Introduction
The Taeniidae family comprises a diverse group of tapeworms, including the genera Taenia, Versteria, Hydatigera, and Echinococcus. Members of the Taeniidae are exclusively mammalian parasites with complex life cycles that involve definitive hosts, usually carnivores, and intermediate hosts, which are often herbivores or omnivores. Although species with zoonotic potential can be found in all genera, only Taenia and Echinococcus are of considerable medical and veterinary, as well as economic importance (Eckert and Deplazes, Reference Eckert and Deplazes2004; Budke et al., Reference Budke, Deplazes and Torgerson2006; Deplazes et al., Reference Deplazes, Eichenberger and Grimm2019).
The genus Echinococcus is known for causing echinococcosis. This disease manifests primarily in two forms: cystic echinococcosis (CE) and alveolar echinococcosis (AE), both caused by the space-occupying larval forms (metacestodes) in internal organs, which can lead to severe morbidity and even mortality in humans (Kern et al., Reference Kern, Menezes da Silva, Akhan, Müllhaupt, Vizcaychipi, Budke and Vuitton2017). A third form, which is almost negligible in comparison, is the neotropical echinococcosis, and only occurs occasionally in Central and South America (Moro and Schantz, Reference Moro and Schantz2009; Deplazes et al., Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson and Jenkins2017).
Echinococcosis is classified by the World Health Organization as a neglected zoonotic tropical disease and can be acquired by intermediate hosts, including humans, through accidental ingestion of infectious eggs via contaminated food, water or direct contact with the definitive host (Eckert and Deplazes, Reference Eckert and Deplazes2004; World Health Organization, 2024).
CE has a worldwide distribution and can be caused by species of E. granulosus sensu lato (s.l.). The complex comprises five species: E. granulosus sensu stricto (s.s.), E. equinus, E. ortleppi, E. canadensis, and E. felidis. Apart from the latter, all have zoonotic potential – albeit to varying degrees. Echinococcus granulosus s.s. has the broadest spectrum of intermediate hosts and is also responsible for 88% of human infections worldwide (Alvarez Rojas et al., Reference Alvarez Rojas, Romig and Lightowlers2014; Romig et al., Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017). Echinococcus canadensis has several genotypes (G6/7, G8, G10), among which G6/7 is primarily responsible for infections in humans (Alvarez Rojas et al., Reference Alvarez Rojas, Romig and Lightowlers2014). Echinococcus ortleppi and E. equinus have only rarely been detected in humans and, in the case of E. equinus, also only recently (Alvarez Rojas et al., Reference Alvarez Rojas, Romig and Lightowlers2014; Kim et al., Reference Kim, Yong, Shin, Lee, Park, Suvonkulov, Kovalenko and Yu2020; Macin et al., Reference Macin, Orsten, Samadzade, Colak, Cebeci and Fındık2021). Echinococcus granulosus s.s., E. equinus, E. ortleppi, and E. canadensis G6/7 are predominantly associated with livestock transmission cycles, with sheep, horses, cattle, camels, and pigs serving as their main intermediate hosts and dogs as definitive hosts. In contrast, E. canadensis G8/G10 and E. felidis are primarily maintained in wildlife cycles, involving cervids and wolves, and warthogs and lions, respectively (Romig et al., Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017; Aschenborn et al., Reference Aschenborn, Aschenborn, Beytell, Wachter, Melzheimer, Dumendiak, Rüffler, Mackenstedt, Kern, Romig and Wassermann2023). However, all the Echinococcus species with domestic life cycles were also found in wildlife (Romig and Wassermann, Reference Romig and Wassermann2024).
The causative agent of AE is E. multilocularis, a wildlife parasite primarily of foxes which uses small mammals – mainly rodents – as intermediate hosts. Other canids, such as wolves, jackals, coyotes, or domestic dogs, can also act as definitive hosts (Romig and Wassermann, Reference Romig and Wassermann2024). This species can be found predominantly in the temperate to cold climate zones in the Holarctic (Deplazes et al., Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson and Jenkins2017). However, recent epidemiological studies indicate a notable increase in AE cases in endemic areas and an emergence of this infection, and therefore a spread of the parasite to new regions across the northern hemisphere (Torgerson, Reference Torgerson2013; Moloi et al., Reference Moloi, Tari, Halász, Gallai, Nagy and Csivincsik2023; Khuroo et al., Reference Khuroo, Khuroo and Rather2024). The causes of this expansion are not yet fully understood, but there are indications that a significant proportion is due to anthropogenic factors (Davidson et al., Reference Davidson, Romig, Jenkins, Tryland and Robertson2012).
In Armenia, CE is widespread and has been detected in livestock such as sheep, goats, cattle, and pigs, as well as in humans (Chobanyan, Reference Chobanyan1965, Reference Chobanyan1974; Davidyants, Reference Davidyants1997; Gevorgyan, Reference Gevorgyan2005; Gevorgyan et al., Reference Gevorgyan, Aghayan, Grigoryan, Saghoyan and Romig2016; Khachatryan, Reference Khachatryan2017). Studies on the prevalence in definitive hosts, including stray, service, and shepherd dogs, as well as foxes, in the country are limited and primarily date back to the 1960s and 1970s. Analysis of fecal samples from 923 dogs, collected post-purgation, revealed a varying prevalence of E. granulosus s.l., ranging from 10.8% to 68%, depending on the study region (Chobanyan, Reference Chobanyan1971). The only genetic characterization to date of metacestodes (hydatid cysts) of Armenian origin confirmed infections caused by E. granulosus s.s. in humans, sheep, and cattle, and E. canadensis G6/7 in pigs (Gevorgyan et al., Reference Gevorgyan, Dinkel, Mackenstedt and Romig2006b; Ebi et al., Reference Ebi, Gevorgyan, Aghayan, Wassermann and Romig2017).
In contrast to CE, it has long been assumed that AE is not endemic in Armenia. Echinococcus multilocularis has never been detected in canids, either domestic or wild, nor had its larval stage been found in humans or other aberrant hosts. This assumption was recently proven wrong by a retrospective study that evaluated all liver surgeries from 2008 to 2020 (Manukyan et al., Reference Manukyan, Avetisyan, Sahakyan, Jimenez, Paronyan, Gevorgyan and Vanyan2022). Over the 12-year review period, 11 human AE cases were discovered, of which only 3 were suspected to be an AE infection at the time of surgery. The misdiagnosis suggests an underreporting of the true number of cases. This is probably due to ignorance; many physicians do not consider E. multilocularis as the cause of liver symptoms, as it was not regarded as endemic (Manukyan et al., Reference Manukyan, Avetisyan, Sahakyan, Jimenez, Paronyan, Gevorgyan and Vanyan2022).
Following these discoveries, a review of the older literature on helminths in potential hosts of E. multilocularis was conducted. This search uncovered a reference to the parasite’s presence dating back 60 years. In his PhD thesis, Chobanyan (Reference Chobanyan1968) presents data from a study examining 6,047 rodents across 14 species for various parasites over a 3-year period (1964–1967). Among his findings, he reported ‘Alveococcus multilocularis’ infections in 3 voles of the species Microtus arvalis and M. socialis, both considered significant intermediate hosts. Unfortunately, the total number of these host species examined was not specified. His search for the parasite in definitive hosts remained unsuccessful. Aside from this report, and the recent human AE cases, no other confirmation of the presence of E. multilocularis in Armenia exists. However, there is evidence of E. multilocularis in natural intermediate and definitive hosts in all of Armenia’s neighbouring countries. In eastern Turkey, for example, wild boars have been found infected with E. multilocularis (Kesik et al., Reference Kesik, Kilinc, Celik, Simsek and Ahmed2020). In order to investigate the presence and distribution of E. multilocularis, it is recommended to examine definitive hosts, as even in highly endemic areas only very small numbers of infected voles, which serve as main intermediate hosts, are usually found (Conraths and Deplazes, Reference Conraths and Deplazes2015; Romig and Wassermann, Reference Romig and Wassermann2024).
Apart from the above-mentioned survey of dogs, no other studies have been conducted in the last 50 years on the presence and distribution of E. granulosus s.l. or E. multilocularis in potential definitive hosts in Armenia. In view of this knowledge gap regarding Echinococcus spp. in free-ranging dogs and wild carnivores, the present study was designed as a pilot survey to gain initial insights and provide a basis for further studies.
Materials and methods
Study area
Fecal samples from carnivores were collected in 16 selected areas in six provinces of Armenia between spring 2017 and summer 2018 (Figure 1). The areas were specifically selected due to their high density of wild carnivores and use as grazing grounds by both livestock and wild herbivores. The sampling sites were located along the primary migration corridors of mammalian predators. These corridors were identified in Armenia within the framework of the designation of regional eco-corridors (Zazanashvili et al., Reference Zazanashvili, Sanadiradze, Garforth, Bitsadze, Manvelyan, Askerov, Mousavi, Krever, Shmunk, Kalem and Devranoğlu Tavsel2020). One corridor includes the Akhuryan-Araks River system, Vayots Dzor, and the Zangezur Mountain systems. Another corridor encompasses the Zangezur, Vayots Dzor, Vardenis, and Gegham Mountain systems.
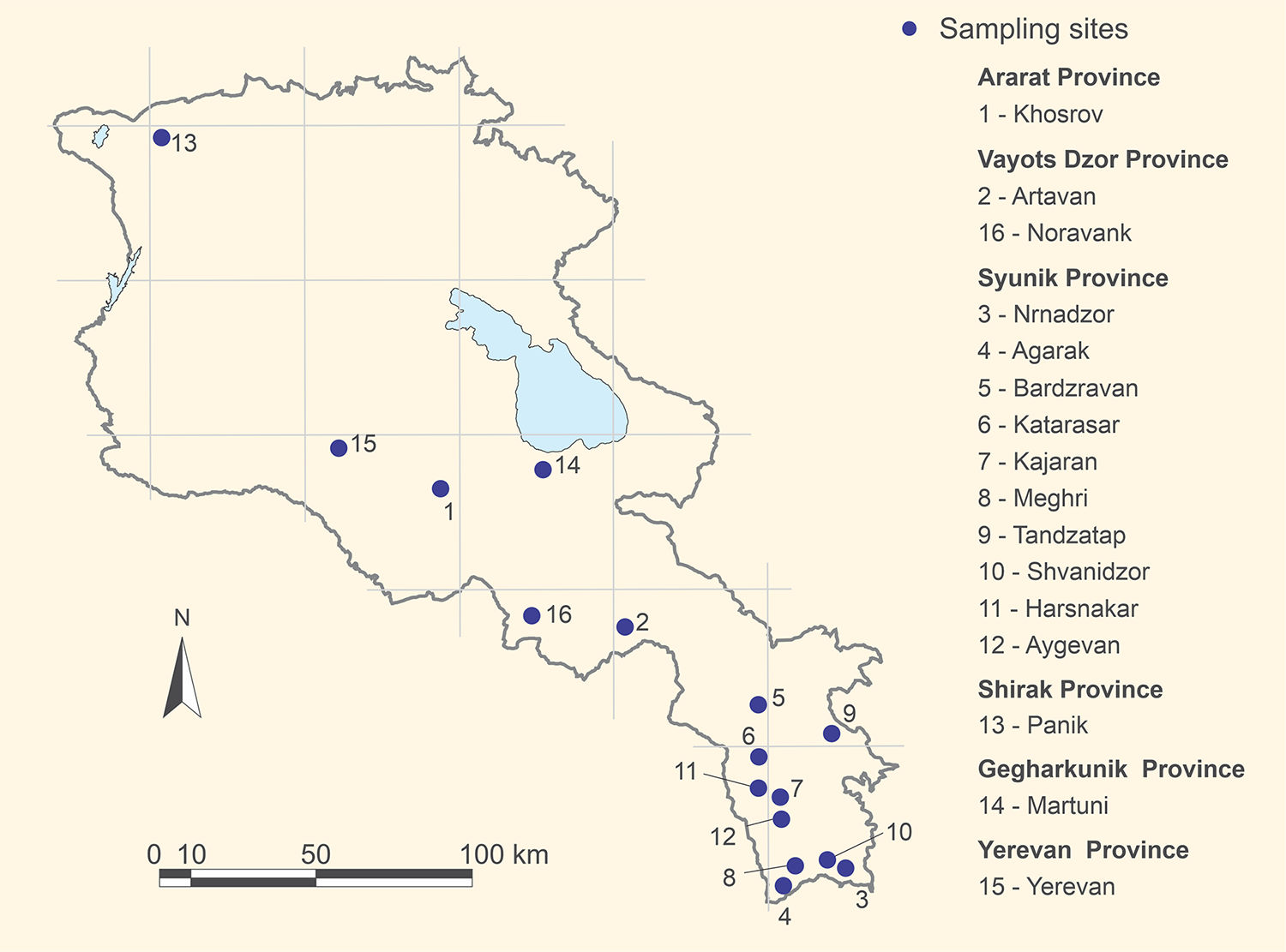
Figure 1. The 16 sampling sites in the 6 provinces of Armenia.
The sampling sites range from 600 to 3900 m above sea level and span five distinct climatic and vegetation zones: semi-desert, juniper woodland, deciduous forest, mountain steppes, and subalpine areas with rocky formations. Most of these sites are situated within national parks and reserves, featuring a rich mammalian fauna that includes large predators such as the leopard (Panthera pardus), lynx (Lynx lynx), brown bear (Ursus arctos), wolf (Canis lupus), red fox (Vulpes vulpes), golden jackal (Canis aureus), and badger (Meles meles), as well as smaller predators such as the stone marten (Martes foina), and their prey species, including the bezoar goat (Capra aegagrus), Armenian mouflon (Ovis gmelini gmelini), roe deer (Capreolus capreolus), wild boar (Sus scrofa), and numerous species of small mammals. In addition to these natural sampling sites, fecal samples were also collected from an urban area, specifically Yerevan, the capital of Armenia. In Yerevan, samples were gathered from the few natural green spaces in the city, such as along the Hrazdan River, which flows through Yerevan. In the urban context, large predators are stray dogs (Canis lupus familiaris).
Sample processing
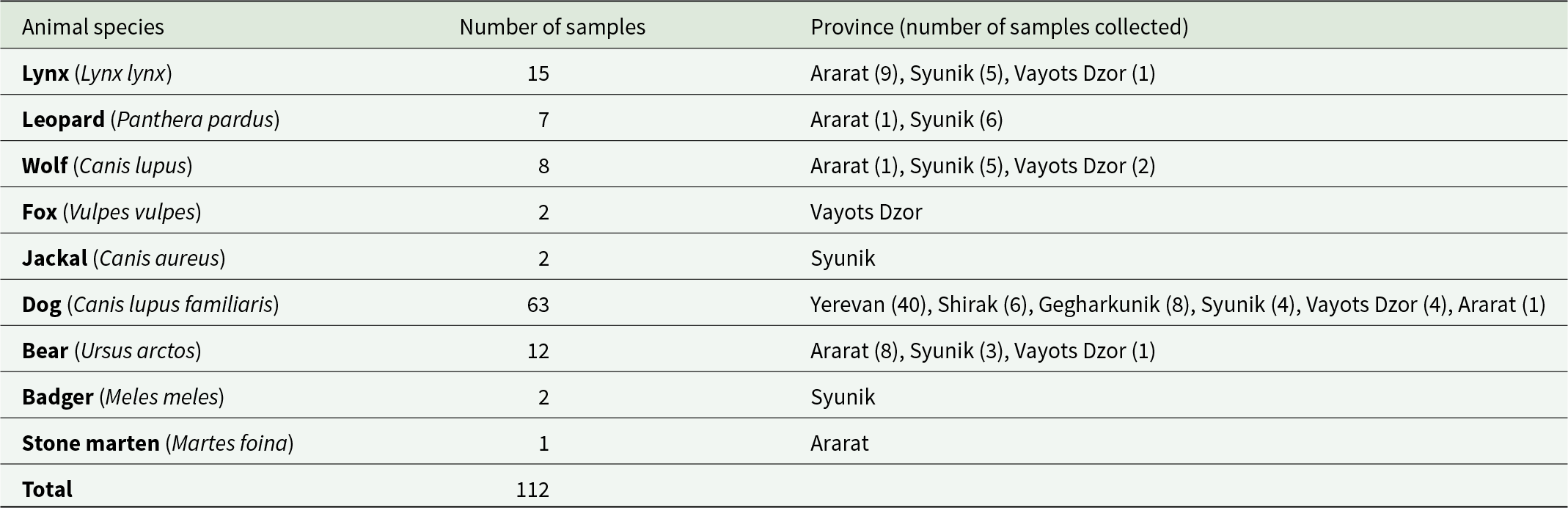
In total, 49 fecal samples from wolves, jackals, lynx, bears, leopards, badgers, foxes, stone martens, and 63 fecal samples from stray dogs in targeted provinces have been opportunistically collected (Table 1). All samples were taken from the ground without interacting with the animals in any way. Identification of the feces was made by experienced mammologists, based on feces characteristics, such as size, shape, colour, and odour, and environmental signs such as footprints (Oshmarin and Pikunov, Reference Oshmarin and Pikunov1990). Each sample collected was stored in separate tubes and labelled. The host species of taeniid-positive samples was later confirmed using molecular methods. All samples were frozen for 1 week at −80 °C to inactivate parasitic stages and stored afterwards at −20 °C until use. The fecal content was visually inspected before performing parasitological analyses. Specifically, the feces were examined for small bones, hairs, berries, and other dietary items to support or question the initial field assessment. Taeniid eggs were isolated from ∼2 cm3 of each fecal sample using the flotation and sieving method described by Mathis et al. (Reference Mathis, Deplazes and Eckert1996). The content of each flotate was placed in a separate Petri dish and screened for taeniid eggs using an inverted microscope. Since the eggs of the different genera of the family Taeniidae cannot be distinguished morphologically and because of the possibility of mixed infections with different taeniid species, individual eggs were isolated and analysed. For this purpose, individual eggs were transferred into 10 µL of 0.02 mM NaOH using a capillary pipette and lysed at 95 °C for 10 min (Hüttner et al., Reference Hüttner, Nakao, Wassermann, Siefert, Boomker, Dinkel, Sako, Mackenstedt, Romig and Ito2008). The lysate was used directly as a template for the polymerase chain reaction (PCR).
Table 1. Hosts, number and origin of opportunistically collected fecal samples from six provinces of Armenia
Molecular analyses of taeniid eggs
The individual eggs were subjected to nested PCR and subsequent sequencing for the identification of the taeniid species as described previously (Hüttner et al., Reference Hüttner, Nakao, Wassermann, Siefert, Boomker, Dinkel, Sako, Mackenstedt, Romig and Ito2008). The first attempts to amplify the complete mitochondrial (mt) NADH dehydrogenase subunit 1 (nad1) gene via nested PCR were unsuccessful, and the amplification of a shorter fragment (<200 bp) of the nad1 gene was carried out. The primers used for the first PCR of the short fragment were forward: 5′-TGT TTT TGA GAT CAG TTC GGT GTG-3′ and reverse: 5′-CAT AAT CAA ACG GAG TAC GAT TAG-3′ and the primer pair for the second PCR was forward: 5′-CAG TTC GGT GTG CTT TTG GGT CTG-3′ and reverse: 5′-GAG TAC GAT TAG TCT CAC ACA GCA-3′. In cases of negative PCR results, a second nested PCR was performed targeting a short fragment (<200 bp) of the mt cytochrome b (cob) gene. Primers for the first cob PCR were forward: 5′-GAG TAC GAT TAG TCT CAC ACA GCA-3′ and reverse: 5′-ATA AGG ATA CTC CGG ATG ACA AC-3′, and for the second PCR forward: 5′-TCG GTG TAT TAA TTC GAA GAT TG-3′ and reverse: 5′-GAT GAC AAC CAC CCA AAT AAG TC-3′ (Hüttner et al., Reference Hüttner, Nakao, Wassermann, Siefert, Boomker, Dinkel, Sako, Mackenstedt, Romig and Ito2008). For the first PCR, a 25-µL reaction mixture containing 10 mM Tris–HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, 200 µM of each dNTP, 10 pmol of each external primer and 0.625 U of Taq polymerase (AmpliTaq; Applied Biosystem) was prepared, and 1 µL of the egg lysate was individually added to the reaction mixture. After amplification, 2 µL of the first PCR product were transferred to a 50-µL reaction mixture containing the same reagents as the first, but 20 pmol of the internal primers and 1.25 U Taq polymerase. Conditions for all PCRs were identical and as follows, initial denaturation at 95 °C for 5 min, 35 cycles of denaturation at 95 °C for 30 sec, annealing at 50 °C for 30 sec, elongation at 72 °C for 30 sec, and final elongation at 72 °C for 5 min. Positive PCR products were purified using the High Pure PCR Product Purification Kit (Roche, Mannheim) following the manufacturer’s instructions and sequenced by GATC Eurofins Genomics (Konstanz, Germany). Sequences were viewed and edited, if necessary, with the program GENtle 1.9.4 (Manske, University of Cologne) and taeniid species identified by comparison with already published sequences in GenBank using the online program BLAST (https://blast.ncbi.nlm.nih.gov).
Molecular confirmation of the host species
Host origin (carnivore species) of fecal samples was determined in the field by shape, colour, and odour of feces, as well as field signs (Oshmarin and Pikunov, Reference Oshmarin and Pikunov1990). Molecular analysis was carried out to confirm the assigned carnivore species of all samples tested positive for taeniid species. For this purpose, total DNA was extracted from fecal samples as described previously (Dinkel et al., Reference Dinkel, von Nickisch-rosenegk, Bilger, Merli, Lucius and Romig1998). For host species identification, nested PCR targeting a fragment of the mt cob gene was performed with subsequent sequencing of the amplicons. Primers and PCR conditions were taken from Hüttner et al. (Reference Hüttner, Siefert, Mackenstedt and Romig2009).
For the differentiation between dog and wolf, the control region located on the mt genome was amplified and sequenced. For the PCR, the previously published primers CR1/CR2R (Sindičić et al., Reference Sindičić, Gomerčić, Galov, Arbanasić, Kusak, Slavica and Huber2011) were used. However, additional primers were designed to perform nested PCR. The primers for the first PCR were forward: ‘CR1’ 5′-CCA CTA TCA GCA CCC AAA GC-3′ and a new reverse: 5′-AGT TTC ATG ATA GTA ACC CCC ACG-3′ and for the second PCR a new forward: 5′-AGC TGA AAT TCT TCT TAA ACT ATT CC-3′ and reverse ‘CR2R’ 5′-CCC GGA GCG AGA AGA GG-3′. The volumes and reagents of the reaction mixtures were identical to those of the taeniid PCR for the first and second PCR, except for the primers. Conditions during both amplifications were initial denaturation at 95 °C for 5 min, 35 cycles of denaturation at 95 °C for 30 sec, annealing at 50 °C for 30 sec, elongation at 72 °C for 60 sec, and final elongation at 72 °C for 5 min. PCR products were purified as described above and sequenced by GATC Eurofins Genomics (Konstanz, Germany). Sequences were viewed and edited, if necessary, with the program GENtle 1.9.4 (Manske, University of Cologne) and carnivore species identified by comparison with published sequences in GenBank using the online program BLAST (https://blast.ncbi.nlm.nih.gov).
Results
Visual examination of the fecal content prior to parasitological analysis either supported the field-based identification of the carnivore species or, in cases where clear support was not possible, revealed no evidence contradicting the initial assessment.
Taeniid eggs were detected in only three of the 112 fecal samples. According to field identification, these samples were attributed to lynx, dog, and wolf. PCR and subsequent sequence analyses confirmed the lynx and dog origins. However, the ∼250 bp sequence of the mt control region from the wolf sample matched 100% with bear DNA in a GenBank comparison (AccNo. X75872; Taberlet and Bouvet, Reference Taberlet and Bouvet1994). Given that the field identification, fecal content examination, and taeniid egg analyses strongly contradict the sample originating from a bear, it is assumed hereafter that the sample was indeed from a wolf.
Of the three taeniid-positive samples, ten eggs each were isolated and subjected to species identification via PCR and sequencing. The resulting sequences were ∼100 bp in length but covered a region that allowed clear differentiation between the species.
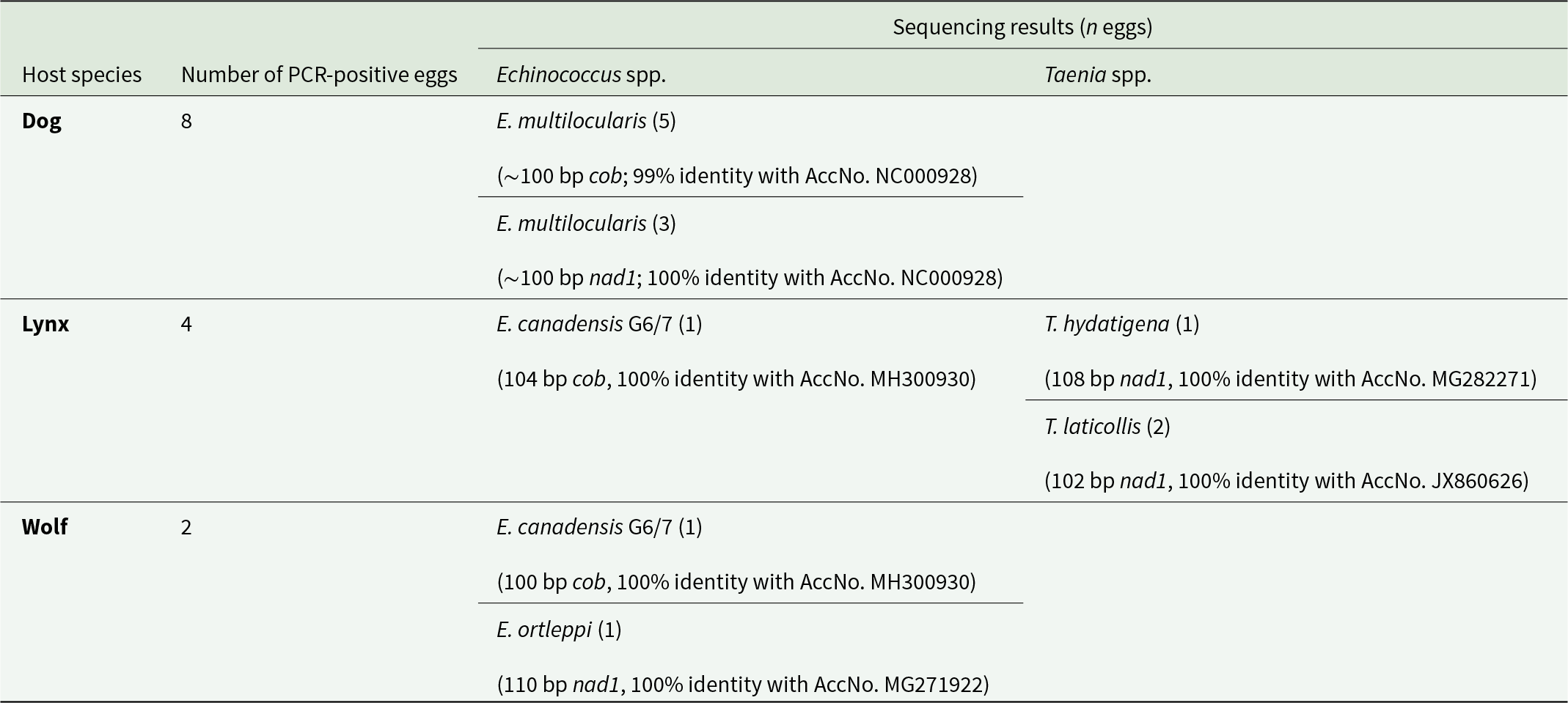
Of the eggs isolated from the dog sample, eight could be amplified, and sequence analysis of the ∼100 bp long fragments showed that all belonged to E. multilocularis. Amplification of the nad1 gene was successful in three eggs, showing 100% identity with GenBank reference sequences (AccNo. NC000928), while the cob gene was successfully amplified in five eggs, with 99% identity (AccNo. NC000928). The five cob sequences displayed one unique substitution at position 876 of the complete cob gene (GenBank AccNo. NC000928; Nakao et al., Reference Nakao, Yokoyama, Sako, Fukunaga and Ito2002). All Armenian sequences exhibit thymine (T) at this position, whereas all corresponding GenBank entries show cytosine (C). However, this is a synonymous substitution and does not affect the resulting amino acid sequence.
Only four eggs of the lynx sample were successfully analysed. Two could be unequivocally assigned to Taenia laticollis (102 bp, 100% identity with GenBank AccNo. JX860626), and one each to T. hydatigena (108 bp, 100% identity with AccNo. MG282271) and E. canadensis G6/7 (104 bp, 100% identity with AccNo. MH300930). Due to the short length of the sequence obtained, it was not possible to differentiate between genotypes G6 and G7 of E. canadensis.
From the single wolf sample, two eggs yielded a positive PCR product, and sequence analysis revealed the presence of E. ortleppi (110 bp, 100% identity with AccNo. MG271922) and E. canadensis G6/7 (100 bp, 100% identity with AccNo. MH300930) (Table 2, Supplementary Figures 1 and 2).
Table 2. Results of genetic characterization of taeniid eggs isolated from various host species
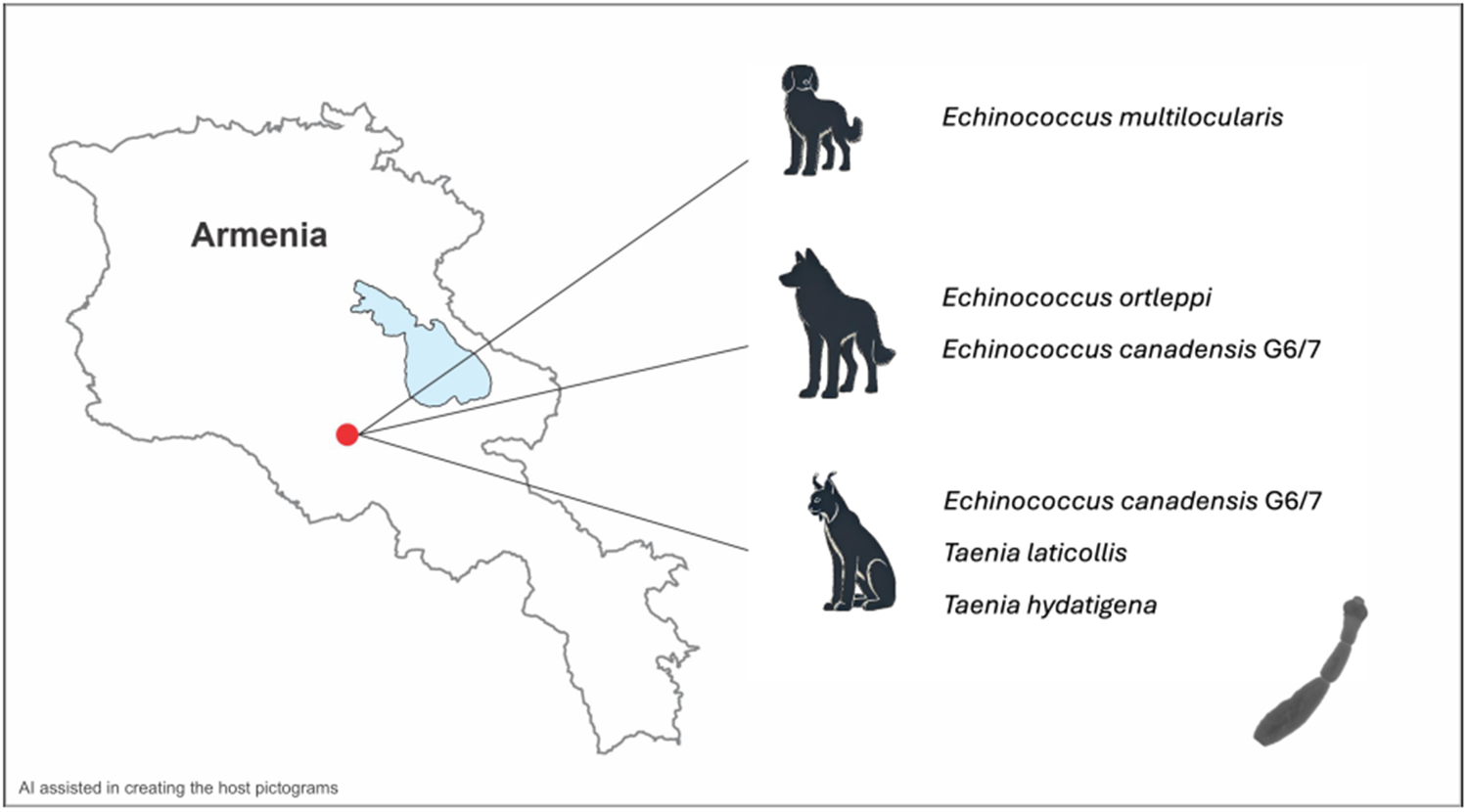
All three taeniid-positive fecal samples were collected from the Khosrov sampling site (Figure 1, sampling site 1).
Discussion
In Armenia, research on echinococcosis received substantial attention in the second half of the 20th century. However, these efforts have since diminished, resulting in today’s rather sporadic and fragmented knowledge of the situation in the country. Although the causative agents of CE are known to be endemic in Armenia, the involved species and their distribution are still poorly understood. A retrospective study, however, revealed an unexpected finding: human infections with E. multilocularis have occurred consistently over at least the past 20 years (Manukyan et al., Reference Manukyan, Danielyan, Gevorgyan, Sahakyan, Vanyan, Andreasyan and Melik-Andreasyan2024). At the time, these cases of AE were misdiagnosed, as it was believed that this parasite was not endemic to Armenia. This study has brought renewed attention to these parasites, underscoring the fact that they represent a neglected public health concern and that epidemiological studies are urgently needed.
The causative agents of CE are easier to study compared to E. multilocularis. Their life cycle is primarily associated with various livestock species that act as intermediate hosts, and post-mortem examinations of these animals can provide valuable insights into the distribution of these parasites. Recent molecular biological studies have confirmed the presence of E. granulosus s.s. and E. canadensis G6/7 in Armenia (Gevorgyan et al., Reference Gevorgyan, Aghayan, Grigoryan, Saghoyan and Romig2016; Ebi et al., Reference Ebi, Gevorgyan, Aghayan, Wassermann and Romig2017). However, despite this molecular information, the available data on these parasites remain limited, particularly regarding their prevalence in definitive hosts, which are primarily domestic dogs, although wild canids can also be infected (Romig and Wassermann, Reference Romig and Wassermann2024). In contrast to E. granulosus s.l., epidemiological studies on E. multilocularis are more challenging to conduct. The life cycle of E. multilocularis is predominantly sylvatic, involving voles as primary intermediate hosts and wild canids predating on these rodents, such as foxes, as definitive hosts. Wolves and domestic dogs can also serve as hosts, albeit to a lesser extent due to differences in feeding habits (Romig et al., Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017). The southern Caucasus is home to a species-rich small mammal fauna. This also encompasses arvicoline and gerbilline rodents, which include the most important intermediate host species for E. multilocularis in Europe (Romig and Wassermann, Reference Romig and Wassermann2024). However, arvicolines of the Caucasus and western Asia have been subjected to numerous taxonomic and nomenclatural changes in the recent past, so the precise geographical repartition of individual taxa is often unclear. According to a recent review, eight arvicoline and five gerbilline species are listed for the territory of Armenia (Wilson et al., Reference Wilson, Lacher and Mittermeier2017), of which no fewer than eight species are confirmed intermediate hosts for E. multilocularis in other parts of their respective ranges, namely Arvicola amphibius, Chionomys nivalis, Microtus irani, M. obscurus, M. mystacinus, M. socialis, Meriones libycus and M. persicus (Romig and Wassermann, Reference Romig and Wassermann2024).
To study the distribution and prevalence of this parasite, examining wild carnivores and/or rodents is necessary, as these animals play essential roles in maintaining the transmission cycle. Since the prevalence among rodents fluctuates and is generally very low, even in areas with high infection rates in foxes, it is recommended to monitor the definitive hosts (Giraudoux et al., Reference Giraudoux, Delattre, Takahashi, Raoul, Quere, Craig, Vuitton, Craig and Pawlowski2002; Conraths and Deplazes, Reference Conraths and Deplazes2015).
However, research on the parasitofauna of wildlife has been nearly absent over the past decades in the country. Consequently, there is a lack of understanding regarding whether, and to what extent, different carnivore species contribute to the transmission of Echinococcus species. The first approach to addressing these knowledge gaps was undertaken in the present study and involved analysing fecal samples from both wild carnivores and free-roaming dogs in Armenia. Fecal samples from eight wild carnivore species and domestic dogs were collected across six provinces of Armenia, including the capital, Yerevan, and analysed for the presence of taeniid eggs. Taeniid eggs could only be detected in three out of the 112 samples. The low detection rate observed suggests a low infection pressure among the animals; however, it is more likely attributable to limitations in the detection method employed. Egg detection following flotation is known to be less sensitive compared to other methods, such as the sedimentation and counting technique, intestinal scraping technique, or total DNA extraction from feces and subsequent PCR (Conraths and Deplazes, Reference Conraths and Deplazes2015; Marchiori et al., Reference Marchiori, Obber, Celva, Marcer, Danesi, Maurizio, Cenni, Massolo, Citterio and Cassini2023). The first two methods involve the direct detection of parasites within the intestine, necessitating the sacrifice of the animal – an approach that is, in most cases, not feasible when studying wild protected species. The extraction of total DNA, on the other hand, would have rendered the detection of double or multiple infections difficult or even impossible. Therefore, individual eggs were isolated and analysed in the present study, prioritizing species identification over sensitivity. Consequently, the number of positive samples reported here is not suitable for estimating prevalence and should be interpreted with caution (Marchiori et al., Reference Marchiori, Obber, Celva, Marcer, Danesi, Maurizio, Cenni, Massolo, Citterio and Cassini2023).
All three taeniid-positive samples originated from the Khosrov Forest State Reserve, one of the oldest protected regions. The Khosrov reserve encompasses a diverse range of ecosystems due to its varied topography and elevation, which ranges from 1070 to 2650 m above sea level. The landscapes include semi-deserts, dry mountain steppes, juniper sparse woodlands, dense oak and hornbeam forests, and subalpine meadows. This ecological diversity supports a rich array of flora and fauna. The area serves as a wildlife corridor and is home to the critically endangered Caucasian leopard, Armenian mouflon, and Bezoar goat, as well as local priority species, such as lynx, wolf, and brown bear. Human habitation within the reserve is minimal; however, several large villages and towns are located in close proximity to the reserve. These settlements practice intensive outrun-type livestock farming near the reserve’s borders, and in some cases, this activity encroaches into the protected area despite existing prohibitions.
The three positive samples were derived from a dog, a lynx, and a wolf from approximately the same area, inside the Khosrov reserve. Host species identification was initially performed by experienced field biologists and through analyses of fecal content. Subsequent molecular confirmation verified the origins of the dog and lynx samples; however, the sample attributed to the wolf yielded a sequence corresponding to a brown bear. Given that field signs, fecal shape, and fecal content all indicated a wolf origin, and the sample contained Echinococcus eggs, it was ultimately assessed as originating from a wolf despite the bear sequence result. To date, no patent infections with Echinococcus spp. have been reported in brown bears, or any bear species, and it is generally assumed that they are not suitable definitive hosts (Romig et al., Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017; Romig and Wassermann, Reference Romig and Wassermann2024). In the Khosrov Forest State Reserve, both wolves and brown bears coexist. The only logical explanation for this result is that the wolf encountered and consumed a bear carcass, with the bear’s DNA subsequently detected through PCR analysis of the copro-DNA. This particular wolf sample contained two Echinococcus species, E. canadensis G6/7 and E. ortleppi. Echinococcus canadensis G6/7 has previously been reported in Armenia in domestic pigs from the same region (Gevorgyan, Reference Gevorgyan2006a; Gevorgyan et al., Reference Gevorgyan, Dinkel, Romig and Mackenstedt2006c). The detection of this parasite in wolves suggests that E. canadensis G6/7 may be also established within Armenia’s wildlife. Wolves are known to be suitable definitive hosts, and in Europe, the parasite’s sylvatic life cycle is maintained through wild boars acting as intermediate hosts (Guerra et al., Reference Guerra, Armua-Fernandez, Silva, Bravo, Santos, Deplazes and Carvalho2013; Umhang et al., Reference Umhang, Richomme, Hormaz, Boucher and Boué2014; Pavia et al., Reference Pavia, De Gori, Ciambrone, De Gori, Musarella and Casalinuovo2020; Kilinc et al., Reference Kilinc, Celik, Kesik, Selcuk, Ahmed and Simsek2023). Sus scrofa, the wild boar, is widely distributed in Armenia, and this could facilitate the establishment of a sylvatic cycle for E. canadensis G6/7. However, it has so far only been documented in domestic pigs. In contrast, E. ortleppi has not been previously documented in the country. The typical hosts for E. ortleppi are domestic dogs and cattle, but also goats and pigs were identified to harbour fertile cysts (Romig et al., Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017). Cattle in Armenia have been reported to be infected with Echinococcus cysts, but so far, only E. granulosus sensu stricto has been identified as the causative agent (Gevorgyan, Reference Gevorgyan2006a). The limited number of cattle isolates examined may not have been sufficient to confirm the presence of E. ortleppi. Studies have shown that in other regions with high prevalences in cattle and where both parasites are present, E. granulosus s.s. predominates (Addy et al., Reference Addy, Alakonya, Wamae, Magambo, Mbae, Mulinge, Zeyhle, Wassermann, Kern and Romig2012). This may also be the case in Armenia. Alternatively, other intermediate hosts, such as the bezoar goat or wild boar, might play a role in the sylvatic life cycle of E. ortleppi. Fertile cysts of E. ortleppi have already been documented in goats and wild boars, and adult worms have been found in wolves (Mbaya et al., Reference Mbaya, Magambo, Njenga, Zeyhle, Mbae, Mulinge, Wassermann, Kern and Romig2014; Mateus et al., Reference Mateus, Gargaté, Vilares, Ferreira, Rodrigues, Coelho and Vieira-Pinto2021; Karamon et al., Reference Karamon, Samorek-Pieróg, Bilska-Zając, Korpysa-Dzirba, Sroka, Bełcik, Zdybel and Cencek2023). A recent study from Poland suggests that cervids may also play a role in the cycle. Echinococcus ortleppi was detected in 10% of the examined wolves, but the parasite has not been found in Poland’s cattle, and cattle are rarely preyed upon by wolves in the country. This indicates the likely presence of another intermediate host (Karamon et al., Reference Karamon, Samorek-Pieróg, Bilska-Zając, Korpysa-Dzirba, Sroka, Zdybel and Cencek2024). Consequently, the presence of suitable hosts for both E. canadensis G6/7 and E. ortleppi suggests that the conditions necessary to sustain a sylvatic cycle for these species are present in Central Armenia. Both species of Echinococcus have also been documented in Namibia, occurring in wildlife cycles independent of domestic animals. Echinococcus ortleppi has been identified in oryx antelopes and jackals, while E. canadensis 6/7 has been detected in oryx and jackals, as well as in African wild dogs. Notably, E. canadensis G6/7 has also been reported in lions and cheetahs, underscoring its broader host range among carnivores (Aschenborn et al., Reference Aschenborn, Aschenborn, Beytell, Wachter, Melzheimer, Dumendiak, Rüffler, Mackenstedt, Kern, Romig and Wassermann2023). This is particularly interesting as the present study also identified this parasite in a felid host – the lynx. To the best of our knowledge, there are only two documented reports of Echinococcus species in lynx. These include E. oligarthra, a species adapted to felids and restricted to Central and South America, identified in a bobcat (Lynx rufus), and E. multilocularis, reported in a Eurasian lynx (L. lynx) in Turkey (Salinas-López et al., Reference Salinas-López, Jiménz-Guzmń and Cruz-Reyes1996; Avcioglu et al., Reference Avcioglu, Guven, Balkaya and Kirman2018). The current detection of E. canadensis G6/7 would be the first finding of a species belonging to E. granulosus s.l. As previously mentioned, the Khosrov area supports suitable intermediate hosts such as wild boar and bezoar goats, which, along with smaller mammals, form part of the lynx’s prey spectrum (A. Malkhasyan, personal communication, 2017, 2025). The identification of E. canadensis G6/7 in both wolf and lynx from the same area suggests the presence of an active transmission cycle of this parasite in Central Armenia.
In addition to E. canadensis G6/7, two other taeniid species, T. hydatigena and T. laticollis, were detected in this lynx. The latter is a well-known tapeworm of lynx, with lagomorphs serving as its intermediate hosts and is here described for the first time in Armenia. Taenia hydatigena, on the other hand, has a life cycle primarily involving canids and ruminants, with pigs also being susceptible (Haukisalmi et al., Reference Haukisalmi, Konyaev, Lavikainen, Isomursu and Nakao2016). Its presence in canids, livestock, and wild ruminants in Armenia, as well as in neighbouring countries, is well documented (Chobanyan, Reference Chobanyan1965, Reference Chobanyan1968, Reference Chobanyan1971; Movsesyan et al., Reference Movsesyan, Nikoghosyan and Petrosyan2013; Kilinc et al., Reference Kilinc, Kesik and Simsek2019; Sarvi et al., Reference Sarvi, Behrestaghi, Alizadeh, Hosseini, Gohardieh, Bastani, Charati, Daryani, Amouei, Spotin and Gholami2020; Modabbernia et al., Reference Modabbernia, Meshgi and Eslami2021; Memarian et al., Reference Memarian, Zahedian, Kwaak, Lippert, Jazayeri, Kordestani, Amirahmadi and Javardi2022; Shamsaddini et al., Reference Shamsaddini, Schneider, Dumendiak, Aghassi, Kamyabi, Akhlaghi, Wassermann, Harandi, Deplazes and Romig2024). Lynx infected with T. hydatigena have been reported previously (Valdmann et al., Reference Valdmann, Moks and Talvik2004; Bridger, Reference Bridger2005), but the identification was based on morphological characteristics, raising the possibility of misidentification with another, possibly unknown, taeniid species present in lynx. Evidence of T. hydatigena infections in felid species is generally limited, as they are not regarded as optimal hosts. However, the presence of T. hydatigena has been genetically confirmed in feces of a domestic cat, as well as snow leopards (Karamon et al., Reference Karamon, Sroka, Dąbrowska, Bilska-Zając, Zdybel, Kochanowski, Rózycki and Cencek2019; Ulziijargal et al., Reference Ulziijargal, Yeruult, Khulan, Gantsetseg, Wandra, Yamasaki and Narankhajid2020). The detection of a T. hydatigena egg indicates that the lynx can indeed be a competent host.
To further investigate these findings, additional sampling in Central Armenia would be essential. This should include fecal sample collection and meat inspections of both wild game and domestic livestock to better understand the transmission pathways of these parasites. However, for the last Echinococcus species identified in this pilot study, E. multilocularis, meat inspection would not be informative. The intermediate host of E. multilocularis are rodents, predominantly voles, and the detection of this cestode in a free-roaming dog represents the first reported case of this parasite in an Armenian carnivore. In the 1960s, the prevalence of Echinococcus in dogs in Armenia reached up to 68% in some areas. At the time, all cases were attributed to E. granulosus sensu lato, though it is possible that some were actually due to E. multilocularis. During the same period, the larval stage of E. multilocularis was detected in voles, albeit in low prevalence (Chobanyan, Reference Chobanyan1968). These historical data, along with the retrospective study by Manukyan et al. (Reference Manukyan, Danielyan, Gevorgyan, Sahakyan, Vanyan, Andreasyan and Melik-Andreasyan2024) on misdiagnosed human cases, indicate that E. multilocularis is not a newly emerging parasite but has been present in Armenia for a considerable period (Manukyan et al., Reference Manukyan, Danielyan, Gevorgyan, Sahakyan, Vanyan, Andreasyan and Melik-Andreasyan2024). The current study also demonstrates that this parasite continues to persist in the area. The detection of E. multilocularis in a dog sample is not surprising, as all canids that consume infected rodents are potential hosts (Romig et al., Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017). However, this finding underscores an increased risk to humans, given the close proximity in which dogs and humans often live.
To summarize the results, the ‘sylvatic’ E. multilocularis is brought closer to humans through rodent-eating dogs, while wild animals in Armenia contribute to sustaining the transmission cycles of the ‘domestic’ E. ortleppi and E. canadensis G6/7. Whether fully sylvatic cycles of the latter two species have already been established remains unclear, but suitable intermediate hosts are present in the region. Further studies will be necessary to clarify this dynamic.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182025100474.
Author contributions
H.G. performed the investigation, formal analysis, conceptualization and writing of the original draft. S.A.A. participated in sampling, as well as manuscript review and editing. M.A. and A.M. conducted field investigations and data collection. M.W. developed the methodology, performed data analysis and contributed to conceptualization and writing the original draft. T.R. supervised the study and reviewed and edited the manuscript.
Financial support
This study has been supported by the Higher Education and Science Committee of the Republic of Armenia (Project 23LCG-1F006) and German Academic Exchange Service (DAAD, Project No. 57378442).
This study has been supported by the Higher Education and Science Committee of the Republic of Armenia (Project 23LCG-1F006) and German Academic Exchange Service (DAAD, Project No. 57378442).
Competing interests
The authors declare there are no conflicts of interest.
Ethical standards
According to Armenian legislation, the collection of fecal samples from the environment does not require special permission.