Introduction
The process of ageing is a series of changes leading to alterations in the homeostasis of cells, chromosomes and tissues over time, which results in the deterioration of the body’s functions. However, the ageing process is complex and influenced by various factors including genetics, energy and nutrient intake, environment and social experiences. The principles of the ageing process, commonly referred to as the hallmarks of ageing(Reference Lopez-Otin, Blasco, Partridge, Serrano and Kroemer1), encompass factors such as genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, and cellular senescence. The ageing process has implications for physical changes, cognitive decline, immune and nervous system functions, and the activity of several organs(Reference Niccoli and Partridge2). In addition, it heightens the risk of age-related diseases such as cardiovascular diseases, neurodegenerative disorders, cancer, diabetes, and weakened immune responses(Reference Guo, Huang, Dou, Yan, Shen and Tang3). The accumulation of senescence and these diseases leads to frailty in the elderly, known as multidimensional syndrome, which includes unintentional weight loss, self-reported exhaustion, reduced grip strength, decreased physical activity and slow walking speed(Reference Fried, Tangen, Walston, Newman, Hirsch and Gottdiener4).
Given the intricate relationship between the ageing process and the changing nutritional needs of individuals, it becomes evident that addressing the nutritional concerns of the elderly is essential for comprehensive healthcare in this population. In addition, the ageing process or the loss of self-care abilities in the elderly may result in inadequate intake of nutrients, which could contribute to frailty, diabetes, cardiovascular diseases, osteoporosis, dementia, and depression. Furthermore, molecular-level changes accumulate from birth to advanced age, contributing to the deterioration of various systems in the body. Therefore, extensive research has been dedicated to preventing or restoring these processes at the cellular level, and the role of folate, vitamin B12, and vitamin D in ageing, in particular, has attracted considerable attention. These vitamins play an important role in ageing and comorbidity leading to frailty in the elderly. The purpose of this article is to review the interplay between folate, vitamin B12, and vitamin D in the ageing process, with a particular focus on their pivotal roles in contributing to frailty and comorbidities in the elderly. This review also delves into the age-related depletion of folate, vitamin B12, and vitamin D, offering insights into the potential mechanisms driving this decline. In addition, the scientific evidence linking low levels of these vitamins to adverse health outcomes, especially frailty in the elderly, is thoroughly reviewed.
Frailty
Frailty, often referred to as a multidimensional syndrome, is associated with several adverse outcomes, including reduced quality of life, higher mortality rates, increased hospitalisation, falls, dementia, and depression(Reference Fried, Tangen, Walston, Newman, Hirsch and Gottdiener4). Major contributors to frailty include engaging in health-risk behaviours, such as lack of exercise, inadequate nutrition, smoking, and alcohol consumption(Reference Fried, Tangen, Walston, Newman, Hirsch and Gottdiener4). Frailty, prevalent in up to 40% of individuals aged 80 years or older, is closely associated with ageing and can lead to dependency and increased susceptibility(Reference Collard, Boter, Schoevers and Oude Voshaar5).
The assessment of frailty in the elderly is crucial for accurate diagnosis and intervention. The Fried method(Reference Fried, Tangen, Walston, Newman, Hirsch and Gottdiener4) is the most common tool, utilising both questionnaires and physical examinations, to screen for frailty. Individuals are rated on the basis of the following criteria: (1) unintentional weight loss of more than 4.5 kg within one year, (2) feelings of exhaustion, (3) reduced grip strength, (4) slow walking speed, and (5) decreased physical activity in daily life(Reference Fried, Tangen, Walston, Newman, Hirsch and Gottdiener4). Meanwhile, the Edmonton Frail Scale(Reference Rolfson, Majumdar, Tsuyuki, Tahir and Rockwood6) and Rockwood’s Frailty Index(Reference Rockwood, Andrew and Mitnitski7) integrate multiple factors such as cognition, general health status, functional independence, medication use, mood, nutrition, bowel, and bladder functions and the performance of life activities for diagnosis. In addition, the Kihon checklist developed by the Japanese Ministry of Health, has been evaluated in Japan, considering social status, mental health and neurological decline through 25 questions(Reference Satake, Senda, Hong, Miura, Endo and Sakurai8).
The significance of frailty in the elderly, both in terms of physical fitness and cognition, underscores the need for preventive strategies. Encouraging and educating them on maintaining a balanced and nutritious diet to ensure adequate intake of nutrients and vitamins is one crucial aspect of preventing various diseases. Addressing frailty comprehensively not only improves an individual’s wellbeing but also alleviates public health and economic burdens.
Vitamin levels in older people
The elderly phase represents a sensitive period in human life, and addressing the issues and needs of this stage is a societal imperative. Transitioning into old age marks a stage in the progression of health challenges and a shift in quality of life. Quality of life is a multi-dimensional concept encompassing aspects such as physical, mental, economic, personal beliefs and interactions with the environment(Reference Cella9,Reference Estoque, Togawa, Ooba, Gomi, Nakamura and Hijioka10) . The elderly often experience inadequate nutritional intake, influenced by various factors such as physiological changes in metabolism, altered dietary preferences, diminished appetite, and psychological and socioeconomic factors. These challenges may lead to deficiencies in essential nutrients, impacting overall health and exacerbating existing health conditions(Reference Amarya, Singh and Sabharwal11).
The physiological and pathological changes that accompany ageing often lead to shifts in vitamin requirements, resulting in an augmented need for specific nutrients. Among these, vitamins D, B1, B6, B12, and folic acid stand out prominently. As individuals age, there tends to be a decline in the body’s ability to synthesise vitamin D efficiently(Reference Gallagher12), coupled with decreased exposure to sunlight owing to factors such as reduced outdoor activity or limited mobility. Moreover, the process of ageing can impact the intestinal concentration of the vitamin D receptor (VDR), potentially leading to a reduction in calcium absorption, as evidenced by studies conducted on ageing rats(Reference Horst, Goff and Reinhardt13). Vitamins B1, B2, B6, and B12 play crucial roles in various metabolic processes within the body, including energy production, neurotransmitter synthesis, and red blood cell formation(Reference Calderon-Ospina and Nava-Mesa14). Ageing may lead to alterations in the absorption and utilisation of these vitamins, necessitating higher intake levels to offset potential deficiencies and support optimal physiological function. Utilising vitamin B6, B12, and folate for diagnosis of vitamin deficiency among community-dwelling and independent elderly, the prevalence of these vitamin deficiencies was variable, ranging from 30% to 55%(Reference Herrmann, Schorr, Bodis, Knapp, Muller and Stein15). The prevalence of vitamin D deficiency among the elderly, defined as levels below 50 nmol/L and 50–75 nmol/L, was 59·7% and 27·5%, respectively(Reference Meshkin, Badiee, Salari, Hassanabadi, Khaleghi and Mohammadi16).
Various observational studies have demonstrated a link between the development of frailty and inadequate intake of specific micronutrients. In the InCHANTI study, frail individuals exhibited notably insufficient levels of vitamin C, D, E and folate, regardless of their overall energy intake, as highlighted by Bartali et al. (Reference Bartali, Frongillo, Bandinelli, Lauretani, Semba and Fried17). This suggests that even when caloric intake is adequate, micronutrient deficiencies can still contribute to the onset of frailty. Similarly, a cross-sectional multi-centre study found a robust association between high intake of various forms of provitamin A, vitamin B6, C, D, E (α-tocopherol) and folate, and a reduced prevalence of frailty(Reference Kobayashi, Asakura, Suga and Sasaki18). Matteini et al. reported that older women with vitamin B12 deficiency, indicated by elevated serum methylmalonic acid, faced a 40–60% increased risk of prefrailty and 1·66–2·33 times higher odds of frailty compared with non-frail older women(Reference Matteini, Walston, Fallin, Bandeen-Roche, Kao and Semba19). Furthermore, decreased serum levels of total carotenoids, α-tocopherol, 25-hydroxyvitamin D (25(OH)D) and vitamin B6 were significantly associated with an increased likelihood of frailty in older women, as reported in a study utilising data and samples from the Women’s Health and Aging Studies (WHAS) I and II(Reference Michelon, Blaum, Semba, Xue, Ricks and Fried20). A 3-year follow-up of participants from WHAS I found that reduced serum levels of 25(OH)D, α-tocopherol and total carotenoids also significantly raised the risk of transitioning to frailty in this demographic(Reference Semba, Bartali, Zhou, Blaum, Ko and Fried21).
Next, this review will focus on the functions and mechanisms of vitamins B9, B12 and D in connection to frailty in the elderly.
Roles of vitamin B9, B12 and D in frailty
Vitamin B9 in frailty
Vitamin B9, also known as folate (natural form) or folic acid (synthetic form), serves as a crucial methyl donor in one-carbon metabolism. It is commonly found in fruits and juices, vegetables, grains, and fortified foods(Reference Subar, Block and James22,Reference Scaglione and Panzavolta23) . The recommended daily intake is 400 µg of dietary folate equivalents (DFE) for individuals aged 19 years and above, 600 µg DFE for pregnant individuals and 500 µg DFE for lactating individuals(24). Vitamin B9 is crucial for various metabolic and cellular functions, including the synthesis and repair of DNA(Reference Xiu and Field25), methylation reactions(Reference Crider, Yang, Berry and Bailey26), cell division(Reference Wagner27), erythropoiesis(Reference Koury and Ponka28), and the pathways involving the synthesis of nucleotides and amino acids(Reference Wagner27,Reference Baggott and Tamura29) . Deficiency in vitamin B9 can lead to several adverse effects on the body, including megaloblastic anaemia(Reference Sukla, Nagar and Raman30,Reference Yadav, Manoli and Madhunapantula31) , cognitive decline(Reference Morris, Evans, Bienias, Tangney, Hebert and Scherr32–Reference O’Connor, Scarlett, De Looze, O’Halloran, Laird and Molloy35), and muscle weakness(Reference Hwang, Sung and Kim36,Reference Wee37) , which can contribute to frailty. Further details on the consequences of hypovitaminosis in B9 can be found in Fig. 1. Moreover, vitamin B9 is associated with various chronic diseases, such as Alzheimer’s disease(Reference Kruman, Kumaravel, Lohani, Pedersen, Cutler and Kruman38,Reference Ma, Wu, Zhao, Ji, Song and Zhang39) , cardiovascular disease(Reference Otsu, Ae and Kuwabara40,Reference Moat, Lang, McDowell, Clarke, Madhavan and Lewis41) , depression(Reference Bender, Hagan and Kingston42,Reference Fava and Mischoulon43) , and cancers(Reference Franco, Seabrook, Nguyen, Leonard and Albrecht44–Reference Mamede, Tavares, Abrantes, Trindade, Maia and Botelho46).

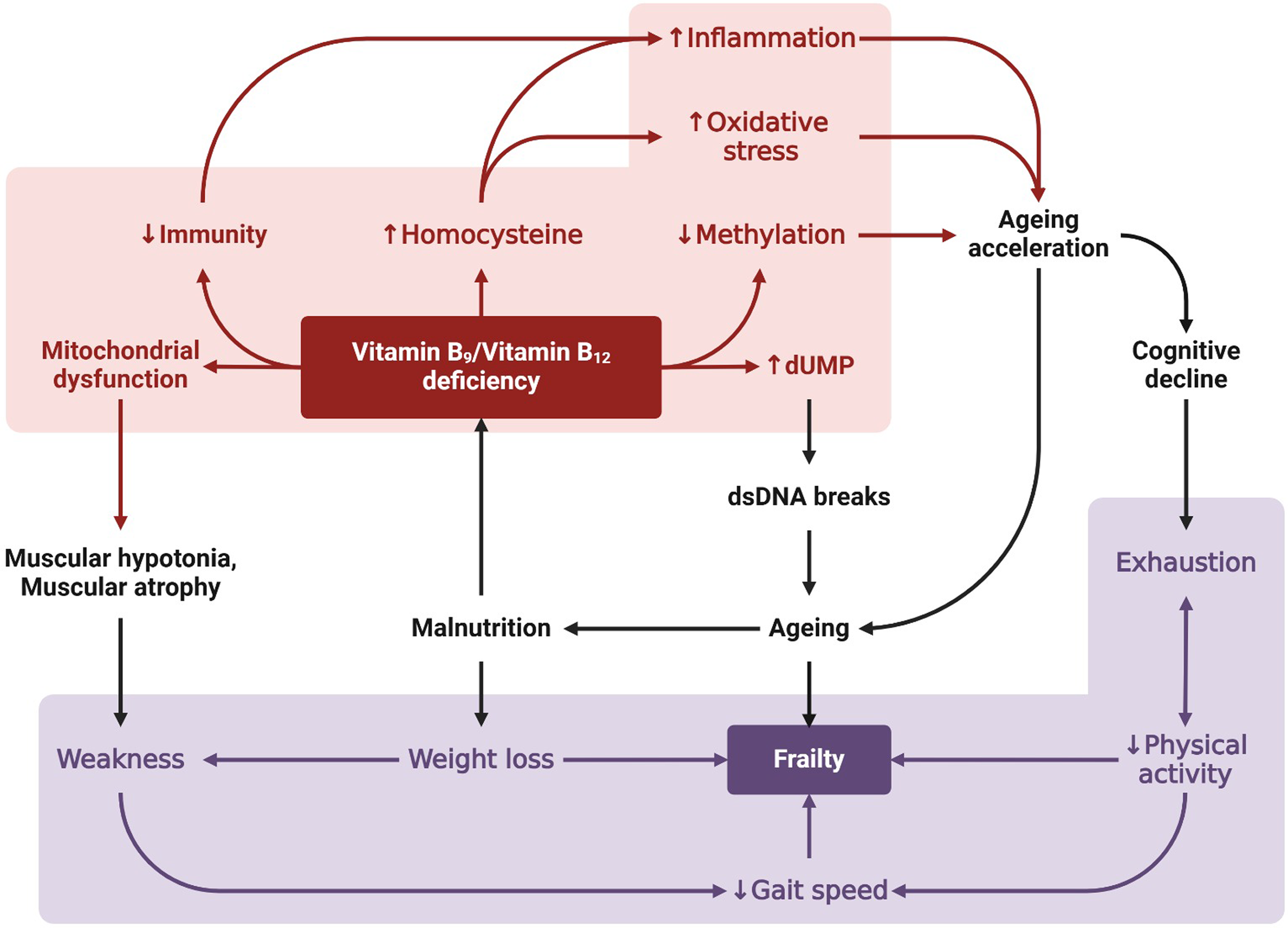
Fig. 1 Mechanisms linking vitamins B9 and B12 to frailty. Deficiency in vitamins B9 and B12, resulting from improper diet and intestinal malabsorption, has a primary metabolic effect to stop the folate and methionine cycles (not shown). As a result, the decrease in methyl donor availability affects gene expression through DNA methylation, leading to a decline in methylation and accelerating ageing. In addition, homocysteine accumulation occurs, which increases oxidative stress and inflammation. This accelerates ageing and is an important factor contributing to frailty and cognitive decline. In addition, hypovitaminosis impairs DNA stability by increasing deoxyuridine monophosphate (dUMP), leading to accelerated ageing. Vitamin B9 and B12 deficiencies also cause mitochondrial homeostasis dysregulation, resulting in muscular hypotonia or muscle atrophy. These dysfunctions affect the physical capacities of the body can be measured by Fried’s phonotypes (weight loss, exhaustion, weakness, slow gait speed and low physical activity) to estimate the severity of the syndrome.
Currently, several studies are exploring the correlation between low levels of vitamin B9 and an elevated risk of frailty among older individuals. Findings from a population-based observational longitudinal study in Singapore revealed that low folate levels were significantly associated with incident frailty(Reference Cheong, Nyunt, Gao, Gwee, Choo and Yap47). Similarly, a retrospective study in Italy found that folate deficiency was associated with severe cognitive impairment, suggesting that assessing B-vitamin status in older adults can provide a practical approach to the preventing and managing cognitive decline(Reference Baroni, Bonetto, Rizzo, Bertola, Caberlotto and Bazzerla48). Moreover, a cross-sectional study revealed a significant positive association between vitamin B9 intake and bone mineral density in aged individuals(Reference Zheng, Luo, Xu and Xue49). A cohort study also demonstrated that vitamin B9 deficiency predicted an increased risk of age-related macular degeneration(Reference Gopinath, Flood, Rochtchina, Wang and Mitchell50). Together, these studies suggest that low levels of vitamin B9 are associated with various health outcomes, including neurological impairment, osteoporosis, and vision impairment, all of which could contribute to frailty risk in older adults. In this review, we summarised the association between vitamin B9 levels and frailty status by compiling human population studies conducted between 2006 and 2023 in Supplementary Table 1. A cross-sectional study in Japanese women found that a high folate diet intake is associated with reduced odds of frailty(Reference Kobayashi, Asakura, Suga and Sasaki18). Furthermore, a low intake of certain micronutrients, including vitamin B9, was significantly and independently related to frailty after adjusting for daily energy intake(Reference Bartali, Frongillo, Bandinelli, Lauretani, Semba and Fried17). Another prospective study (1643 community-dwelling individuals aged ≥65 years) revealed a close association between low vitamin B9 intake and a higher risk (odds ratio = 2·34) of frailty in older adults from Spain(Reference Balboa-Castillo, Struijk, Lopez-Garcia, Banegas, Rodríguez-Artalejo and Guallar-Castillon51). These findings suggest that higher folate levels or intake are associated with a lower risk of frailty, while lower levels are linked to increased severity or prevalence of frailty. Given the significant effect of frailty on the quality of life in patients with chronic heart failure (CHF), researchers have worked on developing a diagnostic model using nutritional indicators to assess frailty in Chinese patients(Reference Gu, Li, Yan, Yin, Lu and Sha52). Their investigation found a connection between abnormal folic acid and vitamin B12 levels and frailty in 123 patients with CHF aged 67·5 years old. Moreover, when considering a potential plasma parameter associated with frailty, findings from Guillotin’s study on 60 older French adults, aged 52–92 years, suspected of having normal pressure hydrocephalus revealed an association between the frailty index and vitamin B9 (Reference Guillotin, Vallet, Lorthois, Cestac, Schmidt and Delcourt53). A cross-sectional study was conducted to assess the prevalence of and explore risk factors for pre-frailty, frailty, and sarcopenia in a national cohort of Dutch childhood cancer survivors diagnosed between 1963 and 2001. Complete assessments of frailty and sarcopenia were obtained from 1114 and 1472 subjects, respectively. The results showed that vitamin B9 deficiency was significantly associated with pre-frailty and frailty, with odds ratios of 1·87 and 2·04, respectively(Reference van Atteveld, de Winter, Pluimakers, Fiocco, Nievelstein and Hobbelink54). However, a cross-sectional study conducted in the Netherlands between 2005 and 2010, involving 404 geriatric outpatients, found no association between plasma concentrations of micronutrients, including vitamin B9, and frailty(Reference Kurkcu, Meijer, Lonterman, Muller and de van der Schueren55). Similarly, the InveCe.Ab study, a longitudinal multidimensional population study in a cohort of community-dwelling older people in Italy, did not observe a relationship between plasma concentrations of vitamin B9 and the cumulative incidence of frailty during the follow-up periods in 2014 and 2018(Reference Guaita, Brunelli, Davin, Poloni, Vaccaro and Gagliardi56). Moreover, results from the population-based KORA-Age study in Germany, conducted in 2008–9 as a follow-up of participants aged 65 years and over, did not find a correlation between folate deficiency and either pre-frail or frail status(Reference Conzade, Koenig, Heier, Schneider, Grill and Peters57). Further research, particularly longitudinal and interventional studies, is needed to establish causality and elucidate the mechanisms underlying this relationship.
Relevant findings from intervention studies have explored the effects of folate supplementation on frailty outcomes in older adults. A systematic review revealed an association between frailty and an elevation of peripheral oxidative stress biomarkers(Reference Soysal, Isik, Carvalho, Fernandes, Solmi and Schofield58), and a meta-analysis of randomised controlled trials (RCT) suggested that folate supplementation can lead to less oxidative stress(Reference Asbaghi, Ghanavati, Ashtary-Larky, Bagheri, Rezaei Kelishadi and Nazarian59). In a Singaporean randomised controlled trial, 246 pre-frail and frail older adults with an average age of 70 years underwent five different 6-month interventions. Among them, 49 participants received daily nutritional supplements containing iron, folate, vitamins B6, B12 and D, and calcium. This intervention led to a reduction in frailty scores, with an odds ratio of 2·98(Reference Ng, Feng, Nyunt, Feng, Niti and Tan60). According to a double-blind RCT conducted between 1999 and 2004 in the Netherlands, 818 elderly volunteers with an average age of 60 years who took vitamin B9 supplements for 3 years had a significant improvement in cognitive function compared with the placebo group(Reference Durga, van Boxtel, Schouten, Kok, Jolles and Katan61).
Overall, observational studies suggest a potential association between folate status or intake and frailty risk. The evidence from clinical trials regarding the effectiveness of folate supplementation alone in preventing frailty remains limited. Further research, particularly large-scale RCT, is needed to elucidate the role of folate supplementation, either alone or as part of a multicomponent intervention, in frailty prevention and management in older adults.
Vitamin B12 in frailty
Vitamin B12, also known as cobalamin, is a hydrophilic B vitamin primarily found in animal-based foods and fermented products. It is synthesised by certain ruminal microbiota and stored in animal tissues, particularly in herbivorous ruminant animals(Reference Watanabe and Bito62). While vitamin B12 is absent in plant-based diets, significant amounts can be found in various fermented foods such as kimchi, thua-nao, natto, miso and soy sauce(Reference Gomes Soares, Bevilaqua, Marcondes Tassi and Reolon Schmidt63). Moreover, some nutritional yeast flakes and breakfast cereals are commonly fortified with vitamin B12. The recommended daily intake of vitamin B12 is 2·4 µg for adults (aged 19 years and older), 2·6 µg for pregnant women and 2·8 µg for women during lactation(24). Vitamin B12 is considered a crucial coenzyme in cellular function, especially for the nervous system(Reference Calderon-Ospina and Nava-Mesa14), erythropoiesis(Reference Koury and Ponka28), and DNA synthesis(Reference Halczuk, Kaźmierczak-Barańska, Karwowski, Karmańska and Cieślak64,Reference Fenech65) . Several symptoms have been closely related to the low level of vitamin B12, such as neuropathy(Reference Stein, Geisel and Obeid66), cognitive impairment(Reference Smith and Refsum67), mood disorder(Reference Hanna, Lachover and Rajarethinam68), sarcopenia(Reference Bulut, Soysal, Aydin, Dokuzlar, Kocyigit and Isik69), musculoskeletal disorders(Reference Fu, Wang and Hu70) and frailty syndrome(Reference Pyrgioti and Karakousis71). So far, vitamin B12 plays a significant role in the maintenance of myelin, the protective covering of nerves(Reference Badar72). Without adequate vitamin B12, myelin and neurotransmitter synthesis are impaired(Reference Calderon-Ospina and Nava-Mesa14), resulting in neuropathy and cognitive impairment, which are significant contributors to frailty in older adults. Cognitive impairment associated with vitamin B12 deficiency can manifest as memory loss, difficulty concentrating and dementia-like symptoms. Furthermore, the imbalance of neurotransmitters, particularly serotonin and dopamine, resulting from vitamin B12 deficiency, can affect mood regulation pathways in the brain. Mood disturbances can contribute to frailty progression by reducing an elder’s motivation and ability to engage in physical activity, social interactions, and self-care practices(Reference Bernal-López, Potvin and Avila-Funes73,Reference Gheorghe, Bălășescu, Hulea, Turcu, Amariei and Covaciu74) . Moreover, several findings support that low vitamin B12 status is strongly associated with age-related diseases such as Parkinson’s disease(Reference Christine, Auinger, Joslin, Yelpaala and Green75,Reference McCarter, Teigen, McCarter, Benarroch, St Louis and Savica76) and cancers(Reference Lacombe, Chabrun, Lacout, Ghali, Capitain and Patsouris77,Reference Arendt, Farkas, Pedersen, Nexo and Sørensen78) . Figure 1 shows the possible links existing between vitamin B12 deficiency and frailty.
Certainly, several studies have investigated the association between low vitamin B12 levels and increased frailty risk. Some studies have shown an association between vitamin B12 and frailty risk or certain components of the Fried phenotype(Reference Soh and Won79,Reference Stolakis, Megas, Panagiotopoulos, Mentis, Antoniadou and Kalivioti80) , whereas others have found no such association(Reference Kurkcu, Meijer, Lonterman, Muller and de van der Schueren55,Reference Conzade, Koenig, Heier, Schneider, Grill and Peters57,Reference Dokuzlar, Soysal and Isik81,Reference Vidoni, Pettee Gabriel, Luo, Simonsick and Day82) . In this review, we summarised the association between vitamin B12 levels and frailty status by compiling human population studies conducted between 2008 and 2023 in Supplementary Table 1.
In a cross-sectional study analysing baseline measures from the combined Women’s Health and Aging Studies conducted in Maryland, the authors suggested that vitamin B12 deficiency, as indicated by elevated methylmalonic acid (MMA) levels, may contribute to the development of frailty syndrome in 703 community-dwelling older women aged 70–79 years(Reference Matteini, Walston, Fallin, Bandeen-Roche, Kao and Semba19). Besides this, the gene variations associated with one-carbon transfer pathways were investigated in more detail using the data (326 women at the baseline) in the Women’s Health and Aging Studies I and II(Reference Matteini, Walston, Bandeen-Roche, Arking, Allen and Fried83). The results revealed that transcobalamin 2 (TCN2) gene variants may lead to decreased vitamin B12 availability, contributing to the risk of the frailty syndrome. A systematic review and meta-analysis, through a search of PubMed, Medline, and Embase databases from inception to June 2024, demonstrated that age-related macular degeneration is associated with elevated homocysteine levels and decreased vitamin B12 levels. The vitamin B12 level in the age-related macular degeneration cases was 64·16 pg/mL (95% CI 19·32, 109·00 pg/mL) lower compared with the controls(Reference Huang, Wang, Kumar Sah, Jiang, Ni and Wang84). A subset of data from the Singapore Longitudinal Aging Study was examined, with 238 participants categorised by age and according to the Fried frailty criteria(Reference Pannérec, Migliavacca, De Castro, Michaud, Karaz and Goulet85). The results showed that ageing and frailty were linked to an increased prevalence of functional vitamin B12 deficiency. These factors lead to intrinsic vitamin B12 deficiencies, which can occur regardless of nutritional intake. In addition, a cross-sectional study was conducted to explore the connection between frailty and vitamin B12 levels in older adults living in the community in Korea(Reference Soh and Won79). Their findings indicated that low vitamin B12 levels increased the incidence of frailty and impacted physical performance. However, when accounting for confounding factors, B12 deficiency did not significantly raise the incidence of frailty. Frailty results from multiple factors, with B12 deficiency being one of them. In addition, a cross-sectional study was carried out to explore the connection between vitamins B12 and D3, balance, and falls among older adults in Greece(Reference Stolakis, Megas, Panagiotopoulos, Mentis, Antoniadou and Kalivioti80). Higher levels of vitamin B12, but not vitamin D3, are linked to better balance, although they are not associated with a reduced number of falls in a sample of community-dwelling older adults. A longitudinal study by Jungert et al. investigated the dynamics and interactions of vitamin B12 and vitamin B9 status in a cohort of community-dwelling older adults with multiple follow-up assessments(Reference Jungert, Zenke-Philippi and Neuhäuser-Berthold86). Their findings indicated that ensuring adequate levels of vitamin B12 and vitamin B9 during early ageing could be beneficial for maintaining sufficient levels in later years. Although addressing vitamin B12 deficiency through dietary interventions may help mitigate frailty risks, the evidence regarding the impact of vitamin B12 supplementation on frailty prevention and management is still limited and somewhat inconclusive. Ma et al. examined whether supplementation with vitamin B9 and vitamin B12 in 240 Chinese participants with mild cognitive impairment (aged >65 years), alone and in combination, improves cognitive performance via reducing levels of peripheral inflammatory cytokines(Reference Ma, Zhou, Li, Zhao, Song and An87). Their studies demonstrated that the combination of vitamins B9 and B12 was significantly superior to either vitamin B9 or vitamin B12 alone. Other studies, conducted in 2,121 Japanese women aged 65–94 years and 1,643 community-dwelling older adults aged ≥65 years in Spain, assessed vitamin B12 intake on the basis of diet history and did not find an association with frailty risk(Reference Kobayashi, Asakura, Suga and Sasaki18,Reference Balboa-Castillo, Struijk, Lopez-Garcia, Banegas, Rodríguez-Artalejo and Guallar-Castillon51) . Moreover, some studies have examined the effects of multicomponent interventions, including vitamin B12 supplementation, on frailty outcomes. For example, Ng et al. conducted a parallel-group, randomised controlled trial in Singapore to evaluate the effects of nutritional supplementation, cognitive training, physical training and a combination of these interventions on reversing frailty among community-dwelling pre-frail and frail older adults(Reference Ng, Feng, Nyunt, Feng, Niti and Tan60). The results demonstrated that individual nutritional supplements, physical training and cognitive training, and their combination, were beneficial in decreasing frailty. Although existing evidence suggests the benefits of vitamin B12 on frailty outcomes in older adults, gaps remain in our understanding of the precise role of vitamin B12 in frailty prevention and management. Future intervention studies are needed in this regard.
Vitamin D in frailty
Vitamin D in incidence and severity of frailty
Calciferol, or vitamin D, is a lipophilic vitamin that functions as a steroid hormone. It naturally exists in two main forms: ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). Vitamin D is acquired through the diet, but it is an inactive form that needs to be further metabolised in the liver and then in the kidney to be able to achieve its effects. Ergocalciferol is synthesised by irradiating ergosterol in plants and fungi with ultraviolet (UV)-B light, while cholecalciferol is derived from the photosynthesis of 7-dehydrocholesterol (7-DHC) within the skin in the presence of UV-B light(Reference Rebelos, Tentolouris and Jude88,Reference Nair and Maseeh89) . Cholecalciferol is primarily abundant in fatty fish, such as tuna, salmon, trout, and fish liver oil. It is also present in smaller amounts in egg yolks, dairy products and beef liver(Reference Roseland, Phillips, Patterson, Pehrsson, Taylor and Feldman90). Many animal-derived foods, such as poultry, pork and beef, contain a notable quantity of 25-hydroxycholecalciferol (25(OH)D3), also known as calcifediol, which is a hydroxylated form of cholecalciferol (inactive vitamin D)(Reference Taylor, Patterson, Roseland, Wise, Merkel and Pehrsson91). Furthermore, most dairy products, particularly milk, some brands of orange juice and ready-to-eat cereals, are fortified with cholecalciferol(Reference Calvo, Whiting and Barton92). The recommended dietary allowance of calciferol for individuals aged 19–70 years old is 15 µg (600 IU), a value that also applies to women during pregnancy and lactation. However, individuals older than 70 years should consume at least 20 µg (800 IU) of calciferol a day to maintain its status(Reference Ross, Taylor, Yaktine and Del Valle93).
In 2007, Holick reported in his review that 1 billion people worldwide had vitamin D insufficiency(Reference Holick94), which would represent 15% of the global population. Almost 10 years later, a study on a European population sample indicated that 13% had insufficient serum 25(OH)D levels(Reference Cashman, Dowling, Škrabáková, Gonzalez-Gross, Valtueña and De Henauw95), although figures worldwide are highly variable depending on the continent(Reference Amrein, Scherkl, Hoffmann, Neuwersch-Sommeregger, Köstenberger and Tmava Berisha96).
We have compiled in Supplementary Table 2 human population studies conducted between 2014 and 2022, from the PubMed database, that assessed the relationship between vitamin D and frailty. Most studies found that vitamin D insufficiency or deficiency was present in frail people, and some longitudinal studies(Reference Vogt, Decke, de Las Heras Gala, Linkohr, Koenig and Ladwig97–Reference van den Berg, Hegeman, van den Brink, Rhebergen, Oude Voshaar and Marijnissen100) concluded that low serum vitamin D levels could be a pre-existing condition of frailty. Nonetheless, how direct and independent this relation could be was never ascertained, leaving the leading cause of frailty elusive. Few longitudinal studies that observed the association were limited to concluding a potential causality(Reference Buta, Choudhury, Xue, Chaves, Bandeen-Roche and Shardell98,Reference Buchebner, Bartosch, Malmgren, McGuigan, Gerdhem and Akesson99,Reference Dai, Yue, Zhang, Wang and Wu101) . In studies that found a negative association between frailty and 25(OH)D(Reference Demircioglu102), the odds ratio of frailty was estimated to be between 2 and 3 in people with insufficient or deficient serum 25(OH)D when compared with normal levels(Reference Vogt, Decke, de Las Heras Gala, Linkohr, Koenig and Ladwig97,Reference Wang, Wang, Zhan, Tang, Huang and Tan103–Reference Zheng, Xu, Wang, Qiu and Xue110) .
A longitudinal study found that progression of frailty was no more associated with 25(OH)D levels in people aged 80 years and older(Reference Buchebner, Bartosch, Malmgren, McGuigan, Gerdhem and Akesson99), which could explain why no association was detected in several cross-sectional analyses using data from populations with mean age above 80 years(Reference Krams, Cesari, Guyonnet, Abellan Van Kan, Cantet and Vellas111–Reference Xiong and Xue113). A different cross-sectional study found no correlation between frailty and serum 25(OH)D in adults with diabetes and chronic kidney disease (mean age of 70 years old)(Reference Adame Perez, Senior, Field, Jindal and Mager114). This suggests that any possible link between vitamin D status and frailty risk may be obscured or confused by underlying medical conditions. Ethnicity may also be a confounding factor in the vitamin D-frailty relationship. The cross-sectional analysis of a database of more than 5,000 North American people aged 60 years or older, found that higher odds of frailty were associated with serum 25(OH)D deficiency (<37·5 nmol/L) in ‘whites’ (3·7 odds ratio), and with deficiency and insufficiency (<75 nmol/L) in ‘non-whites’ (4·0 and 2·7 odds ratios)(Reference Wilhelm-Leen, Hall, Deboer and Chertow115). An important determining factor was sun exposure, with people living in states above the 40° parallel being at higher risk of frailty. Older African American women were also more likely to have lower 25(OH)D and thus be at a higher risk of frailty(Reference Buta, Choudhury, Xue, Chaves, Bandeen-Roche and Shardell98). However, the authors of the study observed no association when adding cardiometabolic diseases as a confounding factor. Schöttker et al. reported, from a large cohort study involving nearly 10,000 participants, that vitamin D levels were associated with self-rated health and frailty by cross-sectional analysis(Reference Schöttker, Saum, Perna, Ordóñez-Mena, Holleczek and Brenner116). However, this relationship was not observed in the longitudinal analysis, except for mortality, letting the authors hypothesise that serum 25(OH)D level could be used as a mortality marker.
To palliate improper nutritional status regarding vitamin D, supplements are usually recommended, but it is inconclusive to prevent frailty. The 4,000 IU/d dose had a lower risk of developing frailty compared with 200 IU/d(Reference Cai, Wanigatunga, Mitchell, Urbanek, Miller and Juraschek117). Bolzetta et al.(Reference Bolzetta, Stubbs, Noale, Vaona, Demurtas and Celotto118) used data from an American dataset of which 3,083 participants (two-thirds of total participants), with a mean age of 62.1 years, had taken a daily dose of vitamin D supplement in the range of 57–600 IU. They were more likely to be women and older, to have a lower BMI (28·3 versus 29·4), a better dietary vitamin D3 intake (145 versus 134 IU/d), a lower energy intake (1,391 versus 1,466 kcal/d) and to suffer less depression. However, after an 8-year follow-up, 362 participants had become frail, but vitamin D supplementation did not affect the incidence rate of frailty. This finding aligns with the following studies. Interventional studies(Reference Cai, Wanigatunga, Mitchell, Urbanek, Miller and Juraschek117,Reference Orkaby, Dushkes, Ward, Djousse, Buring and Lee119) examined the effect of vitamin D supplementation at 2,000 IU/d and found no association with frailty. In addition, it was found in a Swedish cohort study that a serum concentration above 75 nmol/L did not have a particular beneficial effect on frailty(Reference Buchebner, Bartosch, Malmgren, McGuigan, Gerdhem and Akesson99).
Vitamin D in cognitive functions and depression
The role of vitamin D extends beyond bone health, with emerging research suggesting its potential influence on mental health and the nervous system. Numerous tissues, including the brain, have been demonstrated to synthesise active forms of vitamin D. Vitamin D binding proteins (DBP) and VDR have been found in the central nervous system (CNS), specifically in regions linked to depression and mood(Reference Kesby, Eyles, Burne and McGrath120,Reference Caldwell, Londe, Ochs, Hajdu, Rodewald and Gebhart121) . Nonetheless, RCT have shown conflicting findings about how vitamin D supplementation affects the intensity of depression. In the 8-week double-blind RCT, 56 participants with mild-to-severe depression, aged 43 years on average, were given either 50,000 IU/2 weeks of vitamin D supplementation or a placebo. The intervention group experienced a substantial decrease in their depression score, whereas the control group did not(Reference Kaviani, Nikooyeh, Zand, Yaghmaei and Neyestani122). This finding is concomitant with a 12-week double-blind, placebo-controlled, randomised trial on 68 subjects with type 2 diabetes and mild-to-moderate depressive symptoms. After three months of vitamin D supplementation (4,000 IU/d), the intervention group demonstrated a significant decrease in depression score(Reference Omidian, Mahmoudi, Abshirini, Eshraghian, Javanbakht and Zarei123). Furthermore, a 1-year double-blind randomised clinical trial (24,000 IU of vitamin D3/month, 60,000 IU of vitamin D3/month or 24,000 IU of vitamin D3 plus 300 μg calcifediol) on 200 participants, aged 77–78 years on average, showed that participants who achieved 25(OH)D levels in the highest quartile were associated with the best improvements in mental health at 12-month follow-up(Reference Gugger, Marzel, Orav, Willett, Dawson-Hughes and Theiler124). However, there was no benefit of vitamin D on depressive symptoms in four previous clinical trials at doses of 800 IU/d(Reference Dumville, Miles, Porthouse, Cockayne, Saxon and King125), 5,000 IU/d(Reference Dean, Bellgrove, Hall, Phan, Eyles and Kvaskoff126), 50,000 IU/week(Reference Arvold, Odean, Dornfeld, Regal, Arvold and Karwoski127) and 500,000 IU once annually(Reference Sanders, Stuart, Williamson, Jacka, Dodd and Nicholson128).
Vitamin D in muscle functions
VDR has been presented in several tissues, including skeletal muscle(Reference Bischoff, Borchers, Gudat, Duermueller, Theiler and Stähelin129). Therefore, the important role vitamin D plays in preserving healthy muscular function is being increasingly recognised. A prospective cohort study in Australia aimed to screen for aortic aneurysm in men aged 70–88 years, a relationship between vitamin D levels, frailty, and mortality was revealed(Reference Wong, McCaul, Yeap, Hankey and Flicker130). The study determined that vitamin D levels below 52·9 nmol/L were associated with an odds ratio of 1·56 for being frail at 5 years. Low vitamin D levels were found to be predictive of fatigue (worn out or feeling tired most of the time), resistance (inability to climb a flight of stairs), and ambulation (inability to walk 100 m), as well as of all-cause mortality, without establishing a causal relationship(Reference Wong, McCaul, Yeap, Hankey and Flicker130). Gutiérrez-Robledo et al. demonstrated an independent association between 25(OH)D levels and weakness(Reference Gutiérrez-Robledo, Ávila-Funes, Amieva, Meillon, Acosta and Navarrete-Reyes131). In addition, other research studies have highlighted the link between low vitamin D levels and impaired physical performance(Reference Vitale, Caruso, Rapisarda, Cianci and Cianci132,Reference Carrazco-Peña, Farías-Moreno, Del Toro-Equihua, Aguilar-Mancilla, Trujillo-Magallón and Solórzano-Rodríguez133) .
Moreover, the impact of vitamin D supplementation on muscular function has been examined in several RCT. In the 12-week RCT, forty elderly post-menopausal women, aged 70 years on average, were given either 0·5 mg/d of alfacalcidol, a synthetic vitamin D analogue plus 1,500 mg/d of calcium carbonate, or a placebo plus 1,500 mg/d of calcium carbonate. The intervention group effectively improved the quadriceps muscle strength measured by the isokinetic dynamometer device(Reference Songpatanasilp, Chailurkit, Nichachotsalid and Chantarasorn134). A year-long RCT in 261 elderly women (average age of 77 years) showed that daily intake of 1,000 IU of vitamin D2 plus 1,000 mg of calcium citrate positively impacted hip muscle strength and mobility(Reference Zhu, Austin, Devine, Bruce and Prince135). Ceglia et al.(Reference Ceglia, Niramitmahapanya, Da Silva Morais, Rivas, Harris and Bischoff-Ferrari136) argued – through an RCT of vitamin D supplementation at 4,000 IU/d in mobility-limited older women with serum 25(OH)D3 deficiency – that the nuclear VDR concentration in muscle was associated with vitamin D levels. However, Hangelbroek et al.(Reference Hangelbroek, Vaes, Boekschoten, Verdijk, Hooiveld and van Loon137) found that vitamin D3 supplementation at 400 IU/d (a double-blind RCT) did not affect gene expression in the muscle tissue of ten frail older people after 6 months. Moreover, in a 6-month RCT, 65 healthy men, aged 77 years on average, were given 1,000 IU/d of vitamin D3 plus 500 mg/d of calcium or a placebo plus 500 mg/d of calcium. The intervention group did not increase muscle strength or improve physical performance(Reference Kenny, Biskup, Robbins, Marcella and Burleson138). Even when studying the effect of vitamin D supplementation on frailty status, an interventional study(Reference Cai, Wanigatunga, Mitchell, Urbanek, Miller and Juraschek117) analysed the effect of vitamin D3 in individuals aged 70 years and older who had vitamin insufficiency (<75 nmol/L). Over 2 years, they received a daily dose of 1,000 IU of vitamin D3 compared with a control group at 200 IU. The optimal dose of 1,000 IU/d had been determined after comparing with subgroups at 2,000 IU/d, which was deemed to lead to a two-fold increased risk of frailty and slowness. Although the 4,000 IU/d dose of vitamin D3 had a lower risk of developing frailty during follow-up (hazard ratio = 0·22), this might be the result of type 1 error. Therefore, the authors concluded the ineffectiveness of vitamin D3 supplementation in the prevention of frailty.
Vitamin D in immunity
Vitamin D is known to control several important genes that modulate the immune system(Reference Aranow139,Reference Sîrbe, Rednic, Grama and Pop140) . It primarily affects the metabolism, differentiation, maturation, and reaction of immune cells to cytokines and chemokines. Both the 1-α-hydroxylase and VDR genes are expressed more when toll-like receptor (TLR) binding occurs in monocytes and macrophages, leading to increased transcriptional activity of vitamin D(Reference Liu, Stenger, Li, Wenzel, Tan and Krutzik141,Reference Martens, Gysemans, Verstuyf and Mathieu142) . Vitamin D deficiency can lead to immune system dysfunction, which increases the risk of infections such as tuberculosis(Reference Chocano-Bedoya and Ronnenberg143), sepsis(Reference Cutuli, Ferrando, Cammarota, Franchini, Caroli and Lombardi144), coronavirus disease 19(Reference Kaya, Pamukçu and Yakar145), cancers(Reference Seraphin, Rieger, Hewison, Capobianco and Lisse146), and autoimmune diseases(Reference Sîrbe, Rednic, Grama and Pop140). Moreover, vitamin D plays a vital role in the immune response to vaccines such as influenza, measles, rubella, and hepatitis B vaccines(Reference Sadarangani, Whitaker and Poland147).
In this review, we will focus on immunity in the elderly with/without frailty and vitamin D. High serum levels of inflammatory cytokines and acute phase proteins indicate a heightened inflammatory state in frail older persons, corroborating the idea that frailty is associated with a dysregulated immune system(Reference Yao, Li and Leng148). A Chinese study with 288 frailty patients with chronic coronary syndrome identified by cross-sectional analysis that frailty was associated with interleukin-6 (IL-6) and 25(OH)D levels in an independent manner(Reference Dai, Li, He, Huang and Li149). A population-based study conducted in the Netherlands found that there was a correlation between moderately elevated levels of C-reactive protein (CRP) (3–10 µg/mL) and severe vitamin D deficiency (<25 nmol/L) with the development of frailty, whereas no consistent relationships were found between IL-6 and insulin-like growth factor 1 with frailty(Reference Puts, Visser, Twisk, Deeg and Lips150). A retrospective analysis of 237 older adults (mean age of 86.5 years) admitted to an acute care geriatric unit revealed an inverse relationship between serum 25(OH)D and CRP, a marker of inflammation(Reference Boccardi, Lapenna, Gaggi, Garaffa, Croce and Baroni151). In addition, 25(OH)D levels were negatively correlated with the Cumulative Illness Rating Scale Severity Index (CIRS-SI) and Comorbidity Index (CIRS-CI), whereas CRP levels were positively correlated with CIRS-SI and CIRS-CI(Reference Miller, Paradis, Houck, Mazumdar, Stack and Rifai152). RCT are essential for assessing how vitamin D3 affects immunity in older persons, and some studies have started to investigate this connection. In a 14-week intervention study including eighteen older persons given 6,400 IU/d of vitamin D3, this supplementation dramatically improved the response to the cutaneous varicella zoster virus antigen challenge(Reference Chambers, Vukmanovic-Stejic, Turner, Shih, Trahair and Pollara153). In a 3-month RCT, 38 elderly participants, aged 70 years on average, were given 100,000 IU/15 d of vitamin D3 or a placebo. The findings demonstrated that vitamin D supplementation raised the plasma level of transforming growth factor beta in response to influenza vaccination without enhancing the generation of antibodies(Reference Goncalves-Mendes, Talvas, Dualé, Guttmann, Corbin and Marceau154). Collectively, vitamin D may play a modulatory role in the inflammatory and immune pathways associated with frailty in older individuals, making it a potential target for interventions.
Meta-analyses of vitamin D research in frailty
Several observational studies have investigated the clinical relationship between vitamin D serum concentration and frailty. Data obtained can be conflicting, mainly owing to divergences in the types of studies, inclusion/exclusion criteria and analysis methods, but also in the definition of vitamin D deficiency itself. Indeed, although it is commonly admitted that serum 25(OH)D levels of 50–75 nmol/L are insufficient, 25–50 nmol/L are deficient and <25 nmol/L are severely deficient, not all studies use the same scale. Some researchers prefer to test the dose–response effect, whereas others choose to use an incremental scale without associating it with an evaluation of the degree of vitamin deficiency. The threshold of 75 nmol/L was initially chosen as it corresponds to the minimal concentration beyond which serum parathyroid hormone is kept at its minimum through intestinal calcium absorption(Reference Dawson-Hughes, Heaney, Holick, Lips, Meunier and Vieth155). Regarding these discrepancies, meta-analysis proves to be an essential approach to understand the associations between frailty and vitamin D.
The inverse association between vitamin D levels and the risk of frailty in older people is still controversial. Marcos-Pérez et al. concluded that the connection between low 25(OH)D levels and frailty severity, on the basis of their meta-analysis of 26 studies, was an inverse association, especially at the deficient level when comparing frails and pre-frails(Reference Marcos-Pérez, Sánchez-Flores, Proietti, Bonassi, Costa and Teixeira156). A daily intake of 1,000 IU of vitamin D3 was also recommended by Ju et al., in their ten-study meta-analysis, which indicated the protective effect of a 25 nmol/L increase in serum(Reference Ju, Lee and Kim157). This daily dose seemed particularly effective on physical performance(Reference Marcos-Pérez, Sánchez-Flores, Proietti, Bonassi, Costa and Teixeira156). However, on the basis of a large meta-analysis, Bolland et al. showed that vitamin D supplementation was not able to reduce the risk of outcomes such as hip fracture, cerebrovascular disease and cancer beyond 15%(Reference Bolland, Grey, Gamble and Reid158).
In the face of the current state of knowledge, it is not yet possible to define the nature of the effect with certainty (although some research results argue for a threshold effect with a characteristic U-shaped curve(Reference Buchebner, Bartosch, Malmgren, McGuigan, Gerdhem and Akesson99,Reference Wang, Wang, Zhan, Tang, Huang and Tan103,Reference Ensrud, Ewing, Fredman, Hochberg, Cauley and Hillier159) ) the risk of frailty increasing above a certain serum concentration. Nevertheless, cross-sectional studies constitute the majority of research on frailty, and their intrinsic methodology prevents drawing a stronger connection with vitamin D. The causality itself between vitamin D and the risk of frailty is still uncertain, as research has not yet been able to point out which is the stronger contributor. Through a disturbance in calcium metabolism, low vitamin D levels can lead to frailty. However, owing to decreased mobility and outdoor activities, frailty itself frequently results in less exposure to sunshine, which inhibits the skin’s ability to synthesise vitamin D(Reference Marcos-Pérez, Sánchez-Flores, Proietti, Bonassi, Costa and Teixeira156,Reference Ju, Lee and Kim157) . Figure 2 shows the possible links existing between vitamin D deficiency and frailty.

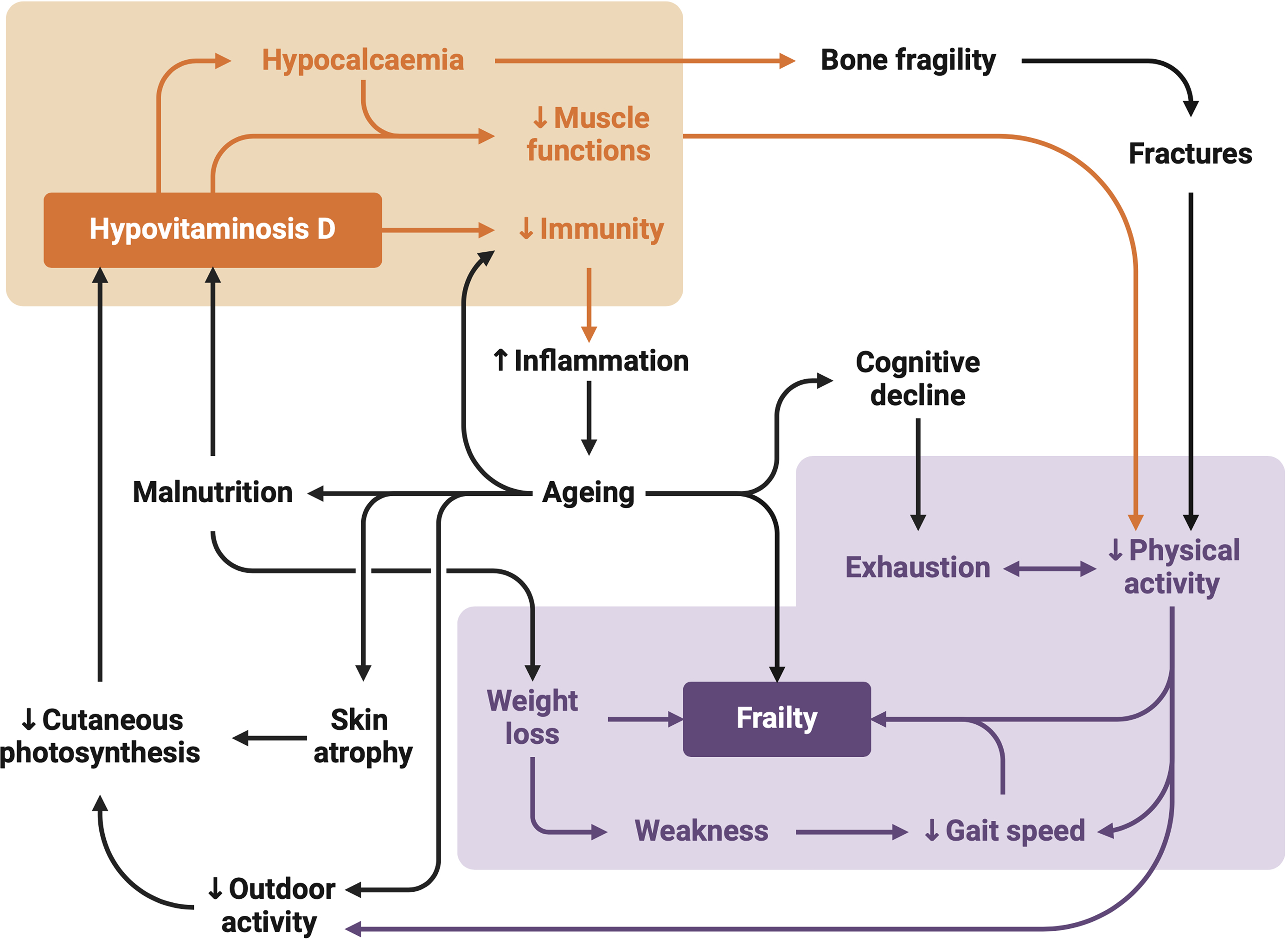
Fig. 2 Mechanisms linking vitamin D to frailty. The ageing process can psychologically affect older adults, leading to behaviours that contribute to lower vitamin D levels. These behaviours include spending more time indoors, reducing exposure to sunlight (an essential factor for vitamin D metabolism) and consuming an insufficient and unbalanced diet. Vitamin D deficiency can result in muscle weakening and bone demineralisation, contributing to the physical component of frailty and increasing the risk of fractures. In addition, low vitamin D levels are associated with a high systemic inflammatory potential of pro-inflammatory factors and cytokines, which accelerate ageing and contribute to frailty and cognitive decline. Frail individuals, affected as such, are less likely to engage in physical activities, especially outdoors, perpetuating the cycle of frailty including low gait speed and weight loss.
Mechanisms linking vitamins to frailty
Vitamins B9 and B12
Vitamins B9 and B12 in methylation
Vitamin B9 and B12 play critical roles in homocysteine metabolism and DNA methylation, both closely related to ageing and frailty in older adults. It is essential for the conversion of homocysteine, an amino acid, into methionine. Methionine is then converted into S-adenosylmethionine, serving as a methyl donor to various acceptors. After these methylation reactions, S-adenosylhomocysteine is produced as a by-product, which is then hydrolysed to regenerate homocysteine. Vitamin B9 provides a methyl group to convert homocysteine to methionine, while vitamin B12 acts as a cofactor to regenerate tetrahydrofolate, an active form of folate, allowing the reaction to proceed. Vitamin B9 or B12 deficiency impairs this conversion of homocysteine to methionine, leading to elevated homocysteine levels(Reference Miller, Nadeau, Smith, Smith and Selhub160,Reference Selhub161) . Elevated homocysteine levels can lead to endothelial dysfunction, impairing blood vessel function and promoting inflammation. In addition, homocysteine can stimulate the production of reactive oxygen species (ROS), leading to oxidative stress(Reference Zou and Banerjee162–Reference Perna, Ingrosso, Lombardi, Acanfora, Satta and Cesare164). This dysregulation in homocysteine metabolism can contribute to the pathophysiology of ageing-related diseases and frailty. Guaita et al. conducted a retrospective longitudinal study to explore the influence of biomarkers on frailty incidence in a community-dwelling older population(Reference Guaita, Brunelli, Davin, Poloni, Vaccaro and Gagliardi56). The results demonstrated that a high homocysteine level is a risk factor for frailty onset in older adults. Furthermore, a cross-sectional study was performed to evaluate the association between inflammatory biomarkers, namely homocysteine and CRP, with frailty, revealing that homocysteine was independently associated with frailty(Reference Álvarez-Sánchez, Álvarez-Ríos, Guerrero, García-García, Rodríguez-Mañas and Cruz-Chamorro165). Deficiency of vitamin B9 and elevated homocysteine levels in the blood have been associated with increased risk of cardiovascular disease(Reference Ma, Peng, Liu, Huang and Luo166,Reference De Koning, Werstuck, Zhou and Austin167) , cognitive decline(Reference Baroni, Bonetto, Rizzo, Bertola, Caberlotto and Bazzerla48,Reference Kim, Choi, Nam, Kim, Oh and Yang168) , osteoporosis(Reference Herrmann, Peter Schmidt, Umanskaya, Wagner, Taban-Shomal and Widmann169,Reference De Martinis, Sirufo, Nocelli, Fontanella and Ginaldi170) , depression(Reference Kim, Stewart, Kim, Yang, Shin and Yoon171,Reference Permoda-Osip, Dorszewska, Skibinska, Chlopocka-Wozniak and Rybakowski172) , and other age-related conditions(Reference Mattson, Kruman and Duan173,Reference Song, Song and Park174) .
The dysregulation of DNA methylation can lead to increased expression of pro-inflammatory genes and decreased expression of anti-inflammatory genes, contributing to chronic inflammation, which is a hallmark of frailty(Reference Pansarasa, Pistono, Davin, Bordoni, Mimmi and Guaita175). Both vitamins B9 and B12 are cofactors in the one-carbon metabolism, i.e. folate metabolism, the homocysteine re-methylation cycle and the transsulfuration pathway. Bellizzi et al. suggested that global DNA methylation in the elderly is correlated with frailty(Reference Bellizzi, D’Aquila, Montesanto, Corsonello, Mari and Mazzei176). Collerton et al. also proposed that the acquisition of aberrant DNA methylation is associated with frailty in older people(Reference Collerton, Gautrey, van Otterdijk, Davies, Martin-Ruiz and von Zglinicki177). This study suggested a potential role for age-related changes in CpG island methylation in the development of frailty. It is currently assumed that the regular intake of micronutrients, particularly vitamin B9 or vitamin B12, can slow down the gradual hypomethylation observed during the ageing process(Reference Choi, Claycombe, Martinez, Friso and Schalinske178,Reference Kuo, Sorond, Chen, Hashmi, Milberg and Lipsitz179) . However, the relationship between vitamin B9 status and DNA methylation levels is complex and is likely influenced by vitamin B9 availability(Reference Bae, Ulrich, Bailey, Malysheva, Brown and Maneval180). Recently, Michels et al. conducted a randomised intervention study to explore the impact of vitamin B9 supplementation on the epigenetic profile (DNA methylation) in healthy participants on an unfortified diet(Reference Michels and Binder181). The results of this study showed that an increase in red blood cell vitamin B9 had a modest impact on the epigenetic clock predicting chronologic age. Furthermore, both folate and vitamin B12 are associated with genome stability, which contributes to frailty(Reference Sanchez-Flores, Marcos-Perez, Lorenzo-Lopez, Maseda, Millan-Calenti and Bonassi182). Folic acid is required for the synthesis of deoxythymidine monophosphate from deoxyuridine monophosphate (dUMP). In conditions of folic acid deficiency, dUMP accumulates, leading to the incorporation of uracil into DNA instead of thymine(Reference Fenech183). There is substantial evidence suggesting that excessive incorporation of uracil into DNA not only leads to point mutations but may also result in the generation of single- and double-stranded DNA breaks, chromosome breakage and micronucleus formation.
Vitamin B12 and immunity
Vitamin B12 plays a vital role in immunomodulation. Research has shown that transcobalamin, a vitamin B12-specific non-glycosylated protein carrier, acts as an inflammatory suppressor by regulating anti-inflammatory cytokines, growth factors and other components within physiological levels(Reference Wheatley184,Reference Lee, Wang, Lin and Lin185) . Vitamin B12 itself serves as a down-regulator of the nuclear factor kappa-light-chain-enhancer of activated B cells, indirectly modulating the expression levels of pro-inflammatory cytokines, growth factors and cell adhesion molecules(Reference Veber, Mutti, Tacchini, Gammella, Tredici and Scalabrino186). Supplementation of vitamin B12 has been shown to increase CD8+ T cell counts and natural killer T-cell activity in vitamin B12-deficient subjects(Reference Tamura, Kubota, Murakami, Sawamura, Matsushima and Tamura187). Studies have demonstrated that vitamin B12 possesses antioxidant activity by reducing cytosolic and mitochondrial superoxide levels in human aortic endothelial cells supplemented with cyanocobalamin(Reference Moreira, Brasch and Yun188) and suppressing superoxide bursts in retinal ganglion cells of Long–Evans rats administered vitamin B12 (Reference Chan, Almasieh, Catrinescu and Levin189).
Regarding monocytes, which can further differentiate into tissue-specific phagocytes such as macrophages or antigen-presenting cells such as dendritic cells, hyperhomocysteinaemia-mediated inflammation triggered by vitamin B12 deficiency activates macrophages(Reference Allen, Stabler, Savage and Lindenbaum190). This increases the production of pro-inflammatory cytokines, particularly tumour necrosis factor (TNF-α), exacerbating the pro-inflammatory environment. In addition to monocytes, vitamin B12 regulates the production of T cells and the balance of the CD4+/CD8+ ratio. Therefore, a deficiency not only impairs the CD4+/CD8+ ratio but also reduces the overall T-cell count. Vitamin B12 deficiency also impacts cytotoxic NK cells owing to impaired haematopoiesis, leading to a decrease in both cell counts and quality, which can be reversed by vitamin B12 supplementation(Reference Tamura, Kubota, Murakami, Sawamura, Matsushima and Tamura187,Reference Erkurt, Aydogdu, Dikilitaş, Kuku, Kaya and Bayraktar191) .
Vitamin B12 in propionate metabolism regulation
Vitamin B12 participates in the mitochondrial tricarboxylic acid (TCA) cycle as a coenzyme, in the form of adenosylcobalamin, for the vitamin B12-dependent enzyme methylmalonyl-CoA mutase (MCM; EC 5.4.99.2). The short-chain fatty acid propionate is first converted to propionyl-CoA, which is then carboxylated to d-methylmalonyl-CoA in a reaction that requires vitamin B7 (biotin) as a cofactor. Methylmalonyl-CoA is subsequently isomerised to l-methylmalonyl-CoA and then converted by MCM to succinyl-CoA, which enters the TCA cycle for energy production. A deficiency of vitamin B12 impairs MCM activity, leading to the accumulation of methylmalonyl-CoA and its hydrolysed product, methylmalonic acid. This disruption affects the catabolism of cholesterols, fatty acids (specifically odd-chain fatty acids and those derived from propionate), and several amino acids (valine, isoleucine, threonine, and methionine), ultimately resulting in metabolic imbalance and potential mitochondrial dysfunction, characteristic of methylmalonic acidaemia(Reference Tejero, Lazure and Gomes192).
Vitamin B12 deficiency impairs MCM activity, leading to increased accumulation of the substrate, methylmalonic acid (MMA)(Reference Tejero, Lazure and Gomes192). MMA is used as a surrogate biomarker for confirming vitamin B12 deficiency, in addition to elevated serum homocysteine and macrocytic anaemia, with serum MMA values exceeding 270 nmol/L considered indicative of vitamin B12 deficiency(Reference Rajan, Wallace, Beresford, Brodkin, Allen and Stabler193). Moreover, elevated circulatory MMA levels are common among the elderly and are associated with increased cardiovascular and all-cause mortality, independent of vitamin B12 status(Reference Wang, Liu, Liu, Tian, Zhang and Cai194).
Pathologies of methylmalonic acidaemia are observed across a broad spectrum of biological systems. An in vitro study showed that MMA induces neuronal damage in embryonic rat striatal cell cultures through the inhibition of mitochondrial complex II and the TCA cycle(Reference Okun, Hörster, Farkas, Feyh, Hinz and Sauer195). In addition to complex II suppression, increased MMA levels also impaired mitochondrial respiration by inhibiting complex I in Wistar rat cerebral cortex homogenates(Reference Brusque, Borba Rosa, Schuck, Dalcin, Ribeiro and Silva196). Moreover, new-born mice injected with MMA demonstrated neurological deficits, caused by increased ROS, TNF-α, and IL-1β, leading to neuroinflammation and apoptosis(Reference Gabbi, Ribeiro, Jessié Martins, Cardoso, Haupental and Rodrigues197). Apart from neurological impacts, methylmalonic acidaemia facilitates non-alcoholic steatohepatitis (NASH) through epithelial–mesenchymal transition-mediated hepatic fibrogenesis(Reference Zhao, Zhu and Sun198,Reference Gomes, Ilter, Low, Endress, Fernández-García and Rosenzweig199) . Moreover, independent of vitamin B12 status, methylmalonic acidaemia is associated with an increased risk of hepatofibrosis in NASH subjects(Reference Li, Huang, Yang, Zhang, Gao and Han200). Skeletal muscles also suffer from increased MMA levels, owing to the high distribution of mitochondria in their tissues(Reference De Mario, Gherardi, Rizzuto and Mammucari201). Methylmalonic acidaemia-mediated inhibition of mitochondrial complex I, II, and the TCA cycle leads to impaired ATP production, causing reduced muscle power and muscular hypotonia(Reference Manoli, Sloan and Venditti202). In addition, mitochondrial dysfunction, oxidative stress, and inflammation caused by metabolic acidosis, including MMA acidaemia, can trigger proteolysis through autophagy or the ubiquitin–proteasome system, leading to muscle atrophy and sarcopenia(Reference Mitch, Medina, Grieber, May, England and Price203,Reference Yin, Li, Jia, Wang, Liang and Yang204) .
Vitamin D
The search for factors of vitamin D deficiency has led researchers to point at the potential routes for frailty and outline its potential mechanism. Research suggests it could contribute to frailty through three motors: cognitive functions, fat-free body mass, and the immune system, with VDR being the starting element of a cascade of reactions supposedly leading to frailty.
Vitamin D transport
The main transport mode of vitamin D in blood, the vitamin D binding protein (DBP), is also a factor that could be linked to frailty. Wang et al. determined that DBP concentration was higher in frail participants(Reference Wang, Wang, Zhan, Tang, Huang and Tan103). More specifically, the first and second quartiles of serum 25(OH)D3 (<31·5 and 31·5 to <41·8 nmol/L, respectively) were associated with an odds ratio greater than 2·5 compared with the highest quartile (≥56·6 nmol/L). In addition, having serum 25(OH)D3 in the first quartile (<31·5 nmol/L) along with serum DBP in the fourth quartile (≥6,686 nmol/L) was associated with an odds ratio of 3·18. This suggests that the interaction with DBP could decrease 25(OH)D3 bioavailability and, therefore, add to the frailty risk. Furthermore, frailty was associated with low cholesterol and triacylglycerols, as well as with high CRP and superoxide dismutase(Reference Xiong and Xue113,Reference Polat, Yalcin, Yazihan, Bahsi, Mut Surmeli and Akdas205) . These findings could indicate a higher inflammatory level in frail older people independently of cholesterol.
Vitamin D cell absorption and signalling
Vitamin D can exert effects on the metabolism of cells at targeted tissues in a genomic or non-genomic way. In the genomic way, the formation of an activation complex between vitamin D–VDR and retinoid X receptor RXR in the cytoplasm leads to nuclear translocation. The complex binds to the vitamin D response element (VDRE), which promotes the expression of vitamin D-regulated genes. VDRE activation has been linked (to name only the most significant and documented ones) to calmodulin, implicated in muscle tone and contraction(Reference Li, Mihalcioiu, Li, Zakikhani, Camirand and Kremer206), and to insulin-like growth factor 1 (IGF-1), which contributes to muscle cell differentiation and proliferation, as well as type 2 muscle fibre development(Reference Barton-Davis, Shoturma, Musaro, Rosenthal and Sweeney207). In addition, IGF upregulates CYP27B1, leading to a rise in serum 1,25(OH)2D levels. The expression of the calcium modulator calbindin-D9k is also modulated via vitamin D(Reference Dardenne, Prud’homme, Arabian, Glorieux and St-Arnaud208).
Non-genomic effects originate from the transportation of free 1,25(OH)2D3 across the plasma membrane, which is facilitated through membrane VDR(Reference Capiati, Benassati and Boland209) and from that of vitamin D–DBP via low-density lipoprotein receptor-related protein 2 (LRP-2)(Reference Abboud, Puglisi, Davies, Rybchyn, Whitehead and Brock210). VDR, once associated with 1,25(OH)2D3, triggers a cascade of mitogen-activated protein kinase (MAPK)-mediated reactions involved in protein synthesis and mitochondrial functions. In consequence, vitamin D deficiency has been associated with decreased mitochondrial calcium uptake and oxygen consumption(Reference Ryan, Craig, Folmes, Wang, Lanza and Schaible211) and is also associated with ROS cytotoxicity(Reference Dzik, Skrobot, Flis, Karnia, Libionka and Kloc212). However, the discovery of protein disulphide isomerase family A member 3 (PDIA3), and subsequent research on it, indicate this membrane-bound receptor is the mediator of non-genomic calcium uptake regulation(Reference Nemere, Garbi, Hämmerling and Khanal213). However, a recent study advocates for both VDR and PDIA3 being implicated in both genomic and non-genomic responses, through different pathways(Reference Nowak, Olszewska, Wierzbicka, Gebert, Bartoszewski and Żmijewski214). Furthermore, MAPK-signalling inhibition and impairment of the proteasome catalytic activity were observed, along with anaerobic capacity, lean mass, and gait speed loss, which are associated with sarcopenia(Reference Sleeman, Aspray, Lawson, Coleman, Duncan and Khoo215). In comparison with the similarities observed in chronic kidney disease, it was proposed that with a long-lasting deficiency in vitamin D, LRP-2 expression itself could decrease, with the consequence of less vitamin uptake(Reference Dusso and Tokumoto216). Taken altogether, these effects produce dysregulation in cellular homeostasis and muscle atrophy.
Target sites
Although VDR has been found in a consequent number of tissues, it is predominant principally in the brain, type 2 fibre skeletal muscle cells and immune cells. VDR is partly responsible for the proliferation of skeletal muscle cells through gene upregulation of the cell cycle and protein production(Reference Bass, Nakhuda, Deane, Brook, Wilkinson and Phillips217). Allele polymorphism of the VDR gene was studied by Arosio et al.(Reference Arosio, Guerini, Costa, Dicitore, Ferri and Mari218) with particular interest in intronic rs7975232 (C/A). Using blood analysis data and a frailty index based on Searle’s criteria(Reference Searle, Mitnitski, Gahbauer, Gill and Rockwood219) from an Italian cohort study (participants aged ≥60 years), they found that women had higher frailty scores, parathyroid hormone (PTH) and inorganic phosphate levels than men, but lower 25(OH)D3 amounts. The homozygous and heterozygous mutations were positively associated with frailty and phosphate levels in women, but no association was found between these mutations and vitamin D or PTH in men. The authors discussed that the difference between sexes could be caused by the imbalanced levels of PTH and 25(OH)D3 responsible for low serum phosphate in women, adding to the observed risk of frailty in women.
With age, the regulation of serum PTH to low levels through 25(OH)D3 mediation becomes less efficient and would require a greater intake of vitamin D than for younger people(Reference Vieth, Ladak and Walfish220). Ageing is associated with vitamin D resistance, characterised by decreased VDR abundance and impaired renal calcium reabsorption. This impacts calcium homeostasis and can lead to an imbalance in calcium levels, which is closely related to bone health in ageing. This resistance is linked to a reduction in VDR expression in various tissues, including bone, intestine, and muscle(Reference Oudshoorn, van der Cammen, McMurdo, van Leeuwen and Colin221). In addition, there is a decrease in the expression of transient receptor potential vanilloid (TRPV) calcium ion channels, specifically TRPV6 in the intestine and TRPV5 in the kidneys(Reference Van Abel, Huybers, Hoenderop, Van Der Kemp, Van Leeuwen and Bindels222). The baseline factors of individual participants highlighted by Lehmann et al., such as age, body weight, vitamin D levels, and triacylglycerol levels, can influence the effectiveness and outcomes of 12 weeks of daily oral doses of vitamin D3 supplementation in relation to frailty, calcium and phosphate metabolism, bone health, and circulating vitamin D metabolites(Reference Lehmann, Riedel, Hirche, Brandsch, Girndt and Ulrich223).
Other factors conditioning vitamin activity
Although vitamin D has well-known direct effects, it is important to recognise that these effects can also be modulated upstream before reaching the target sites. This adds complexity for researchers in establishing causal relationships and for clinicians in developing tailored treatments. For instance, fibroblast growth factor 23 (FGF-23) was found as one of the core proteins identified by proteomic analysis in a Swedish cohort of 1604 women at the age of 75 years. Frailty was associated with increased serum FGF-23 levels over 5 and 10 years, alongside higher odds of frailty. FGF-23, among other core proteins, may contribute to frailty through its effects on multiple biological pathways, including skeletal homeostasis and muscle function, potentially leading to sarcopenia(Reference Mitchell, Malmgren, Bartosch, McGuigan and Akesson224). FGF-23 is produced mainly by osteocytes and regulates the expression of vitamin-D-metabolising enzymes CYP24A1 (up-regulation) and CYP27B1 (down-regulation)(Reference Shimada, Hasegawa, Yamazaki, Muto, Hino and Takeuchi225). It also targets proximal renal tubules where it reduces phosphate reabsorption(Reference Takeshita, Kawakami, Furushima, Miyajima and Sakaguchi226), under the control of PTH, via increasing levels of serum phosphate or 1,25(OH)2D(Reference Bergwitz and Jüppner227). Beben et al. had previously associated FGF-23 as a potential biomarker of functional outcomes in a study involving nearly 3,000 older individuals(Reference Beben, Ix, Shlipak, Sarnak, Fried and Hoofnagle228). The research team demonstrated that higher FGF-23 levels were independently linked to frailty and pre-frailty in older community-dwelling individuals, regardless of demographics, cardiovascular disease, risk factors or kidney function. The activation pathway of FGF-23 requires α-klotho, another hormone expressed in the kidney. Based on a longitudinal study conducted in Italy(Reference Shardell, Semba, Kalyani, Bandinelli, Prather and Chia229) on 649 people aged 65 years and older, the serum α-klotho concentration was inversely associated with frailty odds and exhaustion odds. However, no association was found in a Turkish cross-sectional study with 89 geriatric patients(Reference Polat, Yalcin, Yazihan, Bahsi, Mut Surmeli and Akdas205).
Conclusions
On the basis of the results from clinical studies, it is conceivable that deficiencies in vitamins B9, B12, and D are linked to frailty. However, a definitive conclusion on its extent as a determinant factor cannot be reached at this time. While vitamin supplementation is generally considered beneficial, it is crucial to consider factors such as diet, health status (including age, comorbidities, and metabolic activity), and dosage to improve individuals’ conditions for living longer and healthier lives.
The current overview from recent studies is mostly limited to national-scale research, with variations in study type, recruitment, techniques, and other factors that make comparisons challenging. The conclusions of these studies often only establish associations or correlations. The lack of research on the mechanisms by which vitamin deficiency affects the onset and severity of frailty, along with confounding factors, leaves the direct and potent effects on body physiology unclear. Undertaking research projects with the ambition of studying a broader population would not only increase resources, strengthen international collaboration and share expertise but also generate a body of data expected to allow faster consensus. Prospective studies are rare, and RCTs are even rarer, despite being the most likely to provide results with a high degree of certainty. Their implementation is costly and requires extensive logistics and preparation to account for participants’ non-compliance, dropouts, and the need for long-term follow-up. However, well-designed longitudinal interventional studies in large populations are required to address the gaps left by inconclusive results.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954422425100218.
Acknowledgements
Not applicable.
Financial support
This work was supported by the National Research Council of Thailand (C.S., grant no. N84A670787) and the Siriraj Research Development Fund (C.S., grant no. (IO) R016633018), Faculty of Medicine Siriraj Hospital, Mahidol University.
The National Research Council of Thailand and the Siriraj Research Development Fund had no role in the design, analysis or writing of this article.
Competing interests
The authors declare none.
Authorship
Conceptualisation, funding acquisition and supervision: C.S. Review process: B.P., J.D.B., C.K., Y.B., R.S., P.C., A.D., S.K. and W.K. Original manuscript draft: B.P. and J.D.B. (equally). Manuscript review and editing: C.S., B.P. and J.D.B. Visualisation: C.S., J.D.B. and C.K. All authors read and approved the final manuscript.