Dementia is a clinical syndrome involving difficulties with memory, problem-solving, language and other cognitive functions that impair quality of life and activities of daily living(Reference Robinson, Tang and Taylor1). Although it is common for individuals to experience some cognitive decline with aging, a more marked loss of cognitive functions and dementia are not a normal part of growing older. Instead, dementia is caused by abnormal brain changes that are causal for diseases such as Alzheimer’s disease (the primary cause of dementia), vascular dementia, frontotemporal dementia and Lewy body dementia(2). With population growth and aging, it is estimated that the number of dementia cases will increase markedly in the next 25 years. In 2019, it was estimated that 57 million individuals worldwide were living with dementia. By 2050, it is expected that there will be 153 million dementia cases globally(Reference Nichols, Steinmetz and Vollset3). This will come with a huge economic and social cost, underscoring the need to identify effective dementia prevention and treatment strategies.
Effective treatments for dementia, especially Alzheimer’s disease, have long been sought but remain elusive. Cholinesterase inhibitors and glutamate receptor antagonists e.g. donezepil or memantine are typically recommended for individuals living with Alzheimer’s disease and Lewy body dementia. These drugs can slow the loss of cognitive functions, are widely accessible in high-income countries (but less available in low- and middle-income countries) and have a good side-effect profile(Reference Livingston, Huntley and Liu4). Nevertheless, their benefits are relatively small, and they do not alter the underlying course of disease. In the past 5 years, a new generation of disease-modifying treatments for Alzheimer’s disease have begun to emerge in the form of monoclonal antibodies, which are designed to slow disease progression by removing amyloid-β plaques (believed to play a causative role in the development of Alzheimer’s disease) from the brain. Phase 3 trials of Donanemab and Lecanemab – two promising monoclonal antibodies – have demonstrated modest attenuation of cognitive and functional decline over 18 months with each of these drugs(Reference van Dyck, Swanson and Aisen5,Reference Sims, Zimmer and Evans6) . However, these drugs are currently limited in several respects. Firstly, whilst the benefits are statistically significant, the effects sizes may not be clinically relevant to patients(Reference Muir, Hill and Black7). Secondly, they have notable side effects including brain oedema and microhaemorrhages, especially in APOE ε4 homozygotes(Reference Thambisetty and Howard8). Thirdly, these drugs are very expensive, with an annual treatment cost per patient of $26 500 (∼£21 000) for Lecanemab. A recent modelling study suggests that the cost of Lecanemab would need to fall to around ¼ of its current price to be cost effective(Reference Nguyen, Mital and Knopman9). It is hoped that these drugs will pave the way for more effective therapeutics with fewer side effects and lower cost in the coming years. However, even if more effective treatments emerge, dementia prevention remains a major individual and public health priority. Consumption of a healthy diet is likely to be important in this regard(Reference Stephen, Barbera and Peters10).
Healthy dietary patterns & dementia prevention
The human diet is complex and contains a multitude of bioactive nutrients which can have additive, synergistic or antagonistic effects in the body. Studying the impact of overall dietary patterns is therefore important because it provides insight into how the multiple different components of the human diet collectively impact health(Reference Stevenson, Shannon and Minihane11). Whilst some research has suggested that intake of individual foods (e.g. fish, leafy vegetables or berries) or dietary compounds (e.g. n-3 fatty acids, inorganic nitrate, polyphenols) could impact dementia risk and brain health(Reference Stevenson, Shannon and Minihane11–Reference Moore, Hughes and Ward14), the strongest and most consistent benefits have been reported with consumption of healthy dietary patterns(Reference Townsend, Fairley and Gregory15). In this section, we critically evaluate current research exploring the impact of healthy dietary patterns on cognitive function and dementia risk.
Mediterranean diet
The Mediterranean dietary pattern is widely considered to be the leading dementia prevention diet. It is recommended for the prevention of cognitive decline/dementia by the World Health Organisation(16), due to a low risk of harm and potential for benefit. It is also recommended in public-facing guidelines for dementia prevention produced by major charities such as Alzheimer’s Research UK(17) and the Alzheimer’s Society(18). This dietary pattern is based around the traditional culinary habits of residents from the Mediterranean basin before globalisation of the food industry(Reference Bach-Faig, Berry and Lairon19). Although there is no universal definition of the Mediterranean dietary pattern(Reference Bach-Faig, Berry and Lairon19–Reference Griffiths, Matu and Whyte21), it is based on staple foods which were produced locally and includes seasonally available vegetables, fruits, pulses, nuts and fish. Extra virgin olive oil is typically used in cooking and dressing food, and a small amount of alcohol (especially red wine) is often consumed alongside meals. Unlike the typical Western diet, the Mediterranean dietary pattern contains limited amounts of red and processed meat, high-fat dairy products such as butter and cream, and sugar-rich foods and drinks(Reference Bach-Faig, Berry and Lairon19,Reference Davis, Bryan and Hodgson20) .
Impact on cognitive function
Feart et al.(Reference Feart, Samieri and Rondeau22) were amongst the first to explore associations between adherence to the Mediterranean diet and cognition. Those authors found that higher adherence to a Mediterranean diet was associated with slower decline in performance on the mini mental state exam (MMSE) – an 11-item test designed to measure cognitive impairment – over a 5-year follow up in 1410 adults from Bordeaux, France. Since then, multiple epidemiological investigations have explored associations between adherence to a Mediterranean diet and cognition. Most studies have reported that higher adherence to a Mediterranean diet is associated with greater global or domain-specific cognitive function, or slower rates of cognitive decline, with effect sizes equivalent to several years of cognitive aging(Reference Jennings, Cunnane and Minihane13,Reference Townsend, Logan and O’Neill23–Reference Siervo, Shannon and Llewellyn25) .
Several randomised controlled trials (RCTs) have also explored whether intervention with a Mediterranean diet impacts cognitive function/decline, with findings broadly aligned with the observational evidence. An analysis of data from the PREDIMED Navarra site suggested that intervention with a Mediterranean diet augmented with high levels of nuts or olive oil for ∼6·5 years, v. a low-fat control group, led to better performance on the MMSE and a Spanish version of the Clock Drawing test(Reference Martínez-Lapiscina, Clavero and Toledo26). Meanwhile, data from the PREDIMED Barcelona site – which included a more comprehensive suite of cognitive measures pre- and post-intervention – showed better cognitive trajectories over ∼4·1 years with the same Mediterranean diet interventions v. control(Reference Valls-Pedret, Sala-Vila and Serra-Mir27). Specifically, memory performance was significantly better in the Mediterranean diet plus nuts arm, whilst frontal and global cognitive performance were significantly better in the Mediterranean diet plus olive oil arm, compared with control(Reference Valls-Pedret, Sala-Vila and Serra-Mir27). Several other studies, including modified Mediterranean diets, have also shown cognitive benefits. This includes the ‘MedDairy’(Reference Wade, Davis and Dyer28) (a Mediterranean diet intervention with 3–4 servings/day of diary products) and ‘MedPork’(Reference Wade, Davis and Dyer29) (a Mediterranean diet intervention with 2–3 servings/week of pork) studies from Australia, which demonstrated greater processing speed and improved mood with the Mediterranean diet interventions v. control. Similarly, recent data from the DIRECT PLUS study, conducted in Israel, reported benefits of both a Mediterranean diet and a green Mediterranean diet (augmented with green tea and a green plant-based drink) on neuroimaging parameters v. a control diet, with the greatest benefits observed in the green Mediterranean diet group(Reference Kaplan, Zelicha and Yaskolka Meir30).
The recent MedEx-UK study(Reference Jennings, Shannon and Gillings31), conducted across 3 geographically distinct sites in the UK, showed that intervention with a Mediterranean diet alone, or in combination with increased physical activity, for 6 months resulted in better global cognitive performance and better memory v. a usual care control group. The intervention also improved vascular stiffness and pulse pressure variability in the combined Mediterranean diet and physical activity group, suggesting that the cognitive benefits could be, at least in part, underpinned by improvements in cerebrovascular health.
Despite these promising findings, it is important to note that not all RCTs have reported beneficial effects of a Mediterranean diet on cognition/brain health. For example, neither the MedLey study(Reference Knight, Bryan and Wilson32) (6 month intervention in 137 older Australian adults) nor the much larger NU-AGE study (1 year intervention in 1279 older adults across Europe) reported cognitive benefits of a Mediterranean diet v. control. These results suggest that a Mediterranean diet can, but does not always, lead to tangible cognitive benefits. Potential reasons which could account for between-study differences in findings are explored later.
Impact on dementia incidence
Scarmeas and colleagues were the first to explore associations between Mediterranean diet adherence and Alzheimer’s disease incidence in 2006(Reference Scarmeas, Stern and Tang33). They followed 2258 participants from the Washington Heights-Inwood Columbia Aging Project (WHICAP) cohort in New York for an average of 4 years (range 0·2–13·9 years). During this time, 262 individuals developed Alzheimer’s disease. Compared with participants with the lowest level of Mediterranean diet adherence (tertile 1), individuals with moderate (tertile 2) and higher (tertile 3) adherence to this dietary pattern had 15 % and 40 % lower risk of Alzheimer’s disease, respectively. A later study by the same researchers using data from two cohorts recruited through the WHICAP project (1880 adults followed for an average of 5·4 years), demonstrated that both higher Mediterranean diet adherence (40 % lower risk in highest v. lowest tertile) and greater physical activity (33 % lower risk in highest v. lowest tertile) were associated with lower Alzheimer’s disease risk(Reference Scarmeas, Luchsinger and Schupf34). Participants who had both high Mediterranean diet adherence and high physical activity showed 35 % lower Alzheimer’s disease risk compared with individuals with low levels of both.
Multiple other studies have subsequently emerged exploring associations between Mediterranean diet adherence and either all-cause dementia or Alzheimer’s disease(Reference Fu, Tan and Lee24,Reference Nucci, Sommariva and Degoni35) . Most have been characterised by relatively small sample sizes (e.g. 1000–6000 volunteers) with limited dementia cases (e.g. 20–400 all-cause dementia cases), although several studies leveraging large-scale population cohorts have begun to emerge. A large-scale study by Glans et al.(Reference Glans, Sonestedt and Nägga36) reported no significant associations between Mediterranean diet adherence and either all-cause dementia or specific dementia sub-types in 28 025 Swedish participants (including 1943 dementia cases) over a 19·8-year median follow up. In contrast, in an analysis of data from 60 298 participants from the UK Biobank (including 882 dementia cases), our group observed 23 % lower risk of incident all-cause dementia with the highest (v. lowest) tertile of Mediterranean diet adherence(Reference Shannon, Ranson and Gregory37). Importantly, in that study, there was no significant interaction between Mediterranean diet adherence and polygenic risk for dementia, suggesting that following a Mediterranean diet could lower dementia risk irrespective of genetic vulnerability for this condition.
The overall associations between Mediterranean diet adherence and dementia incidence have been explored in various recent meta-analyses. For example, in a meta-analysis of data from 13 cohort, 6 cross-sectional and 1 nested case-control studies by Nucci et al.(Reference Nucci, Sommariva and Degoni35), higher adherence to a Mediterranean diet was associated with 11 % lower risk of all-cause dementia and 27 % lower risk of Alzheimer’s disease. Meanwhile, in a meta-analysis of 26 prospective cohort studies by Fu et al.(Reference Fu, Tan and Lee24) higher (v. lower) adherence to a Mediterranean diet was associated with 15 % lower risk of MCI and 19 % lower risk of Alzheimer’s disease.
There are currently no RCTs on the effects of a Mediterranean diet on dementia incidence, which is likely due to the logistical (e.g. required study length, number of participants) and financial challenges of conducting such a study.
Ambiguity in the literature
As noted previously, most, but not all, previous investigations have observed beneficial effects of a Mediterranean diet on cognition/dementia risk(Reference Fu, Tan and Lee24,Reference Nucci, Sommariva and Degoni35) . Discordance in the findings between studies could be related to a range of methodological differences, such as the study setting/geographical region, participant cohort (e.g. age, health status, participant education and broader lifestyle characteristics), cognitive test battery employed (e.g. sensitivity of tests, cognitive domains evaluated), duration of study follow up (between dietary assessment and cognitive testing), methods used for assessing dietary intake (e.g. food frequency questionnaire, 24-hour dietary recall) and methodology for quantifying adherence to the Mediterranean diet. Below, we explore three specific factors in detail for which there is reasonable evidence to suggest they could contribute towards this between-study discordance in findings.
Geographical region
Data from a systematic review by Aridi et al.(Reference Aridi, Walker and Wright38) suggests that the Mediterranean diet may be more effective at improving cognitive/brain health in Mediterranean compared with non-Mediterranean regions. Specifically, those authors found beneficial associations between Mediterranean diet adherence and cognitive health in ∼80 % of cohort studies conducted in the Mediterranean region, but only ∼50 % of studies conducted in non-Mediterranean regions. These results are also supported by recent analyses of a pan-European cohort by Gregory et al.(Reference Gregory, Ritchie and Ritchie39), in which dietary and cognitive methods were standardised across study centres. Those authors found that the beneficial associations between Mediterranean diet adherence and cognition were stronger and more consistently observed in Mediterranean v. non-Mediterranean countries(Reference Gregory, Ritchie and Ritchie39). Potential explanations include differences between participants from Mediterranean and non-Mediterranean countries in: (1) the composition or duration of adherence to this dietary pattern (e.g. lifelong v. recent adoption), (2) the absolute level of adherence achieved (with Mediterranean diet scores often several points higher in participants from Mediterranean countries), and (3) participation in other brain healthy lifestyle practices (e.g. being physically active and having large group meals which encourage socialisation) which may be more common in Mediterranean countries. Despite this, many studies have reported cognitive benefits associated with/consequent to consumption of a Mediterranean diet in non-Mediterranean regions (including the UK) with effect sizes that are likely to be clinically and practically meaningful (e.g.(Reference Shannon, Ranson and Gregory37,Reference Shannon, Stephan and Granic40) ).
Mediterranean diet scoring methodology
A range of different tools have been used to define level of adherence to the Mediterranean diet (for review, see Siervo et al.(Reference Siervo, Shannon and Llewellyn25)), and participants can achieve different Mediterranean diet adherence scores depending upon which tool is used. In our previous work using data from the EPIC-Norfolk cohort, we demonstrated that the strength of association between Mediterranean diet adherence and cognition varied depending upon the scoring tool used(Reference Shannon, Stephan and Granic40). Similarly, data from a systematic review by Townsend suggested that beneficial associations between Mediterranean diet adherence and cognitive outcomes were observed more commonly when the Panagiotakos et al.(Reference Panagiotakos, Pitsavos and Stefanadis41) score rather than the Trichopoulou et al.(Reference Trichopoulou, Costacou and Bamia42) score was used for defining level of Mediterranean diet adherence. One possible reason for this may be that the Panagiotakos et al.(Reference Panagiotakos, Pitsavos and Stefanadis41) methodology assigns points ranging from 0–5 for intake of a range of different foods. As such, it can distinguish between small but potentially meaningful differences in intake(Reference Panagiotakos, Pitsavos and Stefanadis41). Conversely, the Trichopoulou et al. score assigns points of 0 or 1 based on intakes above/ below cohort-specific medians, which may be less sensitive at separating those with better/worse diets(Reference Trichopoulou, Costacou and Bamia42). The choice of tool used to measure Mediterranean diet adherence may be particularly relevant when conducting research in non-Mediterranean countries. Here, the dietary targets for achieving points, which are often based on norms in Mediterranean countries, may be unachievable for many individuals in non-Mediterranean countries. On the contrary, tools which award points proportional to the level of intake of a food (such as the Panagiotakos et al.(Reference Panagiotakos, Pitsavos and Stefanadis41) score noted above) may help detect small but potentially meaningful differences in level of adherence to the Mediterranean diet.
Participant cohort
There is evidence to suggest that ‘at risk’ population groups, especially those with elevated CVD risk, may be more responsive to a Mediterranean diet. For example, in our analyses of the EPIC-Norfolk cohort, higher Mediterranean diet adherence was associated with lower risk of poor cognitive performance only in participants with an elevated cardiovascular risk (QRISK2) score(Reference Shannon, Stephan and Granic40). Similarly, RCTs reporting beneficial effects of a Mediterranean diet on cognition (e.g. PREDIMED, MedPork, MedDairy, DIRECT PLUS, MedEx-UK) have typically recruited participants with cardiometabolic co-morbidities (e.g. elevated blood pressure (BP), higher QRISK2 scores, abdominal obesity, dyslipidaemia). In contrast, studies such as MedLey(Reference Knight, Bryan and Wilson32) and NU-AGE(Reference Marseglia, Xu and Fratiglioni43), which found no effects of a Mediterranean diet on cognition, recruited relatively healthy participants.
It is less clear whether genetic risk (e.g. APOE4 genotype or polygenic risk) modifies associations between Mediterranean diet adherence and cognition/dementia risk(Reference Shannon, Ranson and Gregory37,Reference Shannon, Stephan and Granic40,Reference Fote, Geller and Reyes-Ortiz44) , and more research is needed on this topic.
Summary
Overall, the findings from observational studies and RCTs suggest that consumption of a Mediterranean diet could have beneficial effects on later life cognition and dementia risk, which might be particularly pronounced in certain population sub-groups (e.g. those with elevated cardiovascular risk profiles and individuals residing within the Mediterranean basin). However, these effects are not observed in all studies. Therefore, it will be important to establish the specific individuals for/circumstances under which this dietary pattern is beneficial. Such information would be important when designing for targeted or tailored/personalised dietary interventions to improve population health.
MIND diet
The MIND diet was developed to support brain health following extensive literature reviews by scientists at the Rush Institute in Chicago, and includes features from the Mediterranean and DASH diets alongside other potentially neuroprotective foods(Reference Morris, Tangney and Wang45). The MIND diet scoring system awards points for higher intakes of 9 foods which are believed to be brain healthy (wholegrains, green leafy vegetables, other vegetables, nuts, beans, berries, poultry, fish, and olive oil) and lower intakes of 5 foods believed to be unhealthy (fried food, pastries/sweets, red meat, cheese and butter/margarine). Wine was also originally included as one of the brain-healthy dietary components in the MIND diet, but has been omitted in some recent studies, including a large-scale trial of the MIND diet(Reference Barnes, Dhana and Liu46), due to potential health risks associated with alcohol consumption. A unique feature of the MIND diet is that it recommends high intake of green leafy vegetables and berries, which have particularly strong links with cognitive function, whereas Mediterranean diet scores typically award points for overall intake of fruits and vegetables. As this dietary pattern was devised < 10 years ago, fewer studies have explored associations between MIND diet adherence and cognition or dementia risk compared with the Mediterranean diet(Reference Townsend, Logan and O’Neill23)
Morris and colleagues were the first to explore associations between MIND diet adherence and cognition(Reference Morris, Tangney and Wang45). Global cognition and five cognitive sub-domains showed slower rates of decline over a ∼5 year follow up in 960 participants from the Rush Memory and Ageing Project with higher v. lower adherence to the MIND diet. Compared with the low adherence reference group, participants with the highest adherence to the MIND diet showed cognitive function that was equivalent to being 7·5 years younger. The same group also demonstrated 35 % and 53 % lower risk of Alzheimer’s disease with moderate and higher adherence to the MIND diet v. a low adherence reference group in 923 older adults followed for 4·5 years(Reference Morris, Tangney and Wang47). These findings have been substantiated in multiple other observational studies, with a recent systematic review by van Soest et al.(Reference van Soest, Beers and van de Rest48) identifying beneficial effects of a MIND diet on dementia risk, global cognition and episodic memory in 7/10, 3/4 and 4/6 cohort studies, respectively. However, effects on the rate of cognitive decline were less apparent. Interestingly, two comparative studies have suggested that the MIND diet may be more protective against cognitive impairment(Reference Hosking, Eramudugolla and Cherbuin49) and Alzheimer’s disease(Reference Morris, Tangney and Wang47) than the Mediterranean diet. However, this has not been seen in other studies contrasting these two healthy dietary patterns(Reference Seago, Davy and Davy50,Reference Zhang, Cao and Li51) .
Despite largely positive data from observational studies, evidence from trials of the MIND diet is limited, with the two available studies both focusing on energy-restricted MIND diet interventions. Energy-restricted diets may be advantageous because obesity makes a substantial contribution to the population attributable fraction of risk for dementia(Reference Stephan, Cochrane and Kafadar52). Data from a small (n 37) RCT(Reference Arjmand, Abbas-Zadeh and Eftekhari53) conducted in Iran reported benefits of a 3-month energy-restricted MIND diet v. an energy-restricted control on working memory, verbal recognition, attention and a limited number of neuroimaging outcomes (e.g. an increase in grey matter volume in the inferior frontal gyrus). By contrast, a 3-year well powered trial involving 604 older adults contrasting an energy-restricted MIND diet with modest energy restriction alone (control) reported no differences between conditions in effects on cognitive function or MRI-derived neuroimaging markers of brain health(Reference Barnes, Dhana and Liu46). The lack of between-group differences in this study could be related to several factors including (1) potential improvements in diet in the control group who were instructed to restrict their energy intake during the intervention, (2) effect of the MIND diet was undetectable in the presence of energy restriction, and (3) the well-educated participant cohort in whom it may be harder to detect between group differences in cognitive change. It is also possible that a longer period of adherence to the MIND diet may be needed to consistently improve cognitive performance. Additional trials of the MIND diet are clearly needed before firm conclusions can be drawn about the cognitive benefits of this dietary pattern.
Nordic dietary pattern
The Nordic diet is based around foods traditionally consumed in the Nordic region and includes a high intake of root and green leafy vegetables, legumes, fruit and berries, fish, poultry, and wholegrains such as rye, barley, and oats. Rapeseed oil, rich in poly- and mono-unsaturated fatty acids and containing several compounds (e.g. vitamin E and flavonoids) with potential health-promoting properties(Reference Shen, Liu and Wang54), is recommended as the principal cooking fat. Meanwhile, intake of red and processed meat, sweets/confectionary and alcohol is typically low.
Although there is currently limited research focusing on this dietary pattern, data from a prospective cohort study of 2223 adults suggests that higher adherence to the Nordic diet could slow decline in MSSE performance over a ∼6 year follow up(Reference Shakersain, Rizzuto and Larsson55). Meanwhile, another study of 2290 older adults demonstrated increased dementia- and disability-free survival with higher adherence to the Nordic diet(Reference Wu, Shang and Dove56). A similar dietary pattern (following the Nordic Nutrition Recommendations) was also used as the basis for the dietary intervention in the large-scale Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) study. This study, which included 1260 older adults at risk of dementia, showed better cognitive trajectories over a 2 year period with a multi-domain lifestyle intervention(Reference Ngandu, Lehtisalo and Solomon57). Dietary improvement resulted in significantly better executive function over the course of the intervention(Reference Lehtisalo, Levälahti and Lindström58).
Eatwell Guide
The Eatwell Guide is the UK’s healthy eating model, which is designed to help members of the public in the UK achieve a healthy, balanced diet. The Eatwell Guide recommends consumption of a variety of different fruits and vegetables, basing meals on starchy carbohydrates (particularly wholegrains), consuming some beans, pulses, fish and lean meat as protein sources, and consuming a small amount of dairy or dairy alternatives each week. It also recommends using small amounts of unsaturated oils for cooking, and limiting intake of red and processed meat alongside foods that are high in fat, salt and sugar, such as sweets, cakes, crisps and biscuits. There is moderate correlation (r = 0·3–0·4) between Eatwell Guide adherence scores and Mediterranean diet scores(Reference Gregory, Griffiths and Jennings59). Similarities between the two dietary patterns include recommendations to consume primarily plant-based foods and limit intake of red/processed meat and high-fat/sugar/salt foods. Some dissimilarities include the absence of specific recommendations around the intake of typical Mediterranean foods such as olive oil, red wine and sofrito (a tomato-based sauce) in the Eatwell Guide.
As the Eatwell Guide is used as the basis for dietary recommendations in clinical practice and policy in the UK, it is important to understand whether consuming a diet based on the Eatwell Guide is linked to better brain health. Only one small study by our group has been carried out on this topic to date(Reference Gregory, Griffiths and Jennings59). We explored associations between adherence to the Eatwell guide and cognition, neuroimaging and cardiometabolic health markers using cross-sectional data from the UK-based PREVENT Dementia cohort(Reference Gregory, Griffiths and Jennings59). In 517 middle aged (40–59 years) participants, there were no significant associations between adherence to the Eatwell guide and cognitive performance assessed using the Four Mountains Task (a computer-based task which measures allocentric processing) or MRI-derived measures of hippocampal volume or thickness, total intra-cranial volume or white matter hyperintensity volume (an indicator of small vessel disease). The lack of significant associations in this study may be explained by the relatively small size of the cohort (that limits power to detect associations) or by the mid-life age of the cohort, in which it may be difficult to detect associations between diet and brain health markers that may be more apparent in older participants. Interestingly, there were significant associations between higher Eatwell Guide adherence and lower systolic and diastolic BP and BMI, which are known mid-life risk factors for later life cognitive decline and dementia(Reference Livingston, Huntley and Liu4). Therefore, it is possible that longer-term adherence to the Eatwell guide could translate into better cognition or lower dementia incidence in later life. This requires further investigation.
Mechanisms of action
Consumption of a healthy dietary pattern could result in better cognitive function and/or reduced dementia risk through a range of systemic and brain-specific mechanisms, which are reviewed below. There is a lack of high-quality mechanistic data for individual dietary patterns. However, it is likely that healthy dietary patterns work through similar mechanistic pathways given broad overlap between their components (Table 1). Therefore, here we review potential mechanisms for health dietary patterns as a whole, rather than exploring mechanisms for specific dietary patterns individually (Figure 1). As more data emerges, it may be possible to isolate mechanisms specific to, or more pronounced with, different dietary patterns.
Table 1. Key components of the Mediterranean, MIND, Nordic and Eatwell guide dietary patterns
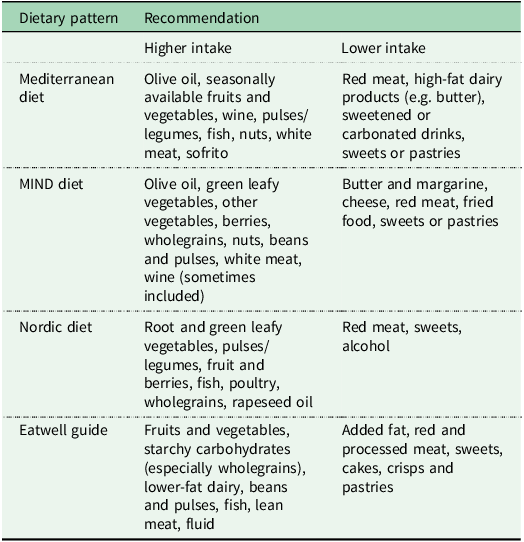
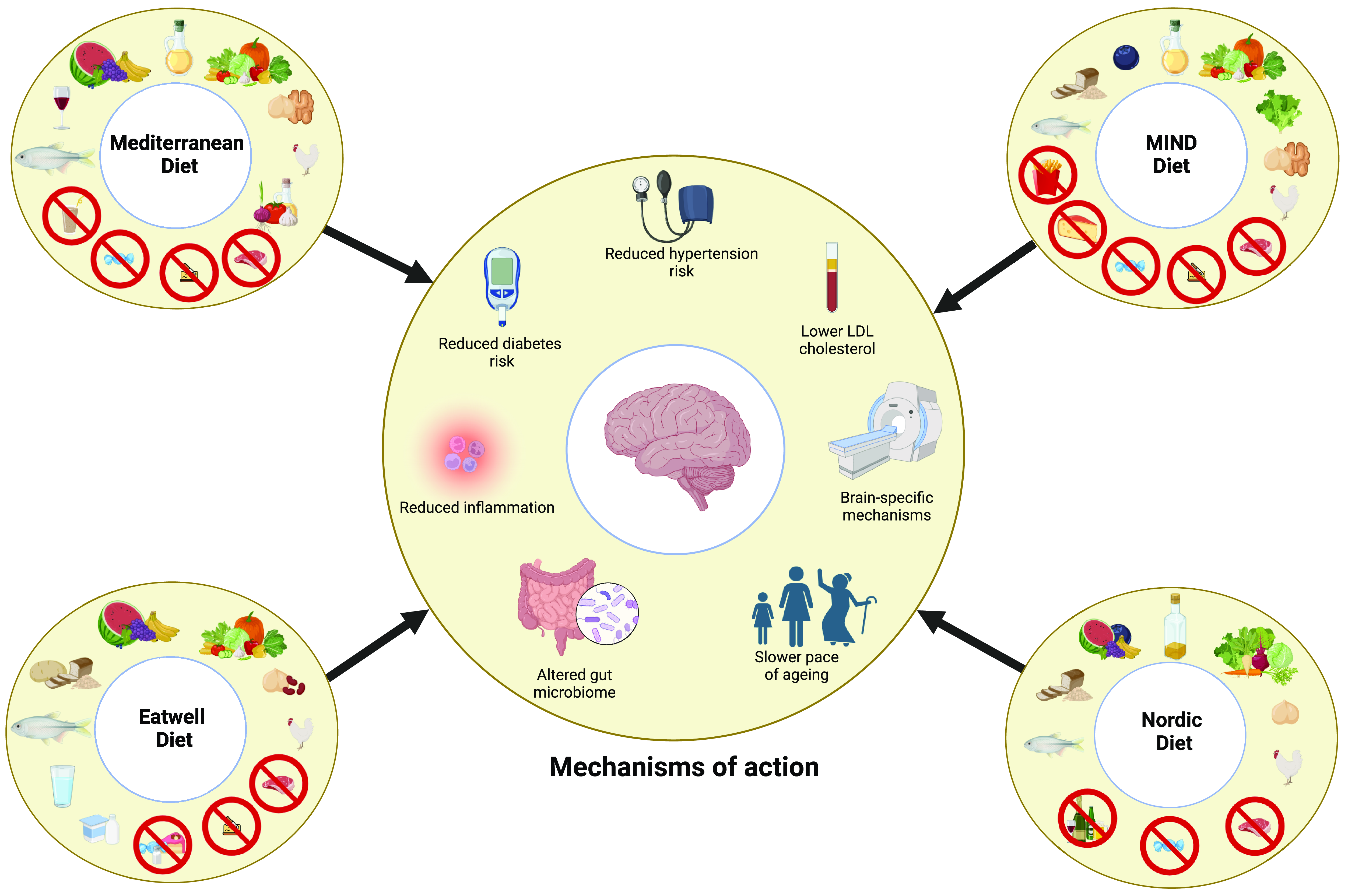
Figure 1. Key components of the Mediterranean, MIND, Nordic and Eatwell dietary patterns and potential mechanisms through which they may lower dementia risk.
Cardiometabolic health
The most recent Lancet Commission Report on Dementia Prevention identified several vascular and metabolic risk factors for dementia. This includes hypertension, obesity, diabetes and high cholesterol(Reference Livingston, Huntley and Liu4), all of which could be targeted through consumption of healthy dietary patterns.
Hypertension affects over 1 billion individuals worldwide and is often undetected due to limited symptoms in most people(Reference Cherfan, Blacher and Asmar60). It is one of the leading risk factor for dementia with a recent meta-analysis estimating the weighted population-attributable fraction for hypertension and dementia to be 7·4 %(Reference Stephan, Cochrane and Kafadar52). This could be related to a greater white matter hyperintensities, smaller brain volumes and altered functional connectivity with hypertension(Reference Lane, Barnes and Nicholas61,Reference Carnevale, Maffei and Landolfi62) . Meanwhile, BP reduction has been shown to significantly lower risk of MCI and combined MCI or probable dementia(Reference Williamson and Pajewski63). Healthy dietary patterns have consistently been associated with modest BP reduction (e.g. 1–5 mmHg), with greater effects emerging with longer duration intervention and in those with elevated baseline BP(Reference Cowell, Mistry and Deighton64,Reference Tomé-Carneiro and Visioli65) . In addition, healthy dietary patterns have been linked with other beneficial cardiovascular changes, including improvements in endothelial function(Reference Shannon, Mendes and Köchl66). This could contribute further towards lower risk of dementia, especially vascular dementia and Alzheimer’s disease, both of which have strong vascular components(Reference Fang, Hsieh and Hu67). The BP-lowering effects of healthy dietary patterns have been linked to the typically high levels of fruit and vegetables, pulses, wholegrains and fish – sources of nutrients with anti-hypertensive properties such as fibre, inorganic nitrate, potassium, magnesium and n-3 fatty acids – alongside the typically low levels of sodium(Reference Cowell, Mistry and Deighton64,Reference Shannon, Stephan and Minihane68,Reference Shannon, Gregory and Siervo69) .
Type 2 diabetes is an established risk factor for cognitive decline and dementia, with risk increasing in accordance with the duration and severity of diabetes(Reference Livingston, Huntley and Liu4). A meta-analysis of 14 studies including > 2 million participants suggested that type 2 diabetes increases all-cause dementia risk by 60 %(Reference Chatterjee, Peters and Woodward70). Imaging studies suggest that diabetes alters regional blood flow (including both hypo- and hyper-perfusion of different brain regions), increases the risk of vascular brain pathology, disturbs brain glucose metabolism, alters grey matter volume and impairs functional networks in the brain(Reference Meng, Liu and Li71,Reference van Harten, de Leeuw and Weinstein72) . Meanwhile, consumption of a healthy dietary pattern has been shown to reduce diabetes risk in a dose-dependent manner in observational studies(Reference Zeraattalab-Motlagh, Jayedi and Shab-Bidar73). Similarly, data from the PREDIMED Rues cohort showed 52 % lower type 2 diabetes incidence over a ∼4 year period with a Mediterranean diet intervention(Reference Salas-Salvadó, Bulló and Babio74). Interestingly, a recent study by Lotan et al.(Reference Lotan, Ravona-Springer and Shakked75) found that greater adherence to a Mediterranean diet was associated with better cognitive trajectories in individuals with type 2 diabetes. Collectively, these data suggest that consumption of a healthy dietary pattern could improve cognition and/or lower dementia risk both by preventing type 2 diabetes and by attenuating the effects of diabetes on the brain in individuals living with this condition.
Both the Mediterranean and MIND diets, even when individuals are allowed to eat ad libitum/ without deliberate energy intake restriction, have been associated with lower risk of obesity and more favourable blood lipid profiles(Reference Fateh, Muhammad and Kamari76–Reference Lotfi, Saneei and Hajhashemy79) – both established risk factors for dementia(Reference Stephan, Cochrane and Kafadar52). This could be related to satiety-inducing effects (e.g. due to high intake of wholegrains, nuts and vegetables), impact on the gut microbiome and lower intake of energy-dense ‘convenience’ foods(Reference Lotfi, Saneei and Hajhashemy79). Similar features of these diets, alongside the high intake of unsaturated lipids through e.g. olive oil and oily fish, could also contribute towards the beneficial impacts on circulating cholesterol concentrations (e.g. lower LDL)(Reference Dinu, Pagliai and Casini80,Reference Martínez-González, Ruiz-Canela and Hruby81) . A recent meta-analysis including data from over 1 million participants suggested that each 1 mmol increase in circulating LDL was associated with an 8 % increased risk of incident all-cause dementia(Reference Wee, Sukudom and Bhat82). Meanwhile, in another meta-analysis, statin use was associated with a 20 % lower risk of all-cause dementia and 32 % lower risk of Alzheimer’s disease(Reference Olmastroni, Molari and De Beni83). The precise mechanisms through which high LDL is linked to dementia risk remains uncertain, but has been proposed to include increased stroke risk (a known dementia risk factor), and increased deposition of amyloid-β and tau in the brain(Reference Wee, Sukudom and Bhat82).
Inflammation
Inflammation – both systemically and specific to the brain (i.e. neuroinflammation) – has been linked to cognitive decline and dementia risk(Reference Tangestani Fard and Stough84). In addition, diets rich in pro-inflammatory nutrients and foods have been shown to elevate markers of inflammation and increase risk of dementia(Reference Griffiths, Matu and Tang12,Reference Shi, Lin and Li85) . For example, in an analysis of data from 166 377 participants from the UK Biobank, Shi et al.(Reference Shi, Lin and Li85) found that for each unit increase in dietary inflammatory index score (which quantifies the inflammatory potential of the diet), the risk of all-cause dementia increased by 4·6 %.In addition, healthy dietary patterns, including the Mediterranean and Nordic diets, have been associated with lower concentrations of circulating inflammatory markers (e.g. c-reactive protein, IL-6 (IL-6) and TNF-alpha (TNF-a))(Reference Shannon, Ashor and Scialo86,Reference Lankinen, Uusitupa and Schwab87) , which have been associated with higher risk of dementia(Reference Metti and Cauley88). Moreover, it has been suggested that the Mediterranean diet could impact the function of microglia – resident immune cells in the brain – promoting a shift from the pro-inflammatory and neurotoxic M1-phenotype to the anti-inflammatory and neuroprotective M2-phenotype(Reference Hornedo-Ortega, Cerezo and De Pablos89).
Gut microbiome
Several lines of evidence suggest that the gut microbiome could play a role in the development of Alzheimer’s disease, and could represent a potential target for prevention/treatment strategies (for review, see(Reference Seo and Holtzman90)). For example, there are notable differences in the gut microbiome of individuals with Alzheimer’s disease, and pre-clinical Alzheimer’s disease, compared with healthy controls(Reference Seo and Holtzman90,Reference Ferreiro, Choi and Ryou91) . Since it is possible that these differences could be due to reverse causality, more convincing evidence on the causal role of the gut microbiome in Alzheimer’s development comes from faecal microbiome transplantation studies. Transplanting the faecal microbiota from wild-type mice to transgenic mice expressing genes associated with Alzheimer’s disease development attenuates the formation of amyloid-β plaques and neurofibrillary tangles in the brain and minimises cognitive impairment(Reference Kim, Kim and Choi92). Recent Mendelian randomisation studies also suggest that the gut microbiome and its metabolites may be causally connected with cognitive performance(Reference Cao, Xing and Liang93). Healthy dietary patterns, particularly the Mediterranean diet, have been shown to impact the composition and metabolism of the gut microbiome, although effects are inconsistent between studies, which may be due to the specific constellation of foods included in this dietary pattern when administered in each study(Reference Kimble, Gouinguenet and Ashor94). There is also limited data on whether the benefits of healthy dietary patterns on cognition may be mediated, at least in part, by changes in the gut microbiome. The NU-AGE study, which involved a 1-year intervention with a Mediterranean-like diet, demonstrated diet-related alterations in the microbiome, with taxa enriched by the diet associated with improvements in cognition(Reference Ghosh, Rampelli and Jeffery95). In contrast, data from the MedDairy study showed modest changes in the abundance of select bacterial taxa with an 8-week Mediterranean diet plus dairy intervention. However, there were no significant associations between the altered taxa and cognitive outcomes(Reference Choo, Murphy and Wade96). Further research on the effects of healthy dietary patterns on the gut microbiome and cognition/dementia risk is needed.
Pace of biological aging
Healthy dietary patterns might also impact cognitive decline/dementia risk by influencing the pace of biological aging. The Mediterranean diet has been proposed to impact at least 9 of the hallmarks of aging(Reference Shannon, Ashor and Scialo86) – that is, key determinants of the aging trajectory including genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. This could contribute towards lower risk of age-related diseases. Moreover, a recent study found that a slower overall pace of aging (quantified using the DunedinPACE epigenetic clock) accounted for 27 % of the effect of a MIND diet and 22 % of the effect of the Mediterranean diet on risk of dementia(Reference Thomas, Ryan and Caspi97).
Brain-specific mechanisms of action
Consumption of healthy dietary patterns may have direct effects on the brain, which are detectable using neuroimaging or post-mortem techniques and could contribute to better cognition and/or lower dementia risk. For example, higher adherence to the Mediterranean and MIND diets, and several of their component foods, has been associated with lower Alzheimer’s disease pathology, including lower amyloid-β load in the brain(Reference Agarwal, Leurgans and Agrawal98–Reference Hill, Goodwill and Gorelik101). Whether this is due to reduced amyloid-β production and deposition, greater clearance, or a combination of both remains unclear. Both the Mediterranean and MIND diets have been associated, albeit inconsistently, with reduced brain atrophy and preserved cerebral microstructure(Reference Barnes, Dhana and Liu46,Reference Gregory, Pullen and Ritchie102–Reference Melo van Lent, O’Donnell and Beiser106) . Using data from post-mortem prefrontal cortex tissue, Li et al.(Reference Li, Capuano and Agarwal107) identified a transcriptomic profile, comprising 50 genes, which was correlated with level of adherence to the MIND diet. In further analyses, they found that this brain transcriptomic profile was associated with slower rates of cognitive decline and a lower risk of dementia. Higher Mediterranean diet adherence has been linked with improved cerebral bioenergetics, including greater brain glucose metabolism(Reference Matthews, Davies and Murray108), alongside greater structural connectivity(Reference Ruiz-Rizzo, Finke and Damoiseaux109,Reference Pelletier, Barul and Féart110) . The Mediterranean diet also elevates circulating levels of brain-derived neurotrophic factor (BDNF), which plays a key role in synaptic plasticity, neurogenesis, gene transcription regulation and intracellular signalling cascade activation(Reference Xue, Waseem and Zhu111,Reference Sánchez-Villegas, Galbete and Martinez-González112) . This could contribute towards the beneficial effects on brain health markers(Reference Xue, Waseem and Zhu111).
Future directions
The limitations of the existing body of evidence, and the potential to harness new research methodologies, create multiple opportunities for future impactful research in this area. These include:
Additional large-scale, longer-duration interventions
At present, there are few intervention studies exploring the impact of healthy dietary patterns on cognitive aging, and none on dementia risk. Most of the current studies have used the Mediterranean diet as the choice healthy eating model and have been relatively short in duration (6–12 months), with some exceptions (e.g.(Reference Martínez-Lapiscina, Clavero and Toledo26,Reference Barnes, Dhana and Liu46) ). They have also typically involved poor representation from minority ethnic communities and individuals from a lower socioeconomic background. As these individuals are disproportionately impacted by dementia(Reference Livingston, Huntley and Liu4), their inclusion in future trials, and the adaptation of trials to ensure the needs of these communities is met, should be a priority. Longer duration (e.g. several years), large-scale (e.g. adequately powered to detect cognitive and/or neuroimaging changes) studies comparing the feasibility, acceptability and efficacy of different dietary patterns on brain health are therefore warranted.
Utilisation of Mendelian randomisation
Whilst financial and logistical reasons mean that the emergence of additional large-scale dementia prevention trials is likely to be slow, Mendelian randomisation provides the opportunity to establish causal links between diet and dementia incidence and is likely to become increasingly popular. This approach uses genetic variants (e.g. single nucleotide polymorphisms) as instrumental variables to make cause-effect inferences about the link between an exposure (e.g. a dietary component/pattern) and outcome (e.g. dementia incidence). Mendelian randomisation studies are already beginning to shed new light on diet-dementia links. For example, a recent finding from Mendelian randomisation studies is that higher intake of alcohol is linearly associated with dementia risk amongst current drinkers(Reference Zheng, Liao and Luo113). This challenges the traditional perspective from observational research, which has posited a ‘J’ shaped relationship between alcohol intake and dementia risk. The findings suggest that there is no safe level of alcohol intake for dementia risk. The growing use of polygenic scores, which reflect overall diet quality as genetic proxies for dietary patterns, will allow causal evidence linking dietary patterns and dementia to be established.
Identification of the optimal window for intervention
At present, there is little agreement on the optimal window for intervening to lower dementia risk. Most interventions have focused on intervening in non-symptomatic individuals in mid-to-later life, although some studies (e.g. LipiDiDiet Trial(Reference Hendrix, Soininen and Solomon114)) have shown benefits of dietary change in older individuals with prodromal Alzheimer’s disease. It is possible that the optimal time window for intervention might also differ between different individuals (e.g. depending upon sex, ethnicity, wider lifestyle factors or genetic predisposition). Recently, there has been a call to shift focus towards earlier adulthood, a time in which lifelong habits may be established and where many dementia risk factors (e.g. diabetes, overweight/obesity, high alcohol intake, smoking, high BP) are already present(Reference Farina, Bridgeman and Gregory115). Identifying the optimal (and most feasible) time(s) to intervene is an important priority for future studies.
Utilising state-of-the-art biomarkers
Blood-based biomarkers such as plasma amyloid-β 42/40 ratio, phosphorylated tau species (e.g. p-tau181 or p-tau217) and neurofilament light chain (NfL) are emerging as promising markers of Alzheimer’s disease risk(Reference Schöll, Verberk and Del Campo116). These biomarkers are viewed as accessible, cost-effective, and highly promising tools which could be used to (a) identify ‘at risk’ groups for trial enrolment, and (b) serve as a surrogate outcome in prevention trials. Their use is likely to become increasingly common in future years. However, various logistical challenges (e.g. establishing suitable cutoffs for different populations, confounding of levels by other health conditions, standardisation of analytic approaches)(Reference Schöll, Verberk and Del Campo116) will need to be overcome before the full potential of these biomarkers can be realised.
Expanding evidence to non-Alzheimer’s dementia
Much of the (observational) research currently available on dietary patterns and dementia risk focuses on either all-cause dementia or Alzheimer’s disease. There are comparatively fewer studies exploring the impact of dietary patterns on vascular dementia – the second most common cause of dementia – and other dementia sub-types(Reference Griffiths, Matu and Tang12). Indeed, a recent systematic review by Griffiths et al.(Reference Griffiths, Matu and Tang12) identified 16 studies focusing on foods or dietary patterns and vascular dementia risk, with only 1–2 studies (in some occasions with contradictory findings) for most dietary exposures. More research on vascular dementia and other dementias is therefore crucial to inform personalised and population-based dementia prevention initiatives.
Diet-drug interactions
As new pharmacological treatments for Alzheimer’s disease (e.g. anti-amyloid immunotherapies) begin to emerge, it is important to understand whether the efficacy and (often severe) side effect profile of these drugs can be mitigated by lifestyle factors, including diet. There is growing evidence that dietary factors, including healthy dietary patterns, have the potential to modulate the effectiveness of anti-cancer immunotherapies(Reference Golonko, Pienkowski and Swislocka117), which provides tentative proof of concept for exploring the interaction between diet and anti-amyloid immunotherapies (and other drugs) in the future.
Conclusion
Current evidence suggests that healthy dietary patterns, especially the Mediterranean diet, represent a potential strategy to improve cognitive trajectories and lower dementia risk in populations, so minimising the enormous economic and human cost of this condition. Additional research, particularly large-scale longer-duration randomised controlled trials, is now needed to establish which dietary approaches are most effective for dementia prevention, their mechanisms of action, when they should be implemented, and whether/how they require tailoring to maximise uptake and efficacy in different population groups.
Acknowledgments
N/A.
Financial support
O.M.S. has received research funding from EPSRC, BBSRC, Rank Prize, MRC, Wellcome Trust, NIHR, OHID, ARUK, the Fruit Juice Science Centre and the Nutrition Society and has consulted for Delta Hat Ltd. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
The authors declare none.
Authorship
O.M.S. conceived this review and led the writing of the manuscript. J.C.M., E.S. and M.S. contributed to the writing and critical revision of the manuscript. All authors approved the final version of this manuscript.




