Like most countries globally, Australia has an ageing population. Age is the most significant risk factor for accelerated cognitive decline. In 2022, dementia (including Alzheimer’s disease) was the leading cause of death in Australian females and the second leading cause of death in males(1) as well as being a major contributor to disability and reduced quality of life (QoL). Approximately two in three people with dementia are living in the community with considerable costs for the 400 000 Australian’s living with dementia, their families and the broader society including healthcare and carer costs(2). Currently, there are no suitable long-term pharmacological treatments or preventative options for dementia, so there is an urgent need to explore non-pharmacological preventative strategies(3).
Diet and physical activity (PA) are two modifiable factors that can reduce the risk or delay the onset of most chronic disease, possibly including dementia(4,5) . Nevertheless, data from historical surveys such as the Australian Health Survey and National Nutrition Survey consistently show that Australians do not meet the government-recommended dietary guidelines, nor do they meet PA recommendations(6,7) . Typically, Australian’s follow a Western style dietary pattern that is low in fruit and vegetables and high in saturated fat and discretionary foods(6) and engage in a high proportion of sedentary behaviour(2,7) . Only 25 % and 41 % of Australians aged 18–64 years and aged ≥ 65 years, respectively, reported meeting the PA guidelines with walking being the most common form of exercise(7). PA is an integral part of healthy ageing, supporting independence and mobility in activities of daily living. The current Australian PA guidelines for older adults only promote 30 min of moderate activity on most days(8).
One strategy to encourage dietary change and increase PA among older adults is to target diet and or lifestyle through interventions and programmes informed by government guidelines. Government dietary guidelines encourage the adoption of more plant-based patterns such as the Mediterranean diet (MedDiet) which was endorsed as part of the USA 2015–2020 and 2020–2025 dietary guidelines for Americans. The MedDiet is known as one of the leading diets for brain health(Reference Sofi, Macchi and Abbate9–Reference Antonia, Antigone and Mark11). It is characterised as a predominantly plant-based dietary pattern rich in extra virgin olive oil, nuts, seeds, legumes, fruit and vegetables with moderate dairy food and low intake of red and processed meat(Reference Davis, Bryan and Hodgson12) which contrasts with the ‘Western Diet’ typically consumed by developed countries like Australia and the USA. International(Reference de Lorgeril, Salen and Martin13–Reference Marseglia, Xu and Fratiglioni15) and national research has shown that older adults can adhere to a Mediterranean style diet (with minimal adaptations) and experience a range of health benefits(Reference Hardman, Meyer and Kennedy16–Reference Murphy, Dyer and Hyde24). As such, it is widely accepted to be a suitable and feasible dietary pattern to promote in Australian populations(Reference Davis, Hodgson and Bryan21,Reference Davis, Bryan and Hodgson22,Reference Murphy and Parletta25–Reference Minihane and Murphy29) , with studies also suggesting dietary habits can be sustained to up to 12 months post-intervention(Reference Murphy, Dyer and Hyde24). Additionally, walking groups are a strategy at a wider population level to promote PA(Reference Kassavou, Turner and French30). Walking groups also have reported additional benefits such as improvements in blood pressure, BMI, heart rate, QoL and total cholesterol, similar to dietary interventions(Reference Kassavou, Turner and French30,Reference Ogilvie, Foster and Rothnie31) .
There is limited information which provides the cost of strategies to support the adoption of a MedDiet and PA through interventions. This is despite evidence suggesting combined interventions, utilising walking as the mode of exercise, provide health benefits in older adults(Reference Hardman, Meyer and Kennedy16). Developing cost-effective community programmes may help to secure future funding for public health initiatives and improve the health of older Australians while preventing cognitive decline. Few economic analyses from MedDiet randomised controlled trial (RCT) exist with the majority of research related to the costs of consuming a MedDiet based on food basket modelling(Reference Bracci, Milte and Keogh32–Reference Flynn and Schiff34) or cross-sectional studies(Reference Saulle, Semyonov and La Torre35) which extrapolate costs from FFQ. A small number of studies do report the costs of MedDiet interventions, with both the HELFIMED and SMILES Australian RCT indicating MedDiet interventions can be cost-effective in populations with depression(Reference Opie, Segal and Jacka36–Reference Segal, Twizeyemariya and Zarnowiecki38); however, no studies have determined the cost of a combined MedDiet and walking intervention and the effect on QoL.
The aim of the present study was to assess the effect of a MedDiet and walking intervention (MedWalk) on QoL over 12 months with the hypothesis that the MedWalk group would be less likely to experience an age-related reduction in their QoL than the control group and then to undertake a cost–consequence analysis to estimate the mean differential cost per person to deliver MedWalk relative to control group costs.
Methodology
The MedWalk intervention
MedWalk was a 12-month dual-site (South Australia and Victoria) cluster RCT implemented predominantly in retirement villages with some recruitment in Victoria occurring from the community due to the impact of the COVID-19 pandemic. The protocol is published elsewhere(Reference Pipingas, Murphy and Davis39). This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Human Research Ethics Committee at Swinburne University (Ref: 20201600-3559, 14/02/2020) and The University of South Australia (Ref: 202844, 16/06/2020). Written informed consent was obtained from all subjects/patients. This trial was registered with the Australia and New Zealand Clinical Trials Registry at https://www.anzctr.org.au/ (ANZCTR 12620000978965).
The primary outcome of which the statistical powering and sample size were based on is change in visual memory and learning, assessed as part of the Cambridge Neuropsychological Test Automated Battery (CANTAB)(Reference Pipingas, Murphy and Davis39) as reported in the protocol paper. The original sample size calculation (n 364) was based on a power and significance level of 80 % and 5 %, respectively, with a moderate effect size (η 2 = 0·06) and an attrition rate of 30 %. Due to the COVID-19 pandemic and recruitment difficulty, power was re-calculated after baseline testing with the adjusted sample size (October 2022) resulting in the smallest detectable effect size increasing to (η 2 = 0·08) but was still adequately powered to address the primary outcome with 7 % attrition. Secondary outcomes include additional CANTAB cognitive outcomes, mood, cardiovascular function, blood biomarkers, QoL and Motivational Interviewing Cognitive Behavioural Therapy (MI-CBT) effectiveness(Reference Pipingas, Murphy and Davis39). Other primary and secondary MedWalk outcomes will be reported elsewhere.
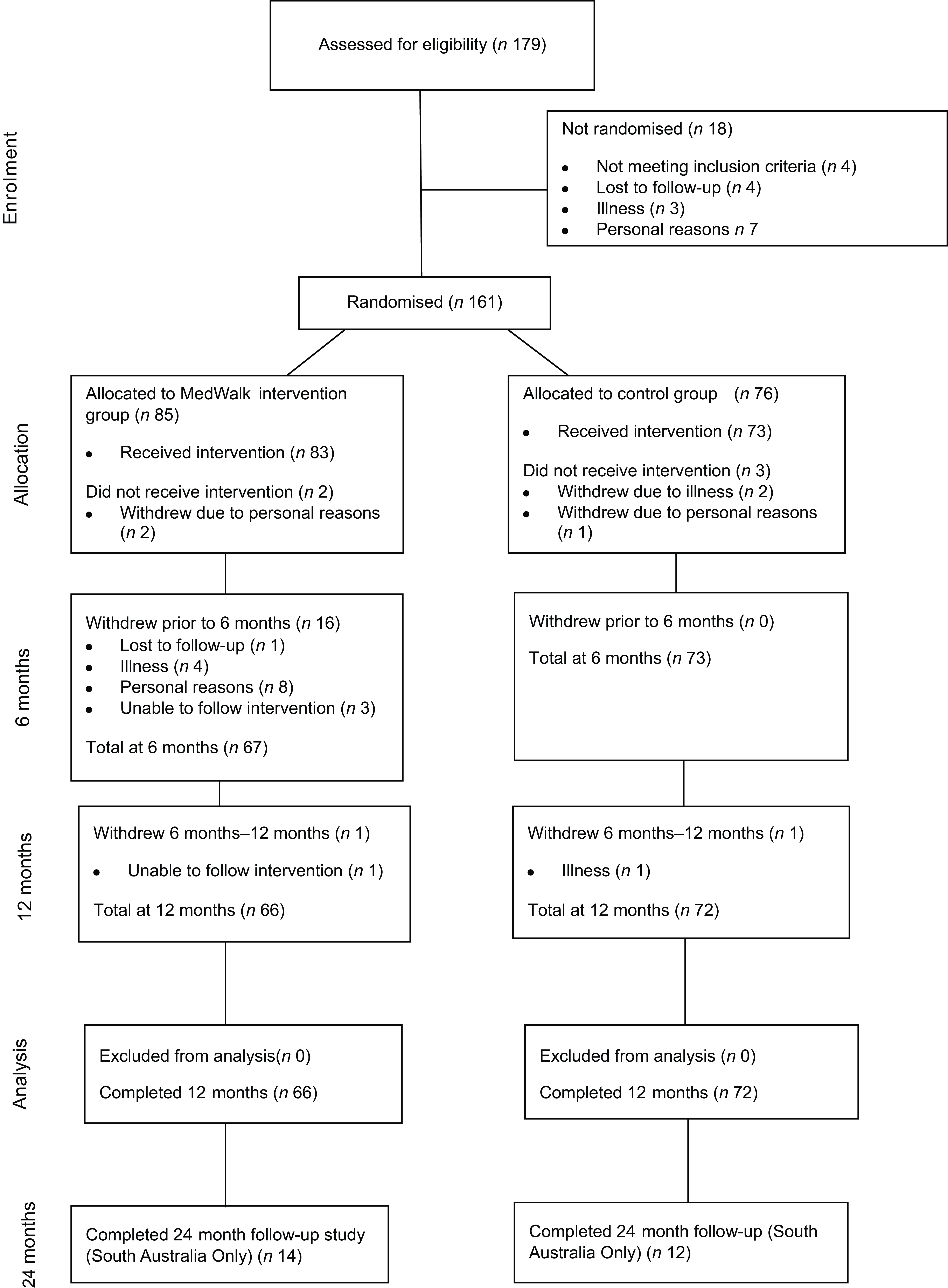
Participants were eligible if they were aged 60–90 years, had a Mediterranean Diet Adherence Score (MEDAS) of < 10/14 and/or did not meet the PA guidelines for older Australians (< 150 min per week)(8). Retirement villages and participants (i.e. ‘clusters’) were randomly allocated to either the MedWalk intervention or control (habitual lifestyle) group (Figure 1) by an independent statistician(Reference Pipingas, Murphy and Davis39).

Figure 1. MedWalk CONSORT diagram. MedWalk, 12-month combined Mediterranean diet and walking intervention; CONSORT, Consolidated Standards of Reporting Trials.
MedWalk was a MedDiet and walking intervention underpinned by the MI-CBT to support behaviour change(Reference Scott, Breckon and Copeland40,Reference Barrett, Begg and O’Halloran41) . The dietary support was based on modified PREDIMED guidelines and included a combination of individual and group dietitian visits where MedDiet counselling and food hampers were provided, and participants received a recipe book(Reference Pipingas, Murphy and Davis39). The walking component included walking groups with an exercise scientist in South Australia and an exercise physiologist in Victoria which were tailored to the participant’s goals and needs to assist with meeting Australian PA recommendations for older adults(Reference Pipingas, Murphy and Davis39).
Intervention participants were also provided with a newsletter (approximately 10 over the 12-month trial) with key study dates and updates and a seasonal MedDiet recipe.
The control group received a short 30-min presentation after the randomisation reveal and were asked to continue with their regular diet and lifestyle activities for the duration of the study. In addition, control participants received a total of $150 in honorarium vouchers(Reference Pipingas, Murphy and Davis39) and were also provided with a newsletter which provided key study dates and updates and a seasonal recipe (not related to the MedDiet) which assisted with maintaining contact with participants.
All participants completed a range of anthropometric and blood pressure measurements, surveys, blood tests, physical fitness tests and cognitive tests at 0, 6 and 12 months(Reference Pipingas, Murphy and Davis39). Baseline and 12-month assessments for all participants were completed in Q4 of 2022 and Q4 of 2023, respectively. A 24-month follow-up study (i.e. 12 months post-intervention) was able to be conducted in South Australia with a small sample of participants with assessments for SA participants completed by Q2 of 2024.
Estimating MedWalk programme costs
The 12-month best estimate intervention costs were calculated by collating resources used in the MedWalk intervention that would be needed to deliver the intervention in a community setting. Staff costs were drawn from project records of staff time allocated to activities, including individual and group diet and exercise visits, and published unit costs.
Staff time allocation not associated with the delivery of MedWalk in a community setting, such as time required for data preparation, data entry and analysis and recruitment activities, was excluded. Further, consumables and testing equipment required for the evaluation of main study outcomes such as FitBits, blood collection consumables, stool collection kits, Actigraph activity trackers, stadiometers and service fees for laboratory assessments were excluded.
Self-Reported measures of Mediterranean diet adherence and physical activity
MedDiet adherence and PA were measured at baseline, 6 and 12 months using the MedWalk Mediterranean Dietary Adherence Score (MW-MEDAS) and the International Physical Activity Questionnaire modified for the elderly (IPAQ-E). The MW-MEDAS was used as a priori assessment of MedDiet adherence with a maximum score of 14 indicating the highest possible adherence. One point is allocated if the participant meets the designated criteria, for example, using extra virgin olive oil as the primary culinary fat, consuming sofrito sauce twice per week, or, consuming ≥ 5 or more serves of vegetables per day. The MW-MEDAS was modified from the original MEDAS to reduce the serve of extra virgin olive oil per day (4 tbsp v. 3 tbsp) needed to acquire a point of adherence to suit an Australian population. Additionally, the serving of legumes per week decreased from 150 g × 3 or more times a week (MEDAS) v. 75 g × 3 or more times a week (MW-MEDAS).
The IPAQ-E determines engagement (minutes of exercise and days of exercise) in PA over the past 7 d according to intensity (light, moderate and vigorous).
Assessment of Quality of Life (AQoL-8D) utility score
QoL utility scores were generated from the Assessment of Quality of Life (AQoL-8D) instrument. The AQoL-8D is an 8-domain 35-item questionnaire offering four or five Likert-style responses depending on the question. Using a published algorithm (www.aqol.com), the total utility score is derived with utility scores ranging from −0·4 (worse than death), 0 (death) to 1·0 (full health). Change in utility score can be combined with time to derive quality-adjusted life years (QALY).
Participants completed the AQoL-8D at baseline and at 12 months using an online database (REDCap). Study data were collected and managed using REDCap electronic data capture tools hosted at The University of South Australia(Reference Harris, Taylor and Minor42,Reference Harris, Taylor and Thielke43) . REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages and (4) procedures for data integration and interoperability with external sources. Data were imported into IBM SPSS version 28.1.1 (IBM Corp), and the published algorithm (www.aqol.com) was used to generate the AQoL-8D utility score.
Group comparison of mean utility scores
Data and residuals were checked for normality. Normality tests indicated utility score data breached the assumption of normality and could not be transformed due to the substantial left skew. To analyse the effect of the MedWalk on QoL, change scores were therefore used (i.e. 12-month utility score minus the baseline utility score) in a general linear model to test for the significance of the group effect. To control for baseline group differences and possible attrition bias, state, sex and binary variables for income meeting needs and health status were included as fixed factors with age (years) and education (years) included as covariates.
Individual change in utility score over time (decrease, maintenance or improvement)
To test the hypothesis concerning the likelihood of reduction in QoL, participants with AQoL utility scores at baseline and 12 months (n 132) were coded as either 0 = a decrease in AQoL, 1 = no change and 2 = an increase in AQoL score with a threshold of ±0·02. A positive change of > 0·02 at 12 months from baseline was considered an improvement, while a decrease at 12 months from baseline of > 0·02 was considered a reduction or worsening in QoL(Reference Maxwell, Özmen and Iezzi44,Reference Richardson, Iezzi and Khan45) . A cross-tabulation test was used to determine significance (P < 0·05) in the distribution of responses. A subsample of the South Australian participants with AQoL-8D also recorded at 24 months was used to compare results at 12 months and 24 months to determine maintenance of any observed effects.
Binary logistic regression: predictors for improvements in utility score
A binary logistic regression analysis was undertaken to determine predictors of AQoL-8D utility score improvement with 1 = an improvement in AQoL score from baseline to 12 months and 0 = no improvement or a reduction in AQoL score with a threshold of ±0·02 as described above. The analysis was completed for participants who had data at both baseline and 12 months (completer analysis). Categorical predictor variables included group, state, sex and binary variables for income meeting needs and health status, while covariates included age and education level in years.
Results
Participants
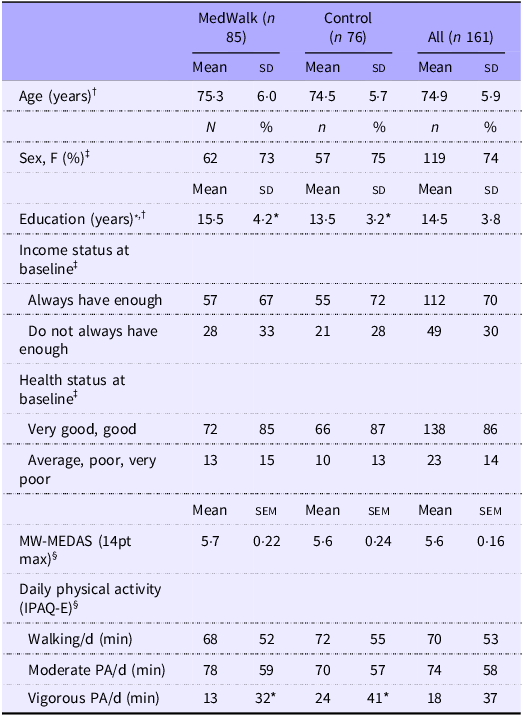
Baseline socio-demographic characteristics for the MedWalk trial (n 157) with baseline cognition data have been previously reported(Reference Pipingas, Murphy and Davis39). Of the overall sample (n 161 participants), included in the current study, 155 had a baseline AQoL-8D utility score and 137 had a 12-month AQoL-8D utility score. Baseline characteristics are in Table 1. The only characteristic for which there was a significant difference between the control and MedWalk groups was years of education (P < 0·001), with the control group averaging 2 years less.
Table 1. Baseline characteristics (n 161) including age, sex, income status and health status of 161 MedWalk and control groups
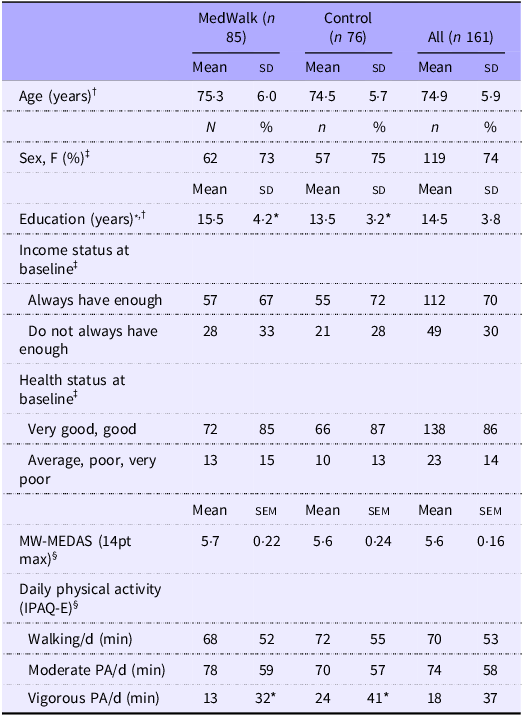
MW-MEDAS, MedWalk Mediterranean Dietary Adherence Score; MedWalk, 12-month combined Mediterranean diet and walking intervention; IPAQ-E, International Physical Activity Questionnaire modified for the elderly; PA, physical activity.
* P < 0·05.
† Variables are expressed as mean (sd).
‡ Categorical variables are expressed as n (%).
§ Variables are expressed as mean (sem).
MedWalk programme costs
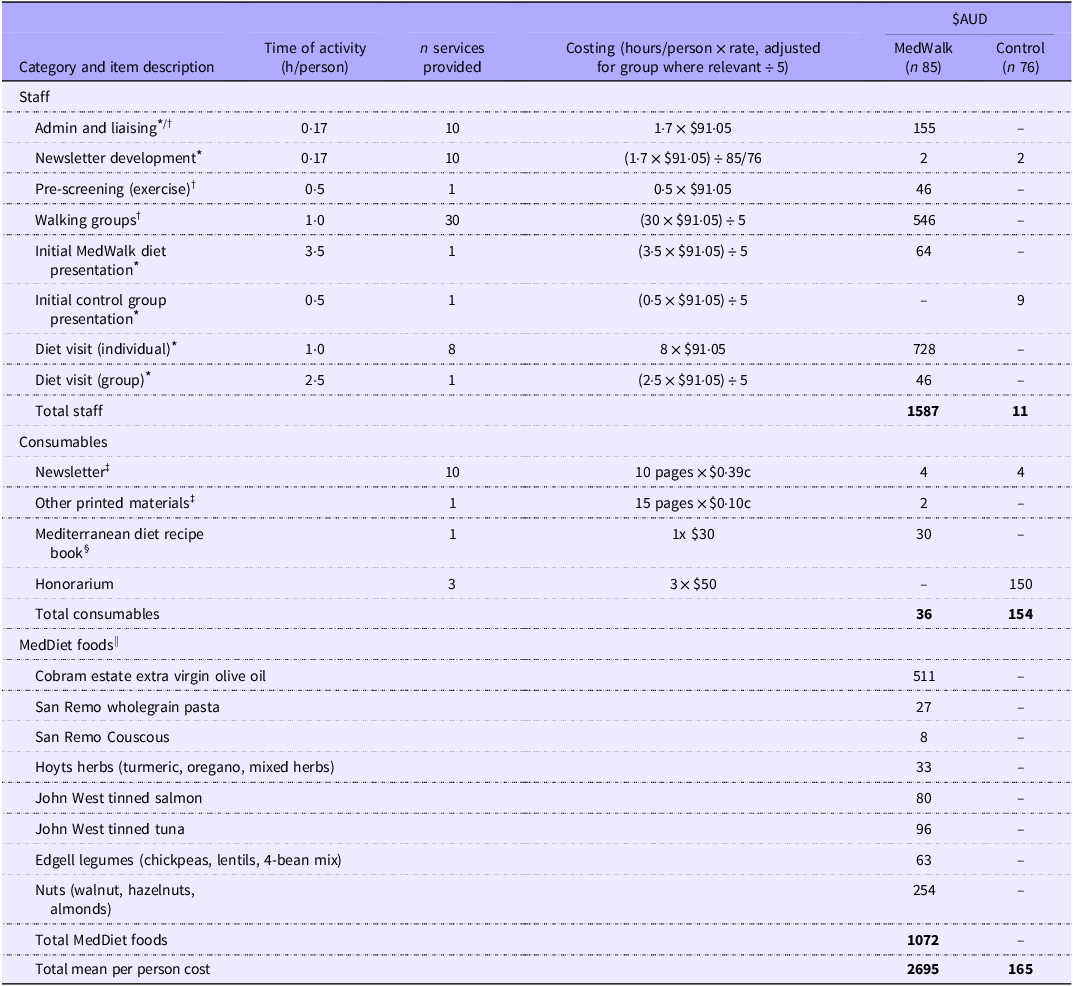
All costs are expressed in $AUD2024 as of September 2024. Total programme costs are summarised in Table 2. The cost of the MedWalk (n 85) group was estimated at $2695 per participant and for the control group (n 76) $165 per participant – a differential of $2530.
Table 2. Best estimate programme costs ($AUD) to deliver MedWalk per participant over a 12-month period for MedWalk intervention and control group
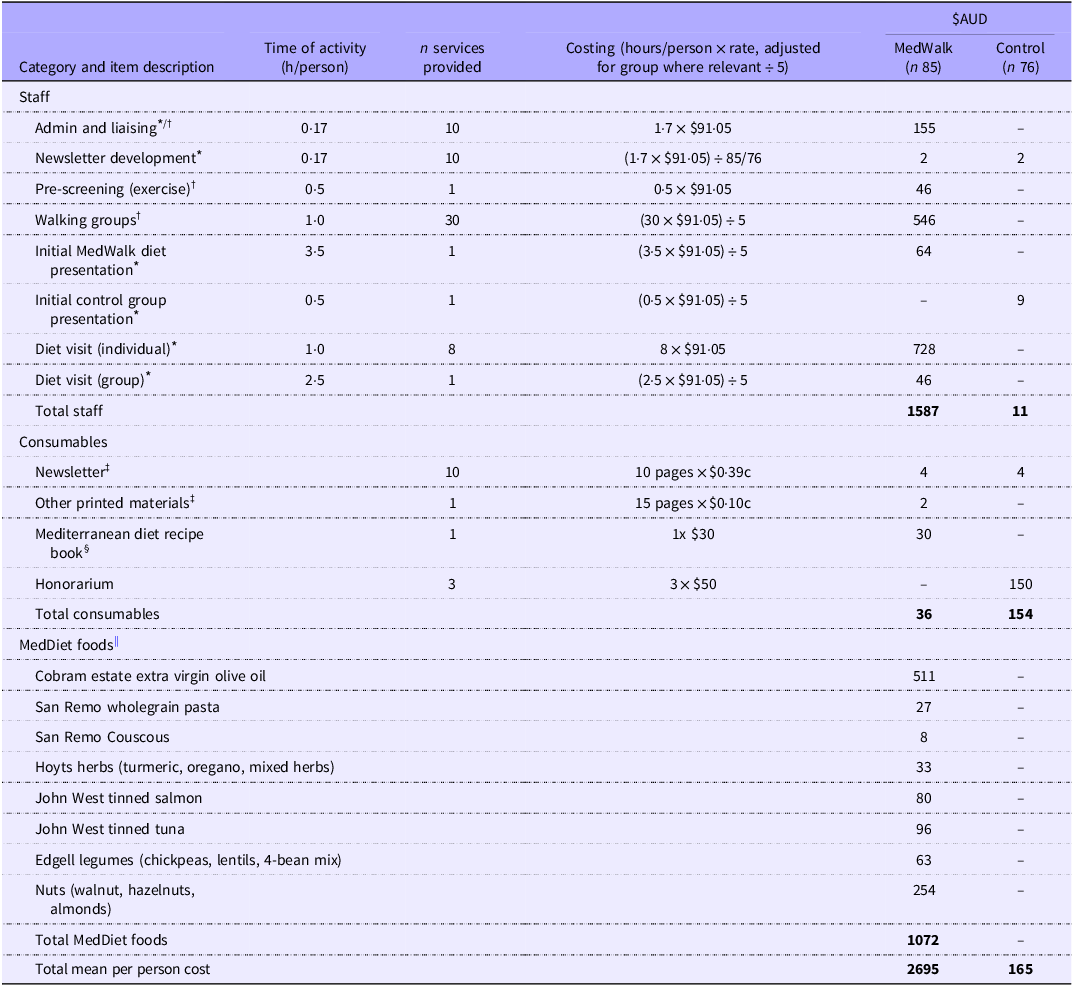
MedWalk, 12-month combined Mediterranean diet and walking intervention. MEDAS, Mediterranean Dietary Adherence Score.
The boldface was to indicate and highlight the totals for each category of cost i.e., Staffing costs, consumables and MedDiet foods.
* Medicare costs of seeing an accredited practising dietitian with a fee of $91.05 (item 81120) for an individual consult of at least 45 min as of September 2024.
† Medicare costs of seeing an accredited exercise physiologist (item 81110) with a fee of $91.05 for an individual consult of at least 45 min as of September 2024.
‡ Printing costs based on 2024 UniSA prices of $0.10c per page black and white double-sided and $0.39c per page colour double-sided; newsletter distributed approx. ten times over the 12 months with 1x A4 page double-sided in colour; other printed materials included MedDiet resources such as sample menus, weekly serve sizes and MEDAS scoring, MedDiet and walking instructions for participants approximately 15x A4 pages single-sided.
§ Mediterranean diet recipe book by Catherine Itsiopoulos, Pan Macmillan Australia.
|| September 2024 AUD$ costs from Coles, Drakes Foodland and Woolworths (South Australia) and stocktake undertaken from South Australian participants. Difference of foods ordered v. foods remaining in supply stock were calculated to determine the number of foods taken by participants over 12 months.
Individual consultations delivered by dietitians (8x individual visits over 12 months) and exercise physiologists were costed based on the schedule fee rate ($91·05 for a consult of at least 45 min) of the Medicare Benefits Scheme (MBS) (https://www.mbsonline.gov.au/) as of September 2024, for item numbers 81120 and 81110 (Table 2). This fee of $91·05 was also used as a proxy for an ‘hourly’ rate for other clinical activities including the delivery of group sessions (1x group diet session and 30x walking group sessions). To translate group sessions to per participant costs, this was based on a mean of five attendees per group which was reflective of attendance rates (Table 2).
Admin and liaising were estimated at 1·7 h over 12 months to book diet and walking group appointments and engage with participant. Consumables including the monthly newsletter, printed materials and a MedDiet recipe book were estimated at $36 for the intervention group and $154 for the control group who just received the newsletter and an honorarium (Table 2). Other printing costs were based approximately 15x A4 pages (MedDiet resources including serve sizes, sample menus and PA information and checklists to assist with individual goals) priced at $0·10c per double-sided black and white page. A newsletter was provided to both MedWalk and control group participants and based on 1x A4 colour page ($0·39c) and distributed approximately 10 times over 12 months (Table 2). Development of newsletters was approximated at a total of 1·7 h over 12 months at the dietitian MBS rate and divided by the number of participants per group to obtain an approximate per participant cost.
The MedDiet food hamper included items such as extra virgin olive oil, wholegrain pasta, canned fish and herbs (Table 2). The cost of these food hampers over 12 months ($1072) were estimated from a stocktake of foods provided (foods offered in hampers less a stocktake of foods not distributed) to a sample of South Australian participants. Prices for food items were based on popular Australian supermarkets Coles online, Drakes and Woolworths. As MedWalk participants in South Australia and Victoria were offered the same food hamper, the South Australian estimate of food provision was used(Reference Pipingas, Murphy and Davis39).
Utility score comparison of groups
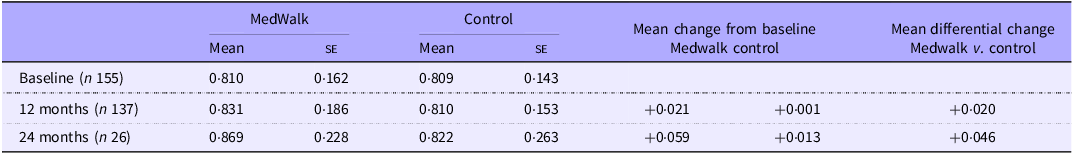
Mean QoL utility scores collected at baseline and 12 months are reported in Table 3. There were no statistically significant changes between groups when controlling for state, sex, income meeting needs, health status, age or education (F(1124) = 0·728, P = 0·395) (online Supplementary Tables S1, S2). For the small subsample for whom there were 24-month data, a clinically significant (a change of ±0·04(Reference Segal, Twizeyemariya and Zarnowiecki38)) differential gain in QoL for the MedWalk group was observed. No general linear model was performed on these data due to the small sample size.
Table 3. Mean AQoL-8D utility score at baseline and 12 months for full MedWalk cohort and 24-month data for South Australian participants only
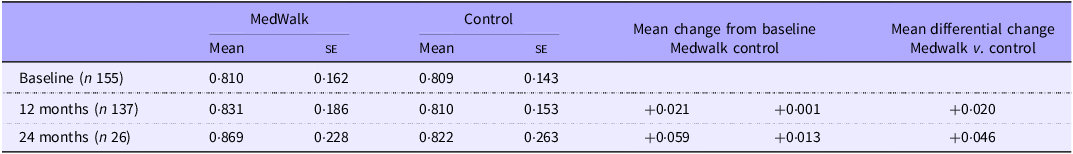
AQoL, Assessment of Quality of Life; MedWalk, 12-month combined Mediterranean diet and walking intervention.
Values are expressed as mean and standard error.
Individual changes in utility score over time
A cross-tabulation test indicated a significant difference between groups for a decrease in QoL over 12 months (χ 2 = 7·85, df = 2, P = 0·020) with a strong relationship (Cramer’s V = 0·244) (Table 4). Control group participants were more likely to experience a fall in QoL compared with MedWalk participants (42·6 % v. 20·3 %; t = 2·8) (Table 4).
Table 4. Individual change in AQoL-8D utility scores for MedWalk and control group

AQoL-8D, Assessment of Quality of Life – 8 Domain; MedWalk, 12-month combined Mediterranean diet and walking intervention.
Data are presented as n (%), difference between groups from χ 2 cross-tabulation test; *Significant adjusted residuals are values larger than 2 or less than −2.
A higher percentage of MedWalk participants improved or maintained their AQoL score over 12 months compared with control participants (34·4 % v. 28·5 %, 45·3 % v. 29·4 %, respectively).
Results from the 24-month sample of South Australian participants (n 23; n 3 did not have data at both 12 and 24 months) indicated similar trends with AQoL utility score, that is, less likely to have decreased and more likely to improve or stayed the same from 12 months to 24 months (Table 4). A cross-tabulation test indicated no significant differences between MedWalk and control groups (χ 2 = 5·095, df = 2, P = 0·078, Cramer’s V = 0·471). Adjusted residuals (2·3) indicated a significant difference between the groups for a decrease in QoL over the second 12-month period, that is, at 24 months (70 % for control and 23·1 % for MedWalk participants) (Table 4).
Predictors in improvement of utility score (binary logistic regression)
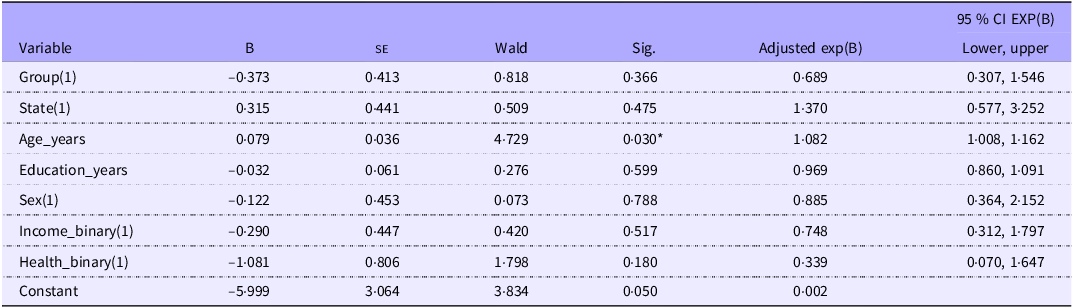
Results from the binary logistic regression in 132 completers indicated that only age in years (χ2(1) = 4·729, P = 0·030) was a significant predictor of utility score improvement (Table 5). The odds of improvement increased by 8·2 % on average for each year of age.
Table 5. Parameter estimates from binary logistic regression with predictors of AQoL-8D utility score

AQoL-8D, Assessment of Quality of Life – 8 Domain; MedWalk, 12-month combined Mediterranean diet and walking intervention.
*P =< 0·05.
Comparisons with Bonferroni adjustment; all variable have 1 df; Group 1 = MedWalk; State 1 = South Australia; Sex 1 = females; Income 1 = not always enough income to meet needs; health status 1 = average, poor or very poor health.
Discussion
The limited cost–consequence analysis of the MedWalk intervention in independent living populations indicates a best estimate cost of $2695 AUD per person to deliver the MedWalk intervention which included a combination of personalised dietary advice from dietitians and a food hamper to support healthy eating and walking group sessions led by exercise physiologists. The control group cost was estimated at $165 per person.
Results from a 2013 systematic review (n 8 studies) indicated that a MedDiet is generally considered cost-effective and affordable; however, the majority of studies included in this review were cross-sectional, narrative reviews and cohort surveys(Reference Saulle, Semyonov and La Torre35). An updated 2024 systematic review of fifteen economic evaluation studies of MedDiet interventions similarly concluded that the MedDiet appears to have mixed but overall promising impacts on savings to healthcare spending and the cost of implementing a MedDiet in terms of QALY; however, more research is needed to confirm these findings(Reference Colaprico, Crispini and Rocchi46). None of the included studies have combined a MedDiet with walking or other forms of PA. This is despite the growing interest and encouragement for adoption of a Mediterranean style diet combined with PA.
While the cost of MedWalk may appear high, it is in fact minimal when compared with pharmaceutical alternatives for dementia and Alzheimer’s disease in prodromal and high-risk groups(47,48) . With some antibody medications either unavailable in Australia(49) or unsubsidised through the Pharmaceutical Benefits Scheme, for many individuals the cost is far too high to access privately(47). Sustainable behaviour changes and lifelong reduced risk through diet and exercise interventions are an important alternative to explore the costs and health outcomes at an individual and health system level. The main contributors to estimated costs for the MedWalk intervention group were the MedDiet food hamper ($1072 per person) and the individual diet visits ($728). While the food hamper was a cost estimated in the programme, it does not in its entirety represent a ‘real resource cost’ to the participant as the foods provided to MedWalk participants will have replaced in part other food purchases. Further, we acknowledge that the food hamper component may be difficult to replicate or not be truly reflective of real-world application, despite the usefulness as tool for behaviour change and often utilised in diet studies. Similarly, staff costs including clinicians to run workshops and allied health workers for the social group from the HELFIMED trial were the highest contributors to programme cost, followed by foods for cooking workshops and hampers(Reference Segal, Twizeyemariya and Zarnowiecki38). The HELFIMED mean per person cost (AUD2017) over 6 months was $411 AUD for the MedDiet group and $195 AUD for the social group and was considered a highly cost-effective programme. Having a longer intervention period (12 months v. 6 months) and providing both a diet and exercise component rather than diet or exercise in isolation (with the inclusion of individual diet visits as opposed to only group visits) explain some of the higher costs associated with the MedWalk trial.
The costs for a MedWalk style intervention could be reduced, especially for the PA component. In a community setting, it is more likely such a programme would be delivered with initial involvement from an exercise physiologist, but the ongoing walking groups could be delivered by an allied health assistant with a Certificate such as IV in Leisure and Lifestyle or lll/IV in Fitness, or a volunteer. Studies that include a walking intervention have reported on the use of peer-led unqualified volunteers, that is, lay-led and passive interventions such as the use of pedometers which have been reported as cost-effective strategies and may have greater feasibility than the use of qualified exercise physiologist(Reference Kassavou, Turner and French30,Reference Ogilvie, Foster and Rothnie31,Reference Kwak, Kremers and Walsh50,Reference Lamb, Bartlett and Ashley51) . For example, a three-arm cluster RCT with a pedometer-based walking intervention in 1023 adults aged 45–75 years (short-term group) and 100 000 adults aged 59–88 years (long-term group) living in the UK found the group who received a pedometer in the post to be more cost-effective than the intervention group who received a 12-week walking programme with practice nurse consultation (£20 000/QALY)(Reference Anokye, Fox-Rushby and Sanghera52). This indicates that QALY can also be achieved with ‘passive’ interventions that encourage walking such as distributing pedometers. Further, a RCT in 106 moderately depressed, obese or overweight women aged > 60 years in Spain found a 6-month walking intervention led by qualified exercise leaders (sport science graduates) to cost a total of €2250 and for each additional QALY, a cost of €311 (95 % CI, €143–€394) compared with usual care(Reference Gusi, Reyes and Gonzalez-Guerrero53).
Lastly, due to the lack of statistical change and clinical significance in AQoL utility score, a cost–utility analysis (cost per QALY gained) and sensitivity analyses were not completed as originally planned. The QoL results suggest the MedWalk intervention participants had an increase in the likelihood of maintaining or improving QoL and avoiding a decline that might be expected in an older population, but mean QoL, that is, utility score was not improved. The lack of improvement could be explained by the reasonably high baseline AQoL utility scores (0·810), similar to that of the population norms for males and females in the 65–74 years age categories (0·84 and 0·81, respectively)(Reference Maxwell, Özmen and Iezzi54) and a ceiling effect occurring due to using the mean as a measure of distribution. Additionally, the MedWalk population is relatively ‘healthy’ compared with other populations in this age demographic. By reporting the costs of combined multimodal interventions, we hope to better understand which components of interventions are driving costs and inform better planning of future studies to identify where potential cost-savings might be appropriate, without compromising the intervention quality and integrity.
MedDiet and PA interventions should continue to recruit populations that could benefit from such interventions at a sample size that is sufficient to detect changes in QoL and include follow-up time points for collection of data to confirm change over time. Although the results do not suggest an increase in QoL, they do align with the hypothesis that a MedDiet combined with PA may slow the decline in QoL compared with a typical Western style diet and sedentary lifestyle. Additionally, trials could consider increased number of group sessions to moderate costs and improve engagement. Further, alternative modes of PA could be explored. Walking groups are a relatively ‘low burden’ and inclusive weight-bearing exercise for healthy populations which also encourage the social aspect of PA, aligning with a Mediterranean ‘lifestyle’. Should a different mode of PA be considered for future studies, that is, resistance training, this may reduce the opportunity for both an increased number of group visits as a cost-saving mechanism and the social aspect that occurs due to the nature of walking within a group.
Limitations
Originally, the MedWalk trial was to be conducted over 2 years (24 months). Due to the impact of the COVID-19 pandemic including lockdowns and restrictions, there were considerable delays to trial commencement and recruitment resulting in the modification to a 12-month trial. The study protocol details the impacts and changes to the MedWalk trial which were considerably more severe in Victoria compared with South Australia(Reference Pipingas, Murphy and Davis39). Despite the loss of the 24-month time point for the whole cohort, MedWalk participants received intensive dietetic support for the first 6 months, so we would expect that the ‘most’ benefit received would be between baseline and the 6-month time point. Nevertheless, South Australia was able to collect 24-month data for a small group of participants to investigate maintenance or change over time.
This limited cost–consequence analysis based on the MedWalk RCT provides further insight as to the feasibility and costs of implementing a MedDiet and walking programme in Australia in a non-Mediterranean population. As our results did not find a differential change in mean utility value, it was not possible to estimate a QALY gain and conduct a cost quality analysis as planned. With the scores high at baseline, the participants appear to have adequate pre-existing QoL with little room for improvement over a 12-month intervention and would be considered a ‘lower risk’ population. A longer intervention period or follow-up period may be needed (i.e. 2–5 years) to determine a substantial impact on QoL. Further, our analyses do not include the combination of programme costs with a health system perspective (i.e. cost-saving on Medicare or the Pharmaceutical Benefits Scheme); therefore, there may be more potential cost-saving mechanisms and benefits we have not measured. These health system results will be reported in future.
It is important to note that the costing in this study reflects how the intervention was delivered which does slightly deviate from the protocol paper, namely in regard to the group visits(Reference Pipingas, Murphy and Davis39). Only one group diet visit occurred, and the walking group costs were based on a 60-min session with an exercise physiologist which may be overestimating staff labour costs when comparing to the walking schedule in the protocol paper (ranging from 20–50 min)(Reference Pipingas, Murphy and Davis39). The additional time allocated to walking groups allows for the exercise physiologist to include light stretching and to warm up with participants as well as social engagement and implementation of MI-CBT. Further as the walking groups were tailored to each intervention site, sessions could be shorter or longer than the advised schedule depending on the participants needs. Additionally, the cost to employ an exercise scientist who delivered the walking groups in South Australia is not covered by the MBS; therefore, the cost of an exercise physiologist was used. Further, dietitians and exercise physiologists delivering the intervention were required to undertake training by an expert in MI-CBT. Hours of training were approximately 3 × 8 h d but were not included in the programme costs as this is not a per-participant cost, but a mandatory staff training to deliver the intervention.
Additionally, the cost of the foods provided to MedWalk participants was based on a stocktake for South Australian participants rather than a percentage compliance or a combined South Australia and Victoria stocktake. Consequently, the Victorian participants and other study populations may take more, or less of the offered foods which could change the estimate for food costs over 12 months. One explanation for participants not taking all of the foods provided is that our cohort was relatively advantaged in terms of socio-economic status. Further, the MedWalk population on average reported that their income provided for their needs rated their general health as good or very good and had tertiary education qualifications. When recruiting ‘worried’ but relatively healthy populations such as retirement village cohorts, this may attenuate the quality-of-life benefits of the intervention.
Future directions
Diet and lifestyle interventions should continue to include QoL measures to provide the opportunity for cost-effectiveness and utility analyses. If possible, follow-up periods or additional interim time points should be used to collect data from participants to strengthen statistical modelling and determine behaviour beyond the intensive portion of interventions and to assess sustainability over time. Alternative modes of exercise could also be explored.
Conclusions
A MedDiet and walking intervention underpinned by behaviour change techniques (MI-CBT) delivered at a cost of $2695 per participant was not associated with a differential improvement in mean QoL scores. Nevertheless, MedWalk was found to prevent a decline in QoL in a relatively healthy independent living population.
Acknowledgements
The authors would like to acknowledge the wider MedWalk collaborative team of associate investigators, scientific committee members and the data safety monitoring board who were involved in the conception and implementation of the MedWalk trial.
The authors would also like to thank the following organisations, their retirement villages, staff and residents for access to facilities, advice and assistance with the trial: in South Australia – ECH (Cumberland Park Community Group and Rotary), Karidis (Acacia Park), Lendlease (Vermont), LifeCare (Marion Rose), Retire Australia (Glengowrie, Tea Tree Gardens and Torrens Grove), RSL Care (Sturt) and Southern Cross (Riverpoint and The Pines); and in Victoria – Abound Communities (Rushall Park and Leith Park), Australian Unity (Campbell Place and Peninsula Grange) and Villa Maria Catholic Homes (St Joseph Mews and Athelstan).
Additionally, the authors acknowledge the contributions to data collection by Jessie Clark, Emily Eversteyn, Bethany Murphy-Aird, Mahima Bedi, Mee Chee Chong, Nicole Echeverria, Gabriella Inguanti, Ashlee Harvey, Kasia Main, Laura Martin, Janis Onuzans, Melissa Rubin, Mahima Shah, Tania Thodis and Nerylee Watson.
ELB was supported by a Research Training Program Scholarship (RTP) funded by the University of South Australia and the Government of South Australia.
The MedWalk trial is funded by a National Health and Medical Research Council (NHMRC) Boosting Dementia Research Initiative grant (GNT1171300). The authors would also like to acknowledge contributions from the following organisations: Cobram Estate for providing Australian extra virgin olive oil gratis to trial participants; SanRemo for providing whole meal pasta and couscous; Hoyts for providing herbs and spices and The Almond Board of Australia for providing almonds.
K. J. M., L. S., E. L. B., C.R.D, and K. A. D. conceived the MedWalk substudy; L. S., E. L. B., K. A. D., K. J. M. and C. R. D. planned the methodology; E. L. B. and L. S. completed the cost analyses; ELB drafted the manuscript. All authors have critically revised the manuscript and have approved the final version for publication.
The authors declare no conflicts of interest
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Human Research Ethics Committee at Swinburne University (ref: 20201600–3559, 14/02/2020) and The University of South Australia (ref: 202844, 16/06/2020). Written informed consent was obtained from all subjects/patients. This trial was registered with the Australia and New Zealand Clinical Trials Registry at https://www.anzctr.org.au/ (ANZCTR 12620000978965).
Data supporting the analyses and results of this paper are available from the corresponding author upon reasonable request.
Consent for publication is not applicable.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114525105333