89), in an adult population with psoriasis(Reference Zanesco, Hall and Gibson91). As the first randomised parallel-arm diet intervention trial in people living with psoriasis in the UK, the METRED-P study aimed to evaluate the feasibility of dietary intervention strategies and generate pilot data on psoriasis severity, life quality, body composition and serological measures subject to the MD, MD with TRE or TRE dietary patterns to inform the design of a future efficacy study. To our knowledge, this combination of intervention arms has not been examined before and is due to be tested for feasibility in individuals with type 2 diabetes(Reference Papamichou, Panagiotakos and Holmes92), bipolar disorder(Reference Johnson, Murray and Kriegsfeld93) and CHD(Reference Iglesies-Grau, Dionne and Latour94).
The rationale for the METRED-P intervention selection is based on the premises that the MD is recognised as one of the healthiest dietary patterns for chronic disease prevention(Reference Romagnolo and Selmin95), cardiometabolic health, and is reputed for its anti-inflammatory effects(Reference Koelman, Egea Rodrigues and Aleksandrova96,Reference Estruch97) , which may be beneficial to people living psoriasis with an underlying pro-inflammatory pathology(Reference Bai, Zheng and Dong98). TRE modifies eating windows but not necessarily diet quality (although reducing the duration of eating windows may indirectly lead to changes in food choices) and promotes optimal circadian alignment where prolonged fasting periods activate favourable autophagic and anti-inflammatory pathways(Reference Longo and Mattson73).
Understanding the effect of a MD and TRE at modulating the symptoms of a systemic inflammatory skin disease such as psoriasis merits further exploration. Current psoriasis management is fully reliant on pharmaceutical treatments and evidence-based dietary advice has the potential to serve as an adjunct to conventional anti-psoriatic therapies. The potential of diet as a therapeutic tool to manage the severity of psoriasis is increasingly gaining recognition(Reference Johnson, Murray and Kriegsfeld93). To date the evidence-base lacks quantity and quality to derive dietary recommendations. It is of paramount importance for research to prioritise RCTs testing candidate dietary approaches such as the MD and TRE, as well as existing dietary guidelines for the general population, to capture the efficacy, acceptability and sustainability of the dietary interventions for psoriasis.
Acknowledgements
The authors are grateful to the Psoriasis Association for supporting this research project.
Author contributions
Sylvia Zanesco drafted the manuscript, and coauthors revised the manuscript. All authors read and approved the final manuscript.
Financial support
This work was supported by the Psoriasis Association (grant number Worktribe ref 1 121 143). This research received no specific grant from any funding agency, commercial or not-for-profit sectors. The Psoriasis Association had no role in the design nor in the writing of this article.
Competing interests
Thiviyani Maruthappu has received honoraria from Abbvie, Almirall, Amgen, Galderma, Pfizer, Novartis, UCB Pharma, L’Oreal and Proctor & Gamble.
Christopher E.M. Griffiths has received honoraria and/or research grants from AbbVie, Almirall, Amgen, Anaptysbio, Artax, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Eli Lilly, Evelo Bioscience, Galderma, GSK, Inmagene, Kyowa Kerin, Janssen, ONO Pharmaceuticals, Novartis, Pfizer and UCB Pharma.
WLH has received research funding from the Alliance for Potato Research & Education and the Almond Board of California, as well as consultancy fees from ZOE Ltd, and funding to attend a conference from Yakult UK Limited.
Sylvia Zanesco and Rachel Gibson declare none.



