Introduction
The Eastern population of the Lesser White-fronted Goose Anser erythropus (hereafter EPLWFG), is shared between Russia, where the birds reproduce and moult, and China, where the majority winters (BirdLife International 2018; Cao et al. Reference Cao, Fox, Morozov, Syroechkovskiy, Solovieva, Fox and Leafloor2018). Far fewer birds winter in Japan, but the numbers have recently increased (Ao et al. Reference Ao, Wang, Solovyeva, Meng, Ikeuchi and Shimada2020; Ikawa and Ikawa Reference Ikawa and Ikawa2009). Recent studies using satellite tracking have revealed the wintering range, wintering habitats, and seasonal migrations of the EPLWFG (Ao et al. Reference Ao, Wang, Solovyeva, Meng, Ikeuchi and Shimada2020; Lei et al. Reference Lei, Jia, Wang, Lei, Lu and Saintilan2019a, Reference Lei, Jia, Zuo, Zeng, Shi and Zhoub; Wang et al. Reference Wang, Fox, Cong, Barter and Cao2012). The summer range has been recognised as a continuous area extending from the Olenyok River (119.2°E) in the west to the Anadyr River (174.8°E) in the east and northwards from 64°N, excluding the Arctic archipelagos (Tian et al. Reference Tian, Solovyeva, Danilov, Vartanyan, Wen and Lei2021). This finding contrasts with previous assumptions of a fragmented summer range (Cao et al Reference Cao, Fox, Morozov, Syroechkovskiy, Solovieva, Fox and Leafloor2018; Morozov Reference Morozov1995; Morozov and Syroechkovski Reference Morozov and Syroechkovski2002). The exclusion of Arctic archipelagos is based on a literature inventory with no LWFG ever reported outside the mainland of Eastern Asia. A study by Tian et al. (Reference Tian, Solovyeva, Danilov, Vartanyan, Wen and Lei2021) does not support the overlap between Western Main and Eastern populations of the LWFG on their breeding grounds and suggests a gap between 103° and 119°E. The Western Main population winters around the Caspian Sea, in the Mesopotamian Lowland, and in south-eastern Europe, and the easternmost edge of the wintering range is on the coast of the Aral Sea (Aarvak and Øien Reference Aarvak, Øien, Fox and Leafloor2018; Romanov and Pospelov Reference Romanov and Pospelov2010). The EPLWFG mainly winters in the middle and lower reaches of the Yangtze River in China (Wang et al. Reference Wang, Fox, Cong, Barter and Cao2012). Thus, the two subpopulations are well separated in winter; their winter ranges lie over 5,000 km apart.
Being classified as “Vulnerable” by the International Union for Conservation of Nature (IUCN) (Birdlife International 2018), the LWFG is listed in the Red Data Book of Russian Federation (category “Endangered”; Morozov Reference Morozov and Amirkhanov2021) and in China, thus receiving a fully legal protection in both key states of the EPLWFG.
Ornithologists have divided the LWFG into three populations worldwide, i.e. the Fennoscandian (now only remains in Norway), Western Main, and Eastern Palearctic populations (Fox and Leafloor Reference Fox and Leafloor2018). Between 1981 and 1999, restocking in Finland and Sweden involved captive breeding birds; however, the programme was interrupted because of a proposal to mix with Greater White-fronted Goose Anser albifrons (Andersson and Holmqvist Reference Andersson and Holmqvist2010; Ruokonen et al. Reference Ruokonen, Kvist, Tegelström and Lumme2000). Recently, Swedish LWFG were found to be genetically distinct from the Fennoscandian and Western Main populations, with no evidence of interspecific introgression into the Swedish LWFG population (Molino et al. Reference Molino, Seth, Gyllenstrand, Widemo, Liljebäck and Svensson2020).
Among the LWFG populations, the critically endangered Fennoscandian population (numbering a few dozen pairs; BirdLife International 2021) has been studied the most for breeding habitats, nesting ecology, reproductive success, moult migration and moult, and juvenile and adult survival rates (Aarvak and Øien Reference Aarvak and Øien2003, Reference Øien and Aarvak2008; Markkola and Karvonen Reference Markkola and Karvonen2020; Marolla et al. Reference Marolla, Aarvak, Hamel, Ims, Kéry and Mellard2023; Øien and Aaevak 2008). This is followed by the Swedish population (fewer than 200 birds), in that nest-site fidelity, predation pressure, productivity, and moulting habitats have been studied (Andersson Reference Andersson, Aarvak and Timonen2004). We typically lack detailed data on the breeding biology of both Western Main and Eastern populations of the LWFG. For the Western Main population, nesting habitats, and some aspects of nesting and post-breeding ecology have been described (Morozov Reference Morozov and Amirkhanov2021; Morozov and Kalyakin Reference Morozov and Kalyakin1997; Rozenfeld et al. Reference Rozenfeld, Kirtaev, Rogova and Soloviev2019) and the nesting association with Peregrine Falcon Falco peregrinus was proposed from observations of nests and newly hatched broods in the vicinities of peregrine nests on Yamal and Gydan Peninsula (Korobitsyn and Tyutenkov Reference Korobitsyn and Tyutenkov2023; Pokrovskaya et al. Reference Pokrovskaya, Sokolova, Erich, Gilg, Sokolov and Sokolov2023). Brood sizes and proportions of juveniles in brood-rearing flocks have been investigated on the Yamal Peninsula and the Polar Ural Mountains (Morozov and Syroechkovski Reference Morozov and Syroechkovski2002). A series of papers by Romanov (2005, 2008, Reference Romanov2015) described brood-rearing habitats, diet, and brood sizes on the lakes of Putorana Plateau. The feeding ecology of western LWFG was investigated in detail (Rozenfeld Reference Rozenfeld2009).
In the EPLWFG breeding and moulting ecology is poorly documented relative to other populations. Few nests have ever been described (Andreev Reference Andreev2001; Degtyarev and Perfilyev Reference Degtyarev and Perfilyev1996). Brood sizes and proportions of juveniles in local populations have been followed in Yakutia (Artiukhov and Syroechkovski Reference Artiukhov and Syroechkovski1999; Degtyarev and Perfilyev Reference Degtyarev and Perfilyev1996; Egorov and Okhlopkov Reference Egorov and Okhlopkov2007) and Chukotka (Solovieva and Vartanyan Reference Solovieva and Vartanyan2011). LWFG summer records during the last decade are available from the Database of Anseriformes Air Surveys and Remote Tracking in Russia (http://rggsurveys.ru/).
Selection of remote and unpopulated rivers for brood rearing (and presumably nesting) was proposed for the EPLWFG (Egorov and Okhlopkov Reference Egorov and Okhlopkov2007; Solovieva and Vartanyan Reference Solovieva and Vartanyan2011). Modelling showed that human disturbance affects summer site suitability, with a decrease in species presence starting around 160 km from human settlements (Tian et al. Reference Tian, Solovyeva, Danilov, Vartanyan, Wen and Lei2021). However, the actual remoteness of breeding, moulting, and summering sites has never been measured. Standard numeric monitoring by ground counts is almost impossible for the EPLWFG (compared with other populations of the species), because birds of this population are able to avoid human presence by summering in the huge and remote areas of the forest, forest–tundra, and tundra of Eastern Siberia and the Far East of Russia. A vast majority of their summer sites are inaccessible for humans. Thus, it is not surprising that knowledge of nesting habitats, breeding phenology, summer movements (including moult migration), moult duration, moulting habitats, nest success, site fidelity, and breeding propensity is still lacking for the EPLWFG. The best way to obtain this information is by the use of tracking techniques. The aim of this study was to provide data on breeding propensity; nest-sites, nesting habitats, and time of nesting; nest success; summer timing, summer movements including moult migration; moult timing, duration and moulting habitats; site fidelity; productivity; the effect of human presence on the selection of nesting and moulting sites. To accomplish this, we combined the results from field surveys with GPS/GSM tracking to generate information on the breeding and moulting ecology of the EPLWFG. Key moulting sites of the EPLWFG were discovered during this study.
Methods
Capturing and tracking methods
We received data for 11 birds of the EPLWFG that were caught during the winter of 2016/7 at East Dongting Lake, China (designated with the prefix BFUL, Supplementary material Table S1), and tagged with transmitters developed by the Hunan Global Messenger Technology Company (Chanhgsha City, China). These transmitters were programmed to record GPS position and speed. The backpack design transmitters measured 55 × 36 × 26 mm and weighed 22 g, approximately 1.6% of the bird’s body mass, with a coordinate recording interval of two hours. All field methods used in this study were consistent with and approved by the Forestry Department of Hunan Province under a scientific research licence (No.11 Xiang Forest Protection 2014) (Lei et al. Reference Lei, Jia, Zuo, Zeng, Shi and Zhou2019b). The captured birds were not aged or sexed due to the field crew’s lack of training. The authors have full access to the data set.
During the summering period, 10 adult LWFG of unknown sex were captured from brood-rearing flocks (nine birds) and non-breeding flocks (one bird) along the Rauchua River (68.8°N 167.7°E) in July–August 2018 (designated with the prefix Lwfg; Table S1). Flightless birds were captured by driving them by motorboat from the water on to the riverbanks, where they were captured by hand. All birds were fitted with backpack solar-powered GPS/GSM transmitters developed by the Ornitela Company (Vilnius, Lithuania). These transmitters weighed 25 g, approximately 1.4% of the bird’s body mass, and had a coordinate recording interval of 10 minutes (Ao et al. Reference Ao, Wang, Solovyeva, Meng, Ikeuchi and Shimada2020). The backpacks in Teflon harnesses were placed over the wings and stitched with floss in situ on the bird, adjusted to breast size, with 1 cm freedom. The authors were granted access to the data set between 25 May and 10 October.
Track analyses
Each EPLWFG track was displayed as a separate layer in the QuantumGIS software, and each track was divided into migration (northbound and southbound), breeding, and moulting. Migration tracks were not utilised for this paper, except for the dates of migration termination (arrival and departure from the breeding or summering ranges). The nesting period was considered to be from 25 May to 5 July.
Breeding behaviour
In geese, it is common for the female to incubate the eggs while the male guards the nest and makes short foraging trips. A pair of geese tracked in 2017 (BFUL059 – a female and BFUL061 – a male) was studied to confirm these behaviours. During the nesting period, the nest-site of a presumed female was identified by an “asterisk” pattern centred on a specific point less than 100 m away. The presence of a breeding male was inferred if the bird remained in a small territory (1 km in diameter if it was a circular territory and a maximum of 12 km long if it was a riverine territory) for more than 10 days. Data on nest positions were analysed using QuantumGIS 3.16.5, which utilised conic coordinates in the Asia_North_Albers_Equal_Area_Conic projection. In this study, a goose was categorised as a non-breeder if its track did not exhibit any of the aforementioned patterns (male or female type) between 25 May and 5 July. Conversely, if a goose displayed a “nesting” pattern, it was considered to be a breeder, indicating a nesting attempt. The breeding propensity was then calculated as the proportion of geese that attempted to nest out of all the tracked geese. There is a method of estimation of geese nest location via overall dynamic body acceleration (Schreven et al. Reference Schreven, Stolz, Madsen and Nolet2021), however it was not suitable for use in our study due to missing acceleration data.
Nest-sites, nesting habitats, and time of nesting
Each bird was categorised as a breeder or non-breeder (see above). Utilising QuantumGIS 3.16.5, we generated heatmaps from breeding tracks from 25 May to 5 July, and each “bubble” on the heatmap was analysed for dates, bubble size, frequency, and regularity of a bird within a bubble. If a bubble was less than 120 m in diameter and a bird visited it for more than 10 days, the site was deemed a female nest. If a 1 km-long bubble was extended along a river or small stream, it was considered the nest-site of a male.
Nesting habitat was identified using the ESRI Satellite map. Nest-sites were classified into geographical elements such as mountain/hill slopes, river valleys, small stream valleys, islands in the river, lakesides, and so on. To determine the dominant vegetation type of the nesting habitat, the nest positions were plotted on the vegetation map of Russia (Yurkovskaya Reference Yurkovskaya and Shoba2011).
The onset of nest-building and laying was considered to begin on the day when the bird was first recorded inside the nest bubble. For geese that successfully nested (see method below), the last day in the bubble was regarded as the day when the brood left the nest.
Nest success
A bird that stays within a relatively small area and does not exhibit significant flights of over 20 km for a period exceeding 80 days is considered to be a successful breeder. This 80-day threshold includes five days for laying an average clutch of five eggs (Degtyarev and Perfilyev Reference Degtyarev and Perfilyev1996), based on a 30-hour egg-laying interval taken from data for the Greater White-fronted Goose (Ely et al. Reference Ely, Dzubin, Carboneras, Kirwan, Garcia and Billerman2020), 25 days for incubation (Kear Reference Kear2005), and 46 days for the period from hatching to fledging of the young (Kear Reference Kear2005). A pre-nesting period of an unknown duration was not taken into account. An unsuccessful nesting or brood-rearing attempt is determined if the above patterns occur within less than 80 days. Failure of breeding by EPLWFG is indicated by an immediate long flight away from the nest-site; successful breeding geese must stay with the goslings, who are unable to fly (Kölzsch et al. Reference Kölzsch, Müskens, Szinai, Moonen, Glazov and Kruckenberg2019).
Summer timing
For non-breeding geese, it is assumed that the spring migration has transitioned into summer activities when the trans-latitudinal movement shifts to trans-longitudinal. Similarly, summering is considered complete when a prominent southbound movement is detected. For breeding birds, the date of arrival at a breeding site was considered to be the beginning of the summering phase. It is also assumed that the summering phase encompasses breeding, moult, and movements between summering sites.
Summer movements including moult migration
Distances for the summer movements along the route were measured, with local movements at staging sites being disregarded for this specific analysis. To validate the summer site, a four-day rule was consistently applied, which meant that the bird should have spent a minimum of four days at the site for it to be classified as a staging site. A staging site was specifically defined by a bubble with a 50-km diameter. The direct distance between staging sites was then calculated, and the migration distance was defined as the cumulative travel distance.
Moult migration was recognised as a pronounced movement occurring in late June to early July, following which the remigial moult commenced (see section below). Return migration from the moulting site was also recorded for some birds; the return migration was considered if a bird returned to within 100 km of the early summer staging or breeding site after the remigial moult.
Moult timing, duration, and moulting habitats
Moulting sites and the period of flightless moult were identified based on a movement pattern characterised by limited or no flight activity. In this context, if the speed of the bird was less than 15 km/hour, and all hourly movements were less than 2 km, it was indicative of the bird’s engagement in moulting activity. The duration of flightless moult was calculated as the period (in days) between the last and the first record of speed over 15 km/hour. Additionally, the time spent on the moulting grounds was measured as the period between the bird’s arrival at, and departure from, the moulting site. Moulting habitats are mainly lakes and rivers and were identified by plotting the moulting tracks on a vegetation map (Yurkovskaya Reference Yurkovskaya and Shoba2011). The length of the river stretch occupied by the bird during the entire flightless period was also measured, excluding birds with the Lwfg prefix in 2018.
Site fidelity
Nesting and moulting site fidelity was recorded if the bird returned to within 10 × 10 km of the same area in the following year/s.
Surveys in Chukotka, Russia for brood size and proportion of juveniles in brood-rearing flocks
During the period of July–August from 2002 to 2021, surveys of rivers were conducted by travelling downstream in a motorboat from the upper reaches of remote rivers, which were accessed by helicopter from the Chaun Biological Station (68.3°N, 170.2°E). A comprehensive description of the study area, survey methods, peak counts for each river, and the proportion of juveniles in brood-rearing flocks during the period 2002–2010 have been published previously (Solovieva and Vartanyan Reference Solovieva and Vartanyan2011; Tian et al. Reference Tian, Solovyeva, Danilov, Vartanyan, Wen and Lei2021). Peak counts along nine previously studied rivers ranged from 0.3 to 3.9 individuals/km, as reported by Tian et al. (Reference Tian, Solovyeva, Danilov, Vartanyan, Wen and Lei2021). Brood sizes were recorded in both single broods and brood-rearing flocks (if a family was recognised in a flock). The proportion of young in the EPLWFG in mid-August, prior to the autumn migration, was calculated based on breeding behaviour, nest success, and brood size obtained from this study (refer to the Results section for further details). The proportion of juveniles in brood-rearing flocks was estimated to be 0.29 in 2002–2010 (Solovieva and Vartanyan Reference Solovieva and Vartanyan2011).
Remoteness of breeding, moulting, and staging sites
We measured the direct distances from each breeding, moulting, and staging site to the nearest human settlement using the Google Earth ruler.
Results
Summary of tracking results
We received summer tracks from 11 individuals (BFUL prefix) out of the 88 EPLWFG captured in winter 2016/7 (12.5%). The tracking data for the remaining 77 birds were not recovered, either due to device malfunction or casualty. Eleven trackers recorded during the entire summer of 2017. Three trackers worked for an additional year (2018), and one also continued working for a third year (2019). There was a pair of geese (BFUL059 and BFUL061) whose tracks were identical in 2017 except for nesting patterns; we considered this to be one track in the summer of 2017. A total of 15 summer records were available for analysis from the birds captured in winter 2016/7.
Additionally, we received summer tracks from 9 out of the 10 individuals (Lwfg prefix) captured in the summer of 2018 (90%, Table S1; see also Ao et al. Reference Ao, Wang, Solovyeva, Meng, Ikeuchi and Shimada2020). The tracks from these birds were analysed by Ao et al. (Reference Ao, Wang, Solovyeva, Meng, Ikeuchi and Shimada2020) for migration chronology and summer staging sites (Table S2). There were nine trackers in the first summer (2018), four trackers in the next summer (2019), and two trackers which continued to work in the third summer (2020).
Thus, we analysed a total of 30 summer tracks from 19 individual EPLWFG, with 9 tracks (Lwfg prefix) representing a part of the moult, post-moulting period, and departure from summer grounds, rather than the full summer. These tracks were not independent as they originated from the same individuals tracked for more than one summer. Some tracks were terminated before the end of the summer (Table S1), so the number of tracks used for each type of analysis differs from the expected 30/19 tracks.
Breeding behaviour
Of the 11 LWFG captured in winter 2016/7 (of unknown age with the BFUL prefix) 36.4% (four birds) were non-breeders during the first year of tracking. Their tracks did not show nest-sites. We assumed that these four non-breeders were AHY/ASY (after hatch year or after second year) birds because they are usually less careful and easier to capture. Among 16 potential breeding attempts by adult geese (a pair is considered as one track), tracked for at least one summer after capture, only one could be classified as a non-breeder (Lwfg06 in 2020; Table S2). Thus, a breeding propensity of 93.8% was estimated for adult EPLWFG and 76.2% for all geese (16 out of 21) arriving in the summer range.
Nest-sites, nesting habitats, and time of nesting
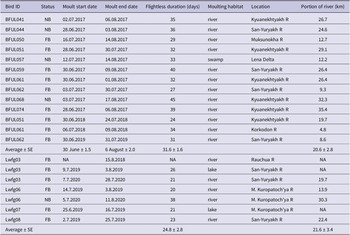
The tracked EPLWFG nested in various habitats, with most nest-sites situated either in the valleys of streams and medium-sized rivers or on mountain slopes facing rivers and streams. Specifically, the nests were placed in stream valleys (six nests), on mountain/hill slopes (three nests), in medium-sized river valleys (three nests), on the shore/island of a lake in a river valley (two nests), or on an ox-bow bank in the river valley (one nest) (Table 1). As a result, 80% of the nests were attributed to the mountainous streams or river valleys, while 20% of nests were situated on hill slopes. Nests were located in various bioclimatic zones, including the northern taiga, sparse forest, sparse forest–tundra, mountain tundra, and flat hypoarctic tundra. Notably, in most of these zones, except for the tundra, the dominant tree species were larches Larix gmelinii and L. cajanderi (Yurkovskaya Reference Yurkovskaya and Shoba2011) (Table 1). The laying onset was estimated in 12 cases (both successful and failed breeding attempts) and the median date was 30 May ± 1 day. Broods left two successful nests on 21 June (BFUL062 in 2018) and 27 June (BFUL065 in 2017).
Table 1. Characteristics of breeding sites of the Lesser White-fronted Goose Anser erythropus revealed by tracking in 2017–2020. Coordinates of sites are given in Table S2
1 Okhotsk-Beringian larch– dwarf pine taiga
2 East-Siberian sparse forest–tundra with larch and dwarf pine
3 East-Siberian tundra with Dryas punctata, D. ajanensis, and steppe fragments
4 Site fidelity is assumed if the bird returned to within 10 × 10 km of the previous nesting site area in the following year/s.
Nest success
Two breeding attempts appeared to be successful: BFUL065 in 2017 and BFUL062 in 2018 seem to have successfully bred and raised their broods to the fledgling stage. The other 13 geese with nesting attempts (both BFUL and Lwfg prefixes) remained at the presumed breeding grounds for an average of 32.2 ± 1.4 days (range 25–42 days) and then flew off on moult migration, so we assumed that they had failed to nest. Only 13.3% of the 15 tracked birds bred successfully in 2017–2020 (a pair BFUL059 and BFUL061 in 2017 was considered as one breeding attempt). None of the eight geese with the Lwfg prefix, which were brood rearing in summer 2018 (presumably successful breeders), bred successfully in 2019 and 2020 (Table S2).
Summer timing
EPLWFG individuals that attempted to nest arrived at their breeding sites on 23 May ± 1.7 days on average (range 15 May to 6 June, Table 2; see Table S2 for the start of the breeding period), while non-breeders delayed their arrival at the summer grounds. For instance, the non-breeding individual BFUL057 moved slowly down the Lena River from 10 June to 26 June, when it reached the Lena Delta. The 26 June was considered to be the date when the spring migration was complete and summering began. An adult individual Lwfg06 was brood rearing (successful breeder) in 2018 and was considered a failed breeder in 2019 (arrival date 26 May). However, this bird arrived on 18 June in 2020, and the track did not show any nesting attempt. As mentioned, this goose may have lost a partner sometime between the breeding seasons of 2019 and 2020. On average, the non-breeding geese reached the first summer stop-over site on 10 June ± 5.8 days (range 26 May–26 June) (Table 2).
Table 2. Timing, number of staging sites (average ± SE, n in parentheses) and movements of the Eastern Lesser White-fronted Geese Anser erythropus on the breeding grounds, 2017–2020

1 All breeders combined (successful and unsuccessful)
2 Including breeding and moulting sites:
3 Known for BFUL062 in 2017 and BFUL062 in 2018.
The duration of summering was shortest for non-breeders, averaging 103.0 ± 7.5 days (Table 2). Failed breeders stayed 22 days longer because they arrived earlier in an attempt to nest, and successful breeders stayed even longer (133 days in BFUL062 in 2018; the second track of successful BFLU065 ended on 5 September 2017 before departure), departing nine days later than failed breeders on average. Successful breeders (BFUL065 and Lwfg03-09) departed on 6 October on average (range 28 September–9 October). Four non-breeders departed on 24 September, on average, and birds that failed to breed departed, on average, on 27 September (Table 2). The three-week difference between arrival (t-test, df = 4, P = 0.029) and a one-week difference in departure (df = 7, P = 0.004) of breeders and non-breeders were both significant. There was a significant difference between the departure times of successful and unsuccessful breeders, with the latter departing eight days earlier (df = 13, P = 0.004).
Summer movements including moult migration
Non-breeding EPLWFG fly within the summering range until they stop to moult (Figure 1). It can be challenging to detect staging sites during their uninterrupted summer movement. Applying the four-day rule, we can conclude that non-breeders used an average of 3.4 ± 0.9 sites per summer and travelled an average of 1,253 ± 248 km (range 544–1,781 km) (Table 1 and Figure 1). Failed breeders used an average of 3.8 ± 0.2 sites per summer, including breeding and moulting sites, and flew an average of 1,281 ± 176 km during the summer (range 296–2,127 km) (see Table 1 and Table S2). Non-breeders and failed breeders travelled similar distances during the summer (the difference was insignificant, P = 0.35). The successful breeders made a short move to another site (average 74.4 ± 14.7 km for all eight successful breeders) after their broods and themselves gained flight ability in mid-August (Table 1). Their autumn staging sites were situated north of the breeding sites, and the duration of stay at these sites averaged 47 ± 2 days (range 35–53 days; n = 8).

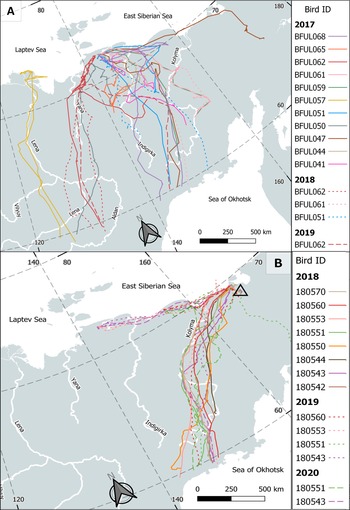
Figure 1. Individual routes of 11 LWFG tracked from wintering grounds (prefix BFUL) in the summering range in 2017–2019 (A) and summer tracks of 8 LWFG tracked from summer grounds (prefix Lwfg) in 2018–2020 (capturing site is shown by open triangle; B). Summer tracks were considered after the bird crossed 60oN during spring migration and before it crossed 60oN during autumn migration. Each bird is denoted by a unique color.
Twelve geese left their nest-sites after nesting or brood failure and began the moulting migration, covering an average distance of 751 ± 114 km in 3.9 ± 0.8 days (range 186–1,330 km, 0.5–8.5 days) (Table S2). For two non-breeders, moult migration was part of their continuous, non-stop flight, while the other two flew distances of 475 and 1,245 km to reach their moulting grounds (Figure 1). An adult non-breeder (lwfg06 in 2020) flew 580 km from Rauchua River (breeding site in previous years) to the moulting site. All birds migrated to the north or north-west from their breeding or staging sites to the moulting grounds.
Out of 16 tracks of failed breeders on the moult migration, seven (43.7%) tracks indicated a return migration to their early summer staging site after completing the moult (Table S2). The distance of the return migration was equal to that of the moult migration. The return migration took an average of 7 ± 3 days (range 2–29 days; n = 7) and included a staging on the way for three out of four geese. On average, the return migration was slower than the moult migration (average 4 ± 1 days) of the same individuals in the same years.
Moult timing, duration, and moulting habitats
The EPLWFG that successfully breed moulted near their nest-sites and formed brood-rearing flocks of up to 70 individuals, often mixed with Bean Geese Anser fabalis (our observations in West Chukotka). After hatching, successful breeders do not fly long distances and take flight alongside their young, making the duration of their flightless moult unclear. In this study, successful breeders typically began flying on the median date of 13 August (range 8–16 August; n = 9; Table 3). In West Chukotka, the earliest young and brood-rearing adults were observed to fly between 6 August and 12 August (2010–2019).
Table 3. Flightless moult timing and habitats of non- (NB) and failure breeding (FB) Lesser White-fronted Geese Anser erythropus in 2017–2020. Coordinates of moulting sites are given in Supplementary material Table S2 (site status = mo). The portion of river used for moulting by individual geese was measured along the river ignoring visits to nearby lakes
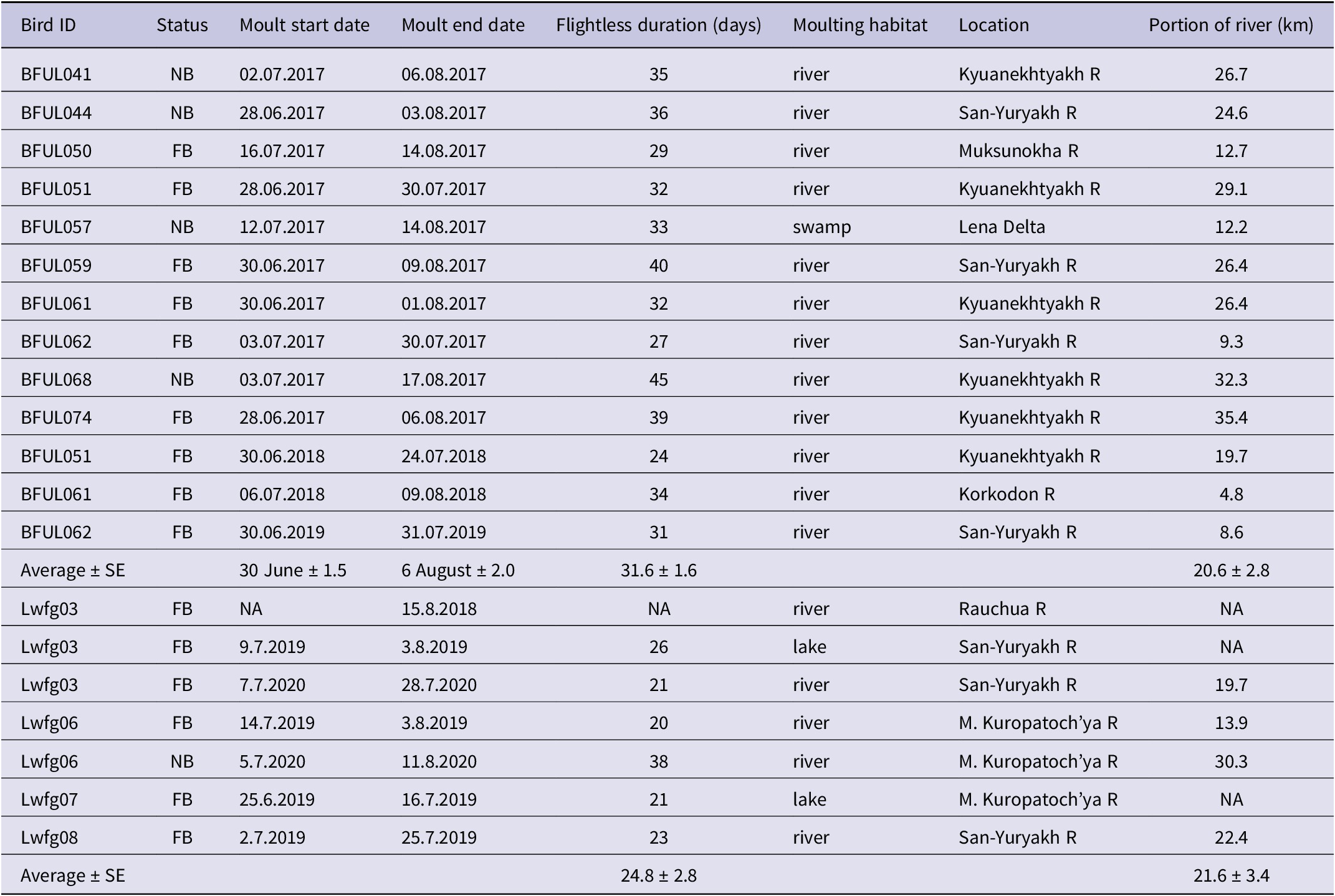
Out of 20 tracks of non- and failed breeders (excluding bird Lwfg03 in 2018 from Rauchua River), 14 cases (70%) of moult were observed along the lower reaches of the San-Yuryakh and Kyuanekhtyakh Rivers at 72.3°N, and three cases (15%) of moult were observed along the Malaya Kuropatoch’ya River at 70.8°N (Table 3 and Table S2; see also Lwfg01 in Ao et al. Reference Ao, Wang, Solovyeva, Meng, Ikeuchi and Shimada2020). These key moulting sites are situated in the northern hypoarctic tundra zone and are characteristic of lowland polygonal marshes (Yurkovskaya Reference Yurkovskaya and Shoba2011). Non- and failed LWFG with the BFUL prefix were flightless for an average of 31.6 ± 1.6 days (range 24–40 days; Table 3), while geese with the LWFG prefix were flightless for an average of 24.8 ± 2.8 days (range 21–38 days). The difference between the groups is statistically significant (t-test; P = 0.012). The earliest a bird (regardless of prefix) became flightless was on 25 June, and the latest a bird finished moult was on 17 August. EPLWFG utilised an average of 20.8 ± 2.9 km of river during the moult (Table 3).
Site fidelity
We utilised data from four EPLWFG (BFUL051, BFUL061, BFUL062, and Lwfg003) tracked for more than one season for estimating nest-site fidelity. All four returned to their previous breeding area in subsequent year/s. Distances between consecutive nests of the same bird were estimated in three cases, averaging 2 km (Table 1). BFUL062 (presumably male), tracked for three years, nested within 4 km along a small stream (the exact nest position is unclear) in all three summers. This bird was successful (fledgling young) only once, in 2018; breeding failed in 2017 and 2019. All five adult geese captured in the summer of 2018 on the Rauchua River also made nesting attempts there in 2019 and 2020. Lwfg06, which did not breed in 2020, also visited the Rauchua River briefly. Thus, we estimate that breeding-site fidelity is 100% in adult LWFG and does not seem to depend on the breeding success of the previous season.
Five out of six (83.3%) of failed breeding adult LWFG also demonstrated fidelity to their moulting site. One goose (BFUL061 in 2018) seemed to have missed the time to reach the moulting site on the Kyuanekhtyakh River (used in 2017) after losing a brood on 3 July 2018 and becoming flightless on 6 July (Table 3). If we exclude the case of BFUL061, fidelity to the moulting site could be estimated to be 100%.
Brood size and proportion of juveniles in brood-rearing flocks
According to our observations in West Chukotka, the LWFG brood averaged 3.69 ± 0.19 young, aged 5–7 weeks old, in 2003–2021 (range 2–7; n = 45). The proportion of juvenile birds was 46.9% of all the birds reported during the surveys at brood-rearing habitats (medium-sized rivers of the West Chukotka). Recently, the annual productivity of EPLWFG can be estimated at 0.49 young per adult pair. We estimated the proportion of young in the EPLWFG population in mid-August (prior to fledging) as 16.5%. This was determined by halving the overall pair productivity of 0.49, which was calculated from multiplication of the breeding rate (76.2%), nest success (13.3%), and number of young per successful brood (3.69). Brood survival from hatch to 5–7 weeks old is unknown and thus it is not considered in the estimation of productivity.
Remoteness of breeding, moulting, and staging sites
There is no difference in remoteness (P = 0.17) between the Rauchua breeding site, where EPLWFG returned to nest in the years following the capture year, and other breeding sites, discovered by tracking, The average distance of the summer staging sites from the nearest village was 69.7 ± 4.8 km (n = 46). The remoteness of the breeding sites was higher, averaging 144 ± 1.8 km (n = 16), and the moulting sites were the most remote, situating on average 159 ± 14.5 km (n = 20) from the nearest village. There was a significant difference among the groups (One-way ANOVA, F 2.78 = 27.2; P <0.001).
Discussion
This paper presents new data on the nesting and moulting ecology of the relatively unknown EPLWFG obtained from GPS/GSM tracking and field surveys. It provides estimates of breeding propensity, nest success, productivity, and nesting and moulting site fidelity for the first time. Tracking the geese led to the discovery of remote moulting sites important for most of the population, which were previously unknown. Literature data on nesting habitats and the summer timing of EPLWFG were scarce and this paper fills the gap in knowledge.
Breeding propensity
Boom et al. (Reference Boom, Schreven, Buitendijk, Moonen, Nolet and Eichhorn2023) found that tracking was an effective way to document breeding behaviour of geese, which is also true for the EPLWFG. Breeding propensity is one of the most mysterious demographic parameters and is almost impossible to quantify in wild birds, even in marked populations (Jean-Gagnon et al. Reference Jean-Gagnon, Legagneux, Gilchrist, Bélanger, Love and Bêty2018). This parameter could be estimated as the proportion of birds that commenced nesting upon reaching the breeding grounds. Among the 16 potential breeding attempts by adult EPLWFG, only one failed, presumably after losing its partner. This individual arrived at the nesting site late on 18 June, compared with 26 May in the previous year (see Lwfg06 in 2020 in Table S2). Breeding propensity in adult geese in the Arctic varies from 22% to 100%, primarily depending on spring weather conditions (Boom et al. Reference Boom, Schreven, Buitendijk, Moonen, Nolet and Eichhorn2023; Reed et al. Reference Reed, Gauthier and Giroux2004; Sedinger et al. Reference Sedinger, Chelgren, Ward and Lindberg2008). Geese in temperate latitudes are less dependent on spring weather for their breeding intention (Boom et al. Reference Boom, Schreven, Buitendijk, Moonen, Nolet and Eichhorn2023). The EPLWFG breeds in the sub-Arctic and boreal forest where spring conditions seem to be favourable for the annual nesting effort of all adult individuals. However, the small number of tracked individuals in this study does not allow for accurate estimates of breeding rates. Any way we estimate breeding propensity in the EPLWFG in 93.8% in adults and 76.2% in all tracked birds.
Nest-sites, nesting habitats, and time of nesting
All geese (LWFG prefix) captured as brood-rearing adults in summer on the Rauchua River (tundra biome) returned to the same site in the following year/s, so we did not analyse their nesting habitats. All seven EPLWFG tracked from wintering grounds (BFUL prefix and including Lwfg01 from Ao et al. Reference Ao, Wang, Solovyeva, Meng, Ikeuchi and Shimada2020) nested in taiga or forest–tundra biomes (sometimes in intrazonal mountain tundra), and none of them nested in the tundra biome (Table 1). This pattern is consistent with previously known nesting or brood-rearing records from taiga and forest–tundra on the Omolon and Muna Rivers (Andreev Reference Andreev2001; Egorov and Okhlopkov Reference Egorov and Okhlopkov2007) and the “forest edge” in Yakutia (see reviews in Degtyarev and Perfilyev Reference Degtyarev and Perfilyev1996; Morozov and Syroechkovski Reference Morozov and Syroechkovski2002). The breeding population of the Chaun-Rauchua Lowland, of the northern tributaries of the Anadyr River (Tian et al. Reference Tian, Solovyeva, Danilov, Vartanyan, Wen and Lei2021), and a single record of a brood near Sellyaskaya Bay of the Laptev Sea (Bysykatova and Krapu Reference Bysykatova, Krapu, Soloviev and Tomkovich2009) were the only breeding EPLWFG recorded from the tundra zone. Low altitude mountains, river valleys, and small stream valleys seem to be the major nesting habitats (Morozov and Syroechkovski Reference Morozov and Syroechkovski2002) (Table 1). It is unclear if the EPLWFG is associated with Peregrine Falcon in nesting, as was proposed for the Western Main population (Korobitsyn and Tyutenkov Reference Korobitsyn and Tyutenkov2023; Pokrovskaya et al. Reference Pokrovskaya, Sokolova, Erich, Gilg, Sokolov and Sokolov2023). Nesting Peregrine Falcons are common on cliffs along rivers with brood-rearing EPLWFG in West Chukotka (based on our data).
The median date of commencement of egg-laying is 30 May, which is consistent with egg-laying on 20–25 May for the first pairs in Norway (Øien and Aarvak Reference Øien and Aarvak2008) and slightly earlier than, on average, 4 June (21 May–13 June) in Finland (Markkola and Karvonen Reference Markkola and Karvonen2020). However, our estimate of egg-laying onset was obtained via spatial–temporal analyses of tracks, in which the first visit to the nest-site may have happened earlier than laying onset, thus our estimate may be biased (earlier) by a few days. More tracking of the EPLWFG is required for modelling the climatic predictors of timing of breeding. Finnish LWFG were found to start egg-laying independently of local weather conditions (Markkola and Karvonen Reference Markkola and Karvonen2020).
Nest success
The 13.3% proportion of successful breeders was lower than the long-term average of 46% in the Fennoscandian population (Aarvak and Øien Reference Aarvak, Øien, Fox and Leafloor2018). In Fennoscandia there was culling of red fox Vulpes vulpes, wolverine Gulo gulo, and lynx Felis lynx, and brown bear Ursus arctos and wolf Canis lupus were absent. All the above-mentioned predators, with Arctic fox V. lagopus and sable Martes zibellina, are common, and sometimes numerous, in the breeding range of the EPLWFG. Lower nest success would appear to result from high predation rates from mammalian and avian predators, however documentation of nest predation is absent for the EPLWFG. Nest success in Finland was highly dependent on cumulative positive temperature and cold spells during incubation, with the latter acting negatively on reproductive success (Markkola and Karvonen Reference Markkola and Karvonen2020). The entire breeding range of the EPLWFG is situated in a colder climatic zone than Finland, and there are greater chances of low temperatures in June and cold spells on any day in summer (https://www.climate-charts.com/World-Climate-Maps.html#temperature). This, in part, may explain the low nest success obtained in this study. Tracking of additional individuals is required to study climatic effect on nest success.
Summer timing
An average breeding EPLWFG arrives at its breeding site at 64–70.5°N on average 17 days earlier than the Greater White-fronted Goose nesting at a similar longitude but at 71–73°N (Table 2); Greater White-fronted Goose arrive on average 162 ± 10 Julian date (Deng et al. Reference Deng, Zhao, Fang, Xu, Wang and He2019). The summering duration in breeding of LWFG is 23–30 days and longer than that of Greater White-fronted Goose, with the departure of LWFG delayed by 6–13 days (for both failed and successful breeders). This is probably due to the more southerly breeding sites of LWFG, allowing for an earlier arrival and longer stay than the Arctic-nesting Greater White-fronted Goose.
LWFG assumed to be younger than two years old and not participating in reproduction arrive later and depart earlier than the adult birds, distinguished by their breeding attempts (Table 2). The three-week difference in arrival and one week difference in departure of breeders and non-breeders were both significant in this study. We lack the tracking data for the same individual as a non-breeder and then becoming a breeder in the following year, while we may get some insight from an individual tracked from the wintering ground in Poyang Lake, China (Lwfg01 in Ao et al. Reference Ao, Wang, Solovyeva, Meng, Ikeuchi and Shimada2020; not included in the analyses in this study due to lack of permission). It arrived at the summer grounds on 30 June in the first year when it was presumably a sub-adult bird; it arrived at the summer grounds on 25 May in the following year when it had reached reproductive age, presumably two years old (see Cramp and Simmons Reference Cramp and Simmons1977 for the age of first reproduction). There was a significant difference between the departure times of successful and unsuccessful breeders, with the latter departing eight days earlier. However, this difference might be biased due to seasonal differences in autumn conditions. In two individual birds tracked for more than one summer (Lwfg006 and Lwfg007), the departure after unsuccessful breeding (26 September 2019) was earlier than that after successful breeding (9 October 2018). However, by contrast, a third individual (Lwfg002 failed to breed in both years) departed on 8 October 2018 and 27 September 2019. The later departure in 2018 is more likely a result of a warmer autumn compared with that of 2019. Following the “frost wave hypothesis” (Xu and Si Reference Xu and Si2019), we suggest that the LWFG leaves summer areas after the onset of frost, which varies between years.
Summer movements including moult migration
The EPLWFG are highly mobile during the summer, using an average of 2 sites for successful breeders, 3.4 sites for failed breeders, and 3.9 sites for non-breeders (see Table 2). Their mobility makes conservation efforts on summer grounds less effective than one would expect unless a network of seasonally protected areas is developed. However, prior to the development of modern bird-tracking techniques, their movement patterns were unclear (Figure 1). Early reconstructions of the summer range based on records of adult birds, nests, and broods were incomplete and suggested a patchy range (Morozov and Syroechkovski Reference Morozov and Syroechkovski2002), whereas the track-based model suggests the possibility of a continuous summer range (Tian et al. Reference Tian, Solovyeva, Danilov, Vartanyan, Wen and Lei2021). Typically, in non-breeders (mostly birds younger than two years and 6.2% of adult birds), spring migration is terminated when they begin their remigial moult.
Failed breeders did not stay close to their nests and began the long-distance migration to moult sites. Among 13 failed breeders, only one moved a short distance from the nest-site to moult in the same river catchment. This short moult migration may be attributed to the late loss of a nest/brood and a lack of time to reach more distant moulting sites. Summer staging sites are widely dispersed within the summer range, sometimes in proximity to breeding or moulting sites, although not always (Table S2).
Many failed breeding EPLWFG (43.7%; Table S2) migrated back to their early summer breeding/staging site after completing the moult, thus extending their stay in the summer range. Of the five individuals tracked for more than one summer, three, being failed breeders, never returned to the breeding site after moulting, while two always did. It is difficult to explain why they almost precisely return to the breeding site, sometimes covering over 1,000 km on the return migration (Figure 1 and Table S2). One possible explanation could be fidelity to the autumn migration route, where geese are familiar with stop-over sites; however, the consistency of migration routes among years has not been analysed. Another hypothesis suggests checking conditions for the next breeding season, similar to the Arctic Rough-legged Buzzard Buteo lagopus (Curk et al. Reference Curk, Kulikova, Fufachev, Wikelski, Safi and Pokrovsky2022), but the kind of conditions geese are able to evaluate (food availability, habitat status, hydrology, predators) remains unexplained.
Moult timing, duration, and moulting habitats
The core moulting site for the entire EPLWFG was identified through tracking (Ao et al. Reference Ao, Wang, Solovyeva, Meng, Ikeuchi and Shimada2020; this study). It is situated along the lower reaches of the San-Yuryakh and Kyuanekhtyakh Rivers near their joint estuary, where they flow into Omulyakhskaya Bay of the East-Siberian Sea. The site spans an area of 647 km2, approximately 44 × 16 km, and is considered the “absolute pole of inaccessibility” even within the remote and sparsely inhabited Arctic Siberia (P. Nikol’skiy, personal communication). Located 150–200 km from Yukagir village, reaching this area in summer is only possible using expensive transportation means, such as large ATVs or helicopters. It is not surprising then that 70% of non- and failed breeding EPLWFG gather in this area. All EPLWFG migrated 185–1,330 km to reach optimal moulting habitats, indicating that moult migration is typical for this species. An even longer moult migration of at least 1,800 km was reported for Fennoscandian LWFG (the individual PTT24676 was tracked from Norway to the Taimyr Peninsula; Aarvak and Øien Reference Aarvak and Øien2003). Another moulting site, the Malaya Kuropatoch’ya River (a small river 150 km long, flowing into the East-Siberian Sea), was utilised by 15% of failed breeders, specifically by two adults (one of them, twice) known to breed 600 km apart on the Rauchua River. This site is located 272–322 km from the nearest village and is almost inaccessible during the summer, akin to the San-Yuryakh and Kyuanekhtyakh Rivers. In contrast to the breeding habitats, the moulting sites are situated in the tundra zone near the Arctic coast (Yurkovskaya Reference Yurkovskaya and Shoba2011).
In their review of Anatidae-moulting strategies, Fox et al. (Reference Fox, Flint, Hohman and Savard2014) proposed that geese moult in the Arctic to avoid the predators that are abundant at lower latitudes. We would like to emphasise that the moulting sites of the EPLWFG provide both potentially lower predation pressure compared with breeding areas and a lack of human disturbance. The EPLWFG prefer to moult on rivers (17 out of 20 birds; the rest moulted on lakes and swamps; Table 3), likely due to the same safety reasons. We cautiously suggest that the portion of the river they use for moulting, on average 20.8 ± 2.9 km, seemingly satisfies their food requirements. Although the food and foraging ecology of moulting EPLWFG is unstudied, it could possibly be obtained from further analyses of existing tracks.
Moult duration in the LWFG is not well-documented, although Aarvak and Øien (Reference Aarvak and Øien2003) reported that a male from the Fennoscandian population remained at its moulting site on Kolguyev Island for 33 days in 1997. The significant difference between the flightless period in geese with BFUL and Lwfg prefixes is due to differences in the fix interval. The two-hour interval between fixes in BFUL geese does not allow for the detection of first flight activity, as is possible with 10-minute interval fixes. This first flight after the flightless moult may be just a short test of flying ability and might not be related to movement from the moulting site. Field observations suggest that birds stay flightless after completing the remigial moult in order to accompany their late moulting conspecifics. During the flightless period, undisturbed flocks of LWFG may not fly until the last individual has completed its moult. While the nesting biology of geese could be better understood by using trackers with hourly or higher intervals between fixes, a 10-minute fix interval is preferred for details of the remigial moult. The flightless period of the EPLWFG obtained from trackers with a 10-minute interval is approximately 24.8 ± 2.8 days, which is close to the 21 days needed for waterfowl birds to replace their flight feathers (Hohman et al. Reference Hohman, Ankney, Gordon, Batt, Afton, Anderson, Ankney, Johnson, Kadlec and Krapu1992). The moulting window for non-breeding and failed geese lasts from 25 June to 17 August. Successful breeders typically complete their moult around the median date of 13 August, similar to late-moulting failed breeders.
Site fidelity
Adult LWFG showed 100% fidelity to their nesting site despite a previous year nest failure: no evidence of nesting area change was observed during this study. The site fidelity of unsuccessful breeders to the moulting site was also 100%, based on tracking five adult geese for more than one summer. We propose that the summer range of the LWFG includes breeding enclaves comprising hundreds of pairs (known enclaves are the Rauchua River; Solovieva and Vartanyan Reference Solovieva and Vartanyan2011 and the Muna River; Egorov and Okhlopkov Reference Egorov and Okhlopkov2007) surrounded by areas with low breeding densities (single broods or small brood-rearing flocks reported during surveys; see also Tian et al. Reference Tian, Solovyeva, Danilov, Vartanyan, Wen and Lei2021), and concentrated moulting sites. Intriguingly, none of the geese tracked from the wintering grounds nested in the two known breeding enclaves, hinting at the existence of additional enclaves. The tracking results from the wintering grounds suggest the existence of one more breeding enclave on the Sededema River, East Yakutia (BFUL051 in 2017 and 2018 and Lwfg01 in Ao et al. Reference Ao, Wang, Solovyeva, Meng, Ikeuchi and Shimada2020). Given the observed 100% site fidelity to nesting sites in the EPLWFG, identifying additional breeding enclaves should be a conservation priority in the near future.
Brood size and proportion of juveniles in brood-rearing flocks
From 2002 to 2021, the average brood size near the fledging stage was 3.69 ± 0.19 goslings in West Chukotka. This surpasses the average of 3.0 goslings recorded from Norway before red fox control (Øien and Aarvak Reference Øien, Aarvak, Tolvanen, Øien and Ruokolainen2009), the 2.93 goslings in Finnish Lapland from 1989 to 1996 (Markkola and Karvonen Reference Markkola and Karvonen2020), and the 2.86 goslings in the reintroduced Swedish population from 1999 to 2003 (Andersson Reference Andersson, Aarvak and Timonen2004). The EPLWFG appears to be more productive in brood size than the Fennoscandian sub-population. Additionally, Degtyarev and Perfilyev (Reference Degtyarev and Perfilyev1996) observed that a brood averaged 4.2 goslings between hatching and fledging in the Khroma–Indigirka tundra from 1960 to 1962. This brood size is similar to that of the Western Main sub-population, with an average brood of 4.0 goslings (Romanov Reference Romanov2004). In recent findings, the annual productivity of EPLWFG can be estimated at 0.49 young/nesting pair or 16.5 % of young in mid-August. Wang et al. (Reference Wang, Damba, Zhao, Xie, Deng and Ga2021) reported that the proportion of young (after hatch year) EPLWFG in wintering flocks was 15.2 ± 11.2%. These proportions align, assuming a low, seemingly unknown mortality of young during the first migration.
Further evidence of breeding enclaves is the observed proportion of juveniles on the rivers of West Chukotka at 46.9%, which is much higher than the estimated 16.5% of young in the entire EPLWFG. In late summer, adult brood-rearing geese and their young are well separated from the rest of the adult geese who departed from the breeding rivers for moulting.
Remoteness of breeding, moulting, and staging sites
There is no difference in remoteness (P = 0.17) between the Rauchua breeding site, where Lwfg geese returned to nest in the years following the capture year, and other breeding sites discovered by tracking. During ground surveys, all flightless groups of the EPLWFG (broods and moulting non-breeders) were located more than 50 km away from villages or mines in West Chukotka. This distance is significant as local people typically travel along the rivers and seacoast in a one-day motorboat trip to fish, hunt reindeer, and pick mushrooms and berries. Further evidence comes from the largest aggregation of mainly brood-rearing birds, with a peak count of 416 geese in 2018, which occurred along the uninhabited Rauchua River 15 years after the closure of the gold mine and settlement of Baranikha (Solovieva and Vartanyan Reference Solovieva and Vartanyan2011), a finding confirmed by this study. When the mine and village were active, no LWFG were found along the river (see details of the 1983 survey in Krechmar et al. Reference Krechmar, Andreev and Kondratiev1991).
Egorov and Okhlopkov (Reference Egorov and Okhlopkov2007) reported a breeding population on the uninhabited Muna River in Central Yakutia and noted the absence of LWFG along the nearby Molodo River, which has similar habitats but where intensive geological surveys were in progress. The absence of people has been suggested as a key factor in the stability of the breeding population of LWFG in the Putorana Plateau (Western Main sub-population; Romanov Reference Romanov2003).
When selecting staging sites, flying birds appear to be less concerned about human presence: the distance of a staging site to the nearest village is significantly shorter than that of breeding or moulting sites. Even though the EPLWFG summers in the least populated provinces of Siberia (i.e. Yakutia, Chukotka, and Magadan Oblast) with an average density of 0.23 people/km, they actively select the most remote areas for nesting, especially for remigial moult. The model of EPLWFG summer range shows that human presence affects site suitability, with a decrease in species presence starting around 160 km from human settlements (Tian et al. Reference Tian, Solovyeva, Danilov, Vartanyan, Wen and Lei2021). Locating sites situated 160 km or more away from any settlement is not an easy task, even in unpopulated East Siberia, thus the long-distance summer travels of the EPLWFG are not surprising.
Conservation recommendations
-
1. Strong nest-site fidelity of adult birds contributes to the formation of local breeding populations, which might be conservation units if genetic studies support this differentiation.
-
2. The EPLWFG selects the remotest and least human-accessible area for the remigial moult, and the key site was discovered only with the help of remote sensing. To enhance conservation management, we suggest that no ground-based expedition to this area should be undertaken during moulting periods (July–August), although aerial surveys to count birds are possible.
-
3. As summering sites are well protected by their extreme remoteness, conservation efforts are best concentrated on the wintering areas and migration stop-over sites.
-
4. Appropriate high-resolution GPS/GPRS tracking does not seems to reduce breeding behaviour in the EPLWFG and is recommended as the best method for investigating migratory ecology, breeding and moulting habitats, breeding propemsity, and nest success in the remote areas of the summer range. However, extreme care should be taken when tracking rare species such as the LWFG.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0959270924000285.
Acknowledgements
We gratefully acknowledge the contribution of the East Dongting Lake Nature Reserve and the hard work of the field teams in China and Russia, Carlie Strancki and Thomas Ogilvi. Logistic support and transportation to remote rivers of the West Chukotka was provided by Chukotka Gold Mining Co., the subsidiary of Kinross Gold, with personal acknowledgement to Claude Schimper, Evgenia Saevich, and Daria Krivolapova. The following information was supplied relating to ethical approvals (i.e. approving body and any reference numbers): approval by capture of adult birds was conducted with permission from the Ministry of Natural Resources of Russian Federation (#058 from 10 May 2018); tracking of birds was permitted by the Federal Service for Technical and Export Control (Permit No 320). Approval for bird capture and transmitter deployment was obtained from the Forestry Department of Hunan Province, China, under scientific research licence (No.11 Xiang Forest Protection 2014). Field research was conducted with permission from the Bureau of East Dongting National Nature Reserve. Track maps for the birds with the prefix lwfg and tracking information for the birds with the prefix BFUL can be submitted for review but cannot be published due to concern for the safety of endangered birds. Our study was supported by the National Key Research and Development Program of China (2017YFC0405300) and the National Natural Science Foundation of China (Grants No. 31870369). Tracking from breeding grounds (lwfg prefix) was supported via a joint project of DS and FM, State Key Laboratory of Urban and Regional Ecology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. Authors’ contributions: DS and JL contributed equally to this work, and carried out the fieldwork, analysed the data, wrote the paper, and prepared the figures and tables. GD and SV carried out the fieldwork. DB prepared the figures and revised the paper. HT, RF, FM, and CL revised the paper. QZ and GL conceived and designed the experiments, contributed materials/analysis tools, and revised the paper. All authors contributed critically to the drafts and gave final approval for publication.