Introduction
The introduction of exotic and invasive vectors into the United States (US) is not only a concern for their negative ecological and economic effects (Marbuah et al., Reference Marbuah, Gren and McKie2014; Bradshaw et al., Reference Bradshaw, Leroy, Bellard, Roiz, Albert, Fournier, Barbet-Massin, Salles, Simard and Courchamp2016), but also for potential health impacts (Lounibos, Reference Lounibos2002; Juliano and Philip Lounibos, Reference Juliano and Philip Lounibos2005). Several tick species of great concern, due to their potential negative impact on the livestock industry, have invaded the US, including the red sheep tick Haemaphysalis punctata (Tufts and Diuk-Wasser, Reference Tufts and Diuk-Wasser2021), cattle fever ticks, Rhipicephalus (Boophilus) annulatus (Say) and Rhipicephalus (Boophilus) microplus (Canestrini) (Lohmeyer et al., Reference Lohmeyer, Pound, May, Kammlah and Davey2011), and the longhorned tick H. longicornis (Beard et al., Reference Beard, Occi, Bonilla, Egizi, Fonseca, Mertins, Backenson, Bajwa, Barbarin, Bertone, Brown, Connally, Connell, Eisen, Falco, James, Krell, Lahmers, Lewis, Little, Neault, Pérez de León, Randall, Ruder, Saleh, Schappach, Schroeder, Seraphin, Wehtje, Wormser, Yabsley and Halperin2018). The recent discovery of H. longicornis in the US is a public health concern due to its ability to transmit a variety of pathogens that impact humans and other animal hosts (Thompson et al., Reference Thompson, White, Shaw, Garrett, Wyckoff, Doub, Ruder and Yabsley2021). Although H. longicornis is not known to use humans as a preferred host (Ronai et al., Reference Ronai, Tufts and Diuk-Wasser2020), it is able to harbour and transmit pathogens which negatively affects humans (Zhuang et al., Reference Zhuang, Du, Cui, Li, Tang, Zhang, Hu, Tong, Feng and Liu2018; Stanley et al., Reference Stanley, Ford, Snellgrove, Hartzer, Smith, Krapiunaya and Levin2020). Reports in the US found H. longicornis parasitizing a wide array of mammal and bird species (USDA-APHIS, 2023). Previous studies surveying H. longicornis on wildlife hosts found white-tailed deer (Odocoileus virginiana), raccoon (Procyon lotor) and Virginia opossum (Didelphis virginiana) to be heavily infested with this tick species (Thompson et al., Reference Thompson, White, Shaw, Garrett, Wyckoff, Doub, Ruder and Yabsley2021, Reference Thompson, White, Doub, Sharma, Frierson, Dominguez, Shaw, Weaver, Vigil, Bonilla, Ruder and Yabsley2022). Although this tick has been widely reported on companion and livestock animals (USDA-APHIS, 2023), its role in the transmission of pathogens from wildlife to humans and domesticated animals in the US is likely underreported. Cofeeding, attachment of 2 separate tick species feeding together on a host, between H. longicornis and other native tick species on companion and wildlife hosts in the US have been reported (Tufts et al., Reference Tufts, Vanacker, Fernandez, Denicola, Egizi and Diuk-Wasser2019, Reference Tufts, Goodman, Benedict, Davis, VanAcker and Diuk-Wasser2021). Cofeeding ticks can have negative implications for human health because they can obtain pathogens from one another; thus, H. longicornis may obtain pathogens from native tick species or vice versa (Tufts, Reference Tufts2021; Price et al., Reference Price, Ayres, Maes, Witmier, Chapman, Coder, Boyer, Eisen and Nicholson2022). For example, Bourbon virus and Anaplasma phagocytophilum from native cofeeding ticks were found in H. longicornis, which could have been transmitted by ticks feeding in proximity (Cumbie et al., Reference Cumbie, Trimble and Eastwood2022; Price et al., Reference Price, Ayres, Maes, Witmier, Chapman, Coder, Boyer, Eisen and Nicholson2022). Trout Fryxell et al. (Reference Trout Fryxell, Chavez-Lindell, Butler and Odoi2023) found H. longicornis follows similar phenological patterns to native tick species and these species were often found questing together in similar habitats. It is possible that native species could be interacting with H. longicornis in the environment as well as on hosts. Knowing how exotic and native tick species select host species is important for understanding pathogen transmission.
To better understand the community structure of H. longicornis on hosts at wildlife–livestock interfaces this study aimed to identify this species' associations with established tick species and wildlife hosts and producer led integrated pest management (IPM) strategies in eastern Tennessee. Although the goal of the present study was to identify factors associated with H. longicornis abundance and presence, which could be targeted for wildlife on-animal control on farms infested with this tick species, important findings for other tick species were also included. Here, it was hypothesized that established tick species and wildlife host characteristics (e.g. sex, age and weight) and body regions would be associated with H. longicornis abundance and presence, and producer led IPM would decrease the presence of this species on wildlife hosts. Factors identified from this research will be important for cost-effective management regimes for controlling H. longicornis on cow–calf farm settings.
Materials and methods
Site selection
Mammals were trapped in forested and edge habitats on 3 farms (farm 1, farm 2, farm 3), which consisted of upland hardwoods surrounded by fields of fescue in eastern Tennessee. Each farm had an established population of H. longicornis on site and a herd of beef cattle. Producer led IPM strategies were conducted on site at each farm and outlined in Butler and Trout Fryxell (Reference Butler and Trout Fryxell2023). These on-farm management strategies consisted of chemical control in the form of an on-animal acaracide for cattle, cultural control in the form of bush hogging pastures, and mechanical control by removing ticks, in forested, field, and edge habitats, from the environment using a corduroy drag. Farm 1 significantly reduced questing H. longicornis in the environment by 90% over 3 years; they bush hogged their pastures once monthly during the growing season (May–October), used an on-animal acaracide, and frequently removed ticks from the environment by mechanical control. Farm 2 did not reduce questing H. longicornis; they chose to bush hog their pasture once a year in the fall, did not use an on-animal acaracide, and moderately removed ticks from the environment by mechanical control. Farm 3 reduced their H. longicornis population by 68%; that producer bush hogged their pastures yearly in the fall, used an on-animal acaracide, and scarcely removed ticks from the environment by using mechanical control (Butler and Trout Fryxell, Reference Butler and Trout Fryxell2023).
Mammal handling and tick collection
All wildlife were handled following guidelines of the American Society of Mammalogists (Sikes and Gannon, Reference Sikes and Gannon2011) and identified to species using taxonomic keys outlined in Kellogg (Reference Kellogg1939); Schwartz and Schwartz (Reference Schwartz and Schwartz2016). Methods for handling small- and medium-sized wildlife were approved by the University of Tennessee, Knoxville (IACUC #2774-0620). Species, sex, age (subadult and adult), weight (g) and external morphological measurements (mm) were taken on each wildlife host. Small mammals were trapped using Sherman live traps (2″ × 2.5″ × 6.5″, H. B. Sherman Traps, Inc., Tallahassee, FL, USA) baited with rolled oats and black oil sunflower seeds (Owen et al., Reference Owen, Carter and Rutan2015; Butler et al., Reference Butler, Trout Fryxell, Houston, Bowers, Paulsen, Coons and Kennedy2020). Medium-size mammals were trapped using medium (16.5″ × 5.75″ × 5.75″, Tomahawk Live Trap, Hazelhurst, WI, USA) and large (32″ × 10″ × 12″, Tomahawk Live Trap) tomahawk traps baited with sardines and marshmallows (Modarelli et al., Reference Modarelli, Westrich, Milholland, Tietjen, Castro-Arellano, Medina and Esteve-Gasent2020). In 2020–2021, a total of 42 Sherman live traps, 14 medium-sized tomahawk, and 10 large tomahawk traps were placed in forested and edge habitats on each farm. Traps were run for 2-week intervals in every season, which was determined by equinox and solstice, to accommodate differences in tick–host feeding patterns determined by day length (Trout Fryxell et al., Reference Trout Fryxell, Chavez-Lindell, Butler and Odoi2023). Mammals were trapped in every season to understand H. longicornis on-host phenology throughout the year. Raccoons were sedated with 20 mg kg−1 ketamine and 4 mg kg−1 xylazine administered by intramuscular (IM) injection and reversed with 0.375 mg kg−1 atipamezole IM (Eiden et al., Reference Eiden, Kaufman, Oi, Allan and Miller2015; Johnson et al., Reference Johnson, Ellis, Wickham, Selleck and Gilbert2024). Opossums were sedated with 10 mg kg−1 ketamine and 2 mg kg−1 xylazine IM, but a reversal was not used (Stoskopf et al., Reference Stoskopf, Meyer, Jones and Baumbarger1999; Kreeger and Arnemo, Reference Kreeger and Arnemo2018). Small mammals (rodents and squirrels) were sedated in an enclosed plastic box with cotton balls of isoflurane and monitored for sedation every 5 s or until the animal became drowsy (Bennett and Lewis, Reference Bennett and Lewis2022). Ticks were removed from trapped mammal hosts with forceps and stored in 80% ethanol. All ticks were identified to species and life stage using taxonomic keys but were not sized for engorgement status (Yunker et al., Reference Yunker, Keirans, Clifford and Easton1986; Keirans et al., Reference Keirans, Hutcheson, Durden and Klompen1996; Keirans and Durden, Reference Keirans and Durden1998; Egizi et al., Reference Egizi, Robbins, Beati, Nava, Evans, Occi and Fonseca2019).
Statistical modelling
All analyses were performed in Statistical Analysis Software (SAS, ver. 9.4, Cary, NC, USA) with 2-tailed hypotheses (α = 0.05). The best fit model was chosen based on minimizing AIC and BIC as well as the −2 restricted (res) log likelihood statistics. Least squares means (LS Mean) Tukey–Kramer post-hoc pairwise comparisons were used for examination of each categorical variable. All mammal hosts collected were included in each model; further, season and tick life stage were not blocked on due to low sample sizes associated with some categorical variables. A generalized linear mixed model (PROC GLIMMIX) with negative binomial distribution was used to model associations between response variable tick abundance and the presence of each tick species, host morphological body region where ticks were collected and their interaction as a main effect (tick species × body region). Collected ticks were categorized based on host morphological body regions appendage for ticks collected on the foot, leg, or axilla; ventrum for the thorax and abdomen; face for cervical spine, cranium, mandible and rostrum; posterior for anus, inguinal and tail; dorsum for dorsal and lateral regions; and pinna for the external ear regions (Fig. 1). Posterior region was dropped from all models due to low sample sizes. Models used to analyse tick interactions based on morphological body regions used each body part as the unit of observation. Each individual animal was used as a random effect in the model and the variables management type and mammal type were used as covariates. Mammal type represents small or medium mammals and management refers to the tick management strategy used on each farm following Butler and Trout Fryxell (Reference Butler and Trout Fryxell2023). In addition, a hierarchical linear mixed model (PROC GLIMMIX) with a negative binomial distribution was built using each individual animal as a random effect with mammal type and management as covariates. Shannon index of species diversity was used as the response variable H to identify the diversity of tick species across host body regions using the formula (Shannon, Reference Shannon1948; Dejong, Reference Dejong1975), K as a constant and p i is the proportion of the entire body region made up of species i. p i was calculated by dividing the number of ticks collected on each body region by the total sum of ticks collected. Shannon index of species diversity was also calculated for the diversity of tick species on each mammal host.
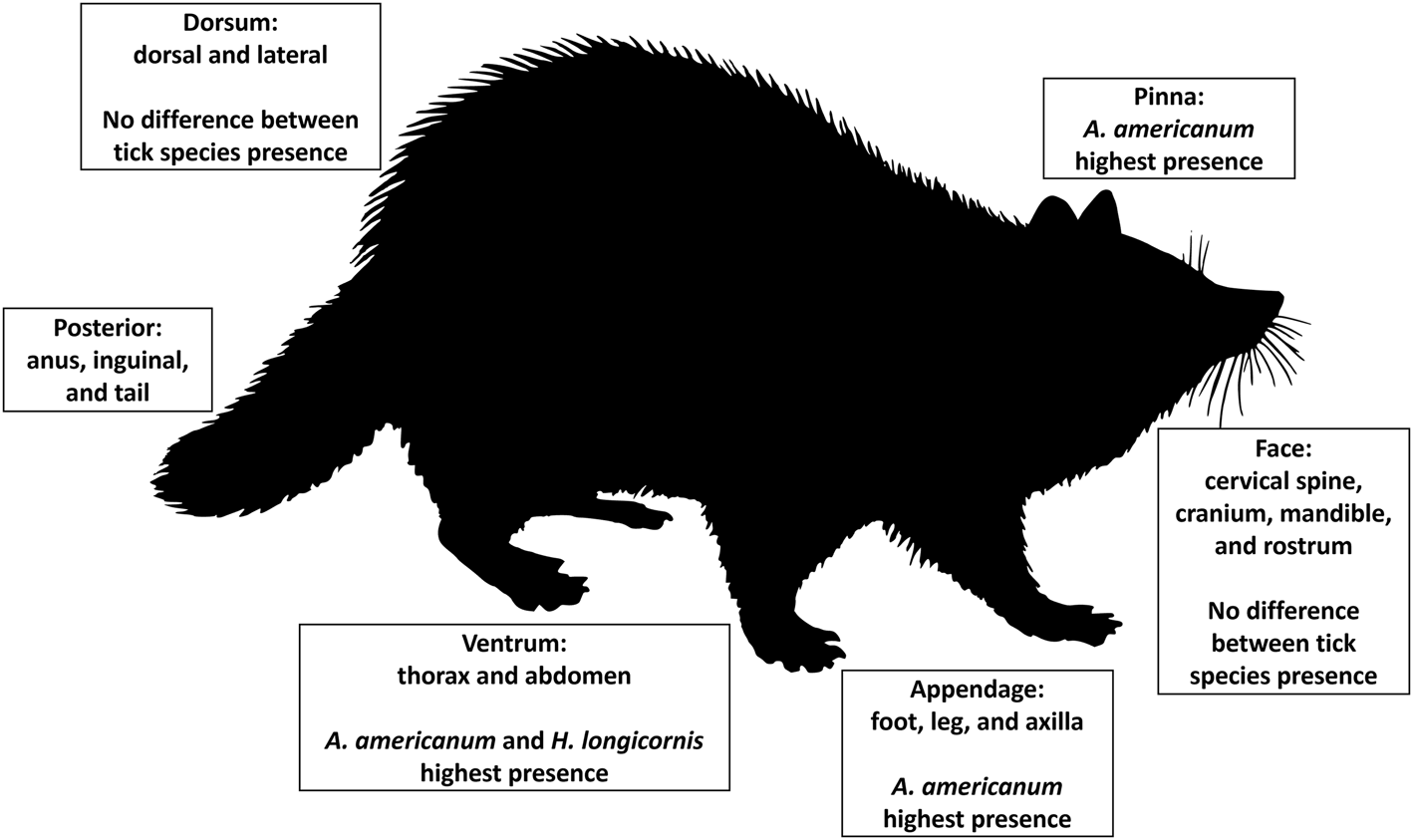
Figure 1. Morphological body regions where ticks were collected on small and medium wildlife hosts on farms in eastern Tennessee in 2020–2021. In addition, significant associations for tick species, Amblyomma americanum, Haemaphysalis longicornis, Dermacentor variabilis, and Ixodes scapularis, were labelled for each body region.
Chi-squared (PROC FREQ) analyses were then run to understand interactions between each tick species occurring on host body region and P values were determined. Additionally, odds ratios and their 95% confidence intervals were examined for each tick species. Finally, a generalized linear model (PROC GLIMMIX) with a negative binomial distribution was constructed with each individual mammal as the unit of observation. This was done to understand relationships between H. longicornis abundance as a dependent variable and the total tick species present and management strategy used by each farm. Host characteristics sex, age, and weight did not contribute to model performance and were removed.
Results
There was a total of 11 088 trap nights resulting in the collection of 329 wildlife hosts comprised of 11 different mammal species with 6040 ticks on farms in eastern Tennessee. Farm 1 had 800 (13.24%) ticks on 31 mammal hosts out of 89 collected, farm 2 had 184 ticks (3.04%) on 21 mammal hosts out of 100 collected, and farm 3 had 5056 (83.70%) ticks on 52 wildlife hosts out of 140 collected. Overall, 40 northern short-tailed shrews (Blarina brevicauda), six southern flying squirrels (Glaucomys volans), one eastern woodrat (Neotoma floridana) and two eastern grey squirrels (Sciurus carolinensis) were captured but zero ticks were present on them (Table 1; Fig. 2). There were 65 Virginia opossums with 843 ticks (mean ± standard error of the mean) (Shannon diversity index) (12.9 ± 4.59) (0.704) and 31 raccoons with 5096 ticks (164.3 ± 53.35) (0.964) collected (Table 1; Fig. 2). Seventeen woodland voles (Microtus pinetorum) had a total of four ticks (0.2 ± 0.18) and a diversity index could not be calculated. Six golden mice (Ochrotomys nuttalli) with five ticks (0.8 ± 0.83) (0.673), seven cotton deermice (Peromyscus gossypinus) with five ticks (0.7 ± 0.28) (0.500), 74 white-footed deermice (Peromyscus leucopus) with 21 ticks (0.2 ± 0.07) (0.878), 36 North American deermice (Peromyscus maniculatus) with 38 ticks (1.0 ± 0.34) (0.680), and 44 eastern chipmunks (Tamias striatus) with 28 ticks (0.6 ± 0.38) (1.272) were collected (Table 1; Fig. 2). There were as few as one and as many as six tick species found feeding together on infested wildlife hosts.
Table 1. Descriptive statistics depicting the mean ± standard error (minimum range–maximum range) and total number of ticks separated by tick species and life stage for wildlife hosts collected on farms in East Tennessee in 2020–2021

40 Blarina brevicada, 6sixGlaucomys volans, two Sciurus carolinensis, and one Neotoma floridana were searched, but no ticks were recovered from those animals.
No male Haemaphysalis longicornis, Ixodes scapularis, Ixodes cookei or Ixodes texanus, or female Amblyomma americanum were collected.
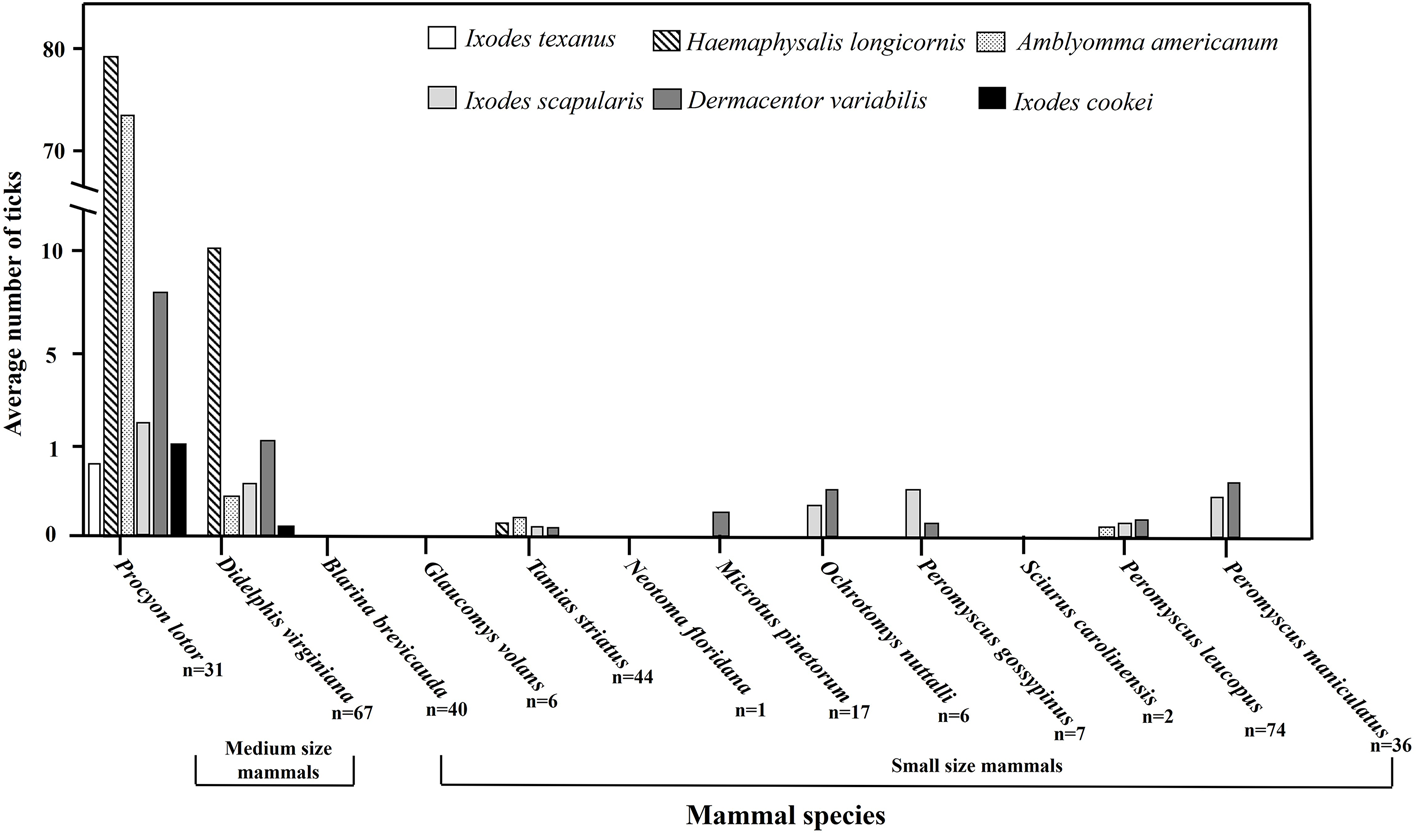
Figure 2. Average number of ticks collected on wildlife hosts on farms in East Tennessee and the total number of hosts collected, denoted as (n = ) under scientific name, in 2020–2021.
Haemaphysalis longicornis were recovered from raccoon more than any other host species; however, Virginia opossum was the second most frequented host and eastern chipmunk was the third (F 2, 137 = 3.75, P < 0.0001). Amblyomma americanum was most abundant on raccoon and second most abundant on Virginia opossum and eastern chipmunk, while the tick was least abundant on the white-footed deer mouse (F 3, 210 = 38.97, P < 0.0001). Dermacentor variabilis was most abundant on raccoon; second most abundant on Virginia opossum and North American deer mouse (F 7, 272 = 10.29, P < 0.0001); third most abundant were golden mouse, white-footed deer mouse, woodland vole, and cotton deer mouse; and least abundant on eastern chipmunks (F 7, 272 = 10.29, P < 0.0001). Ixodes scapularis was most abundant on raccoon and Virginia opossum; second most abundant on cotton deer mouse, North American deer mouse, and golden mouse; and least abundant on eastern chipmunk and white-footed deer mouse (F 6, 256 = 5.92, P < 0.0001). Ixodes cookei was most abundant on raccoon and second on Virginia opossum (F 1, 94 = 17.96, P < 0.0001), whereas I. texanus was only found on raccoon.
Tick species cofeeding interactions differed by host body region
Interactions between tick species abundance × host morphological body regions for all host species (F 12, 201 = 3.75, P < 0.0001) were associated with tick abundance (Table 2). On appendage and pinna regions A. americanum had the highest abundance. There was no difference between A. americanum, D. variabilis, H. longicornis, or I. scapularis on the dorsum or face regions. Finally, A. americanum and H. longicornis had the highest abundance on the ventrum region (Figs 1 and 3). Tick diversity was associated with the host body region where they were feeding (F 4, 131 = 8.90, P < 0.0001). Pinna regions had the highest overall adjusted average diversity, but face and ventrum regions had a higher diversity compared to appendage and dorsum regions (Fig. 4).
Table 2. Total number of ticks, separated by species and life stage, collected on wildlife host's body regions on farms in East Tennessee in 2020–2021

Amblyomma americanum 17 larvae, 17 nymphs, and 2 males; Dermacentor variabilis 2 nymphs, 12 males, and 5 females; Haemaphysalis longicornis 281 larvae and 17 nymphs; Ixodes scapularis 8 larvae, 4 nymphs and 1 female; Ixodes cookei 1 larvae; and Ixodes texanus 1 female did not have the host body region recorded.
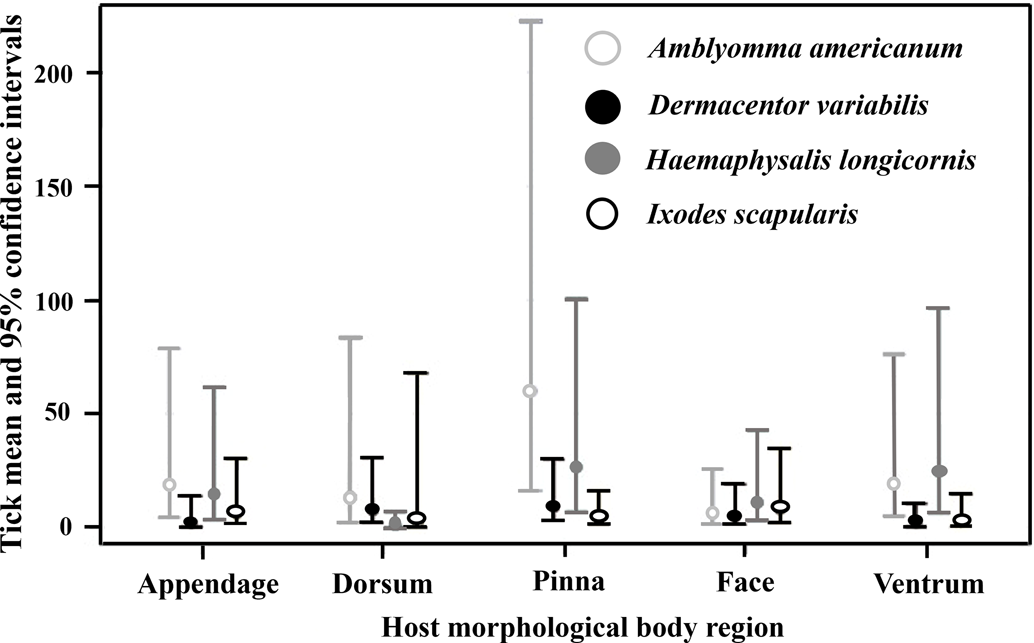
Figure 3. Mean and 95% confidence intervals for where tick species, Amblyomma americanum, Dermacentor variabilis, Haemaphysalis longicornis, and Ixodes scapularis, were collected on wildlife hosts, Procyon lotor, Didelphis virginiana, Tamis striatus, Microtus pinetorum, Ochrotomys nuttalli, Peromyscus leucopus, P. gossypinus, and P. maniculatus, morphological body regions on farms in East Tennessee in 2020–2021.
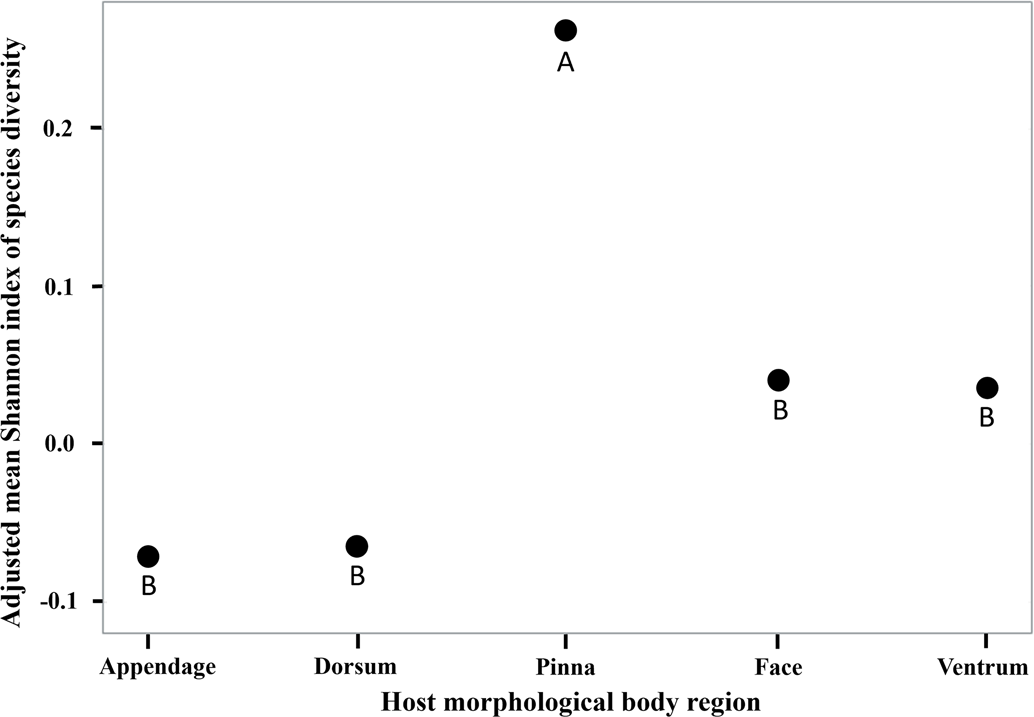
Figure 4. Adjusted mean Shannon diversity index for tick species, Amblyomma americanum, Dermacentor variabilis, Haemaphysalis longicornis, and Ixodes scapularis, collected on wildlife hosts, Procyon lotor, Didelphis virginiana, Tamis striatus, Microtus pinetorum, Ochrotomys nuttalli, Peromyscus leucopus, P. gossypinus, and P. maniculatus, morphological body regions on farms in East Tennessee in 2020–2021. Pinna regions (A) had significantly more diverse tick species compared to host appendage, dorsum, face, or ventrum (B).
Ticks cooccurred differently on wildlife hosts depending on species
The likelihood of finding ticks on different regions when other tick species were present was also compared. There was a 37% decrease in the odds of D. variabilis being present on regions when and where A. americanum was also present (P value = 0.0011; odds ratio 0.3728; 95% Cl 0.2048–0.6787) and a 53% decrease in the odds of H. longicornis being present on a host's body region when D. variabilis was present (P value = 0.0315; odds ratio 0.5319; 95% Cl 0.2990–0.9465). However, there was a 209% increase in the odds of H. longicornis being present whenever A. americanum was also present (P value = 0.0002; odds ratio 3.0932; 95% Cl 1.7002–5.6275). Additionally, H. longicornis abundance was 45 times more likely to increase with a 1 unit increase in the number of tick species on a host (F 1, 325 = 13.11, P < 0.0001) (Fig. 5).
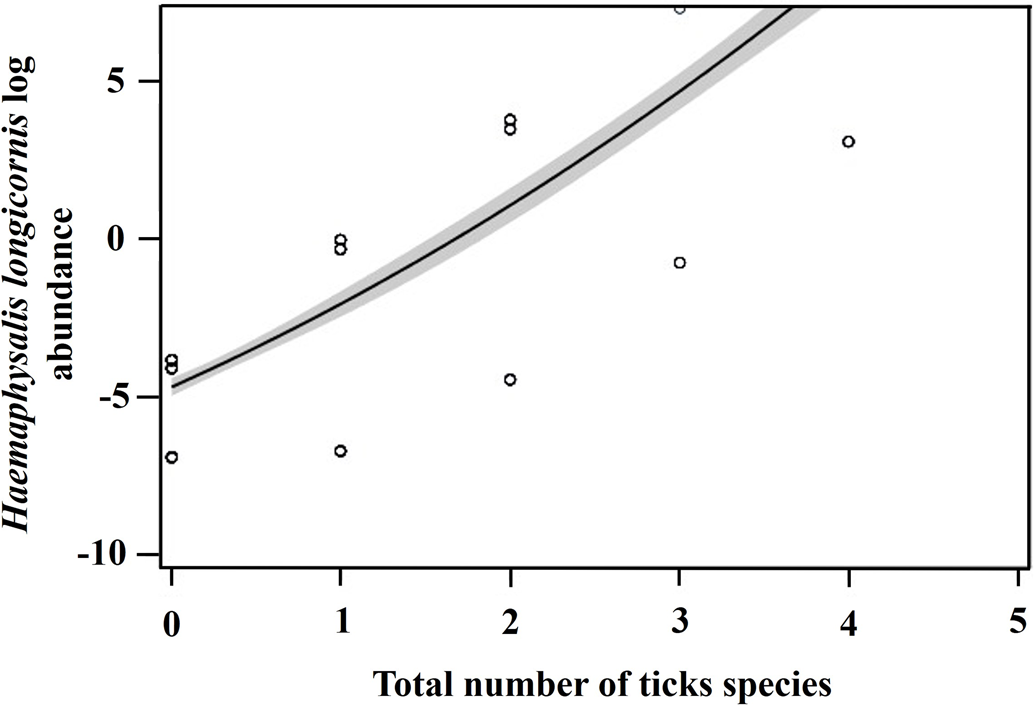
Figure 5. The log abundance of Haemaphysalis longicornis on wildlife hosts and the number of total tick species on farms in East Tennessee in 2020–2021.
Pasture management implications reduced ticks on wildlife
The management strategy used by each producer was also associated with H. longicornis infesting wildlife or on-animal abundance (F 2, 325 = 13.11, P < 0.0001), where higher numbers were found on farms 2 and 3 compared to farm 1 but there was no difference in the number of ticks collected from farms 2 and 3 (Fig. 6). On farm 1 there were 7 total (3 larvae and 4 nymphs) H. longicornis found on one raccoon and five Virginia opossum. Farm 2 had 93 (68 larvae, 24 nymphs and 1 female) H. longicornis on three raccoons and seven Virginia opossum, and farm 3 had 3031 (2879 larvae, 140 nymphs and 12 females) H. longicornis on two eastern chipmunks, eight raccoons, and six Virginia opossum. The percentage (total for a single species/total animals collected) × 100 of wildlife collected on farm 1 was 2.43% for raccoon, 6.39% for Virginia opossum, and 1.82% for eastern chipmunk; on farm 2 it was 1.82% for raccoon, 7.59% for Virginia opossum, and 1.82% for eastern chipmunk; and on farm 3 it was 5.16% for raccoon, 5.77% for Virginia opossum, and 9.72% for eastern chipmunk.
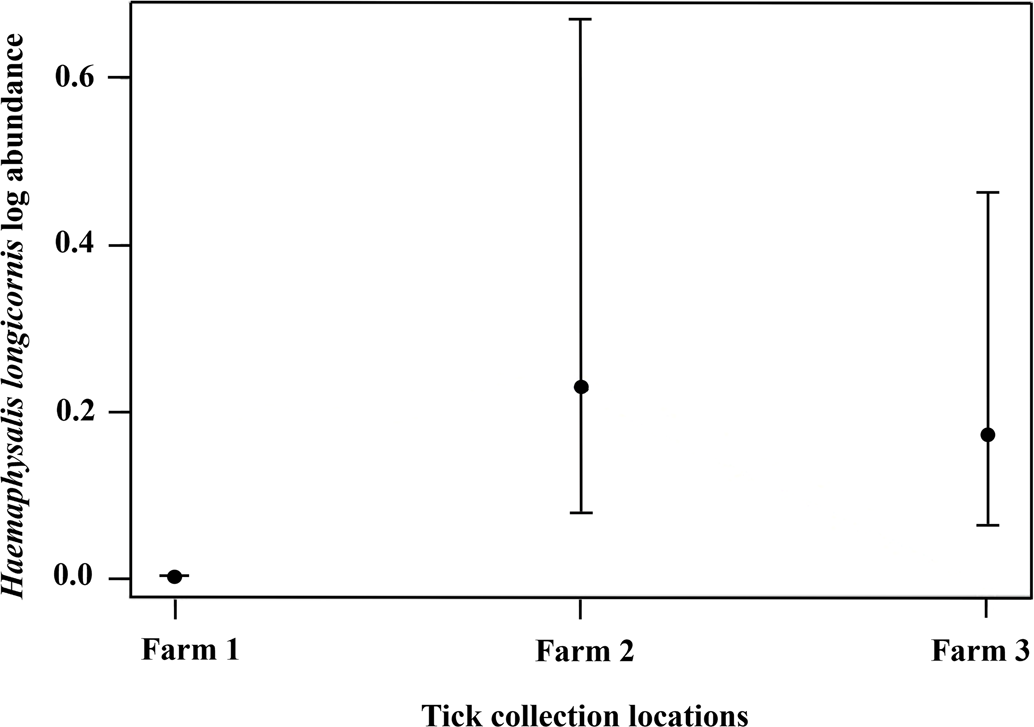
Figure 6. The abundance of Haemaphysalis longicornis on wildlife hosts at three farms in East Tennessee in 2020–2021. Each farm adopted different integrated pest management techniques to control tick populations in the environment which ultimately affected the abundance on wildlife hosts. Farm 1 bush hogged monthly, dragged for ticks frequently, and used on-animal control for cattle; farm 2 used bush hogged yearly, dragged for ticks moderately, and used on-animal control for cattle; farm 3 bush hogged yearly, dragged for ticks scarcely, and did not use an on-animal control for cattle.
Discussion
There is a need to better understand tick ecology because of their effects on a variety of animal hosts including humans, wildlife, companion animals, and livestock, but the invasion of H. longicornis makes the need more urgent (Dinkel et al., Reference Dinkel, Herndon, Noh, Lahmers, Todd, Ueti, Scoles, Mason and Fry2021). Discerning interactions between invasive and established tick species are imperative for understanding tick community structure on hosts. This study successfully identified wildlife and established tick species associated with the presence of H. longicornis which can be targeted for tick surveillance and management. Although ixodid tick species only spend 10% of their total life cycle on hosts compared to the environment (Needham and Teel, Reference Needham and Teel1991), knowing which host body regions are associated with tick attachment allows us to develop important tools for reducing large populations at a single time, which is especially important for a clonal species. In this study, tick and host body regions associated with H. longicornis presence that can be targeted for their control were identified. For example, targeting raccoon and Virginia opossum host's pinna, on farms infested with H. longicornis, would be an important on-animal control measure for exposing a larger abundance of ticks and tick species to acaracides.
Here wildlife host species types that were associated with tick parasitization but can also be evaluated as targets for on-farm tick control were identified. Haemaphysalis longicornis and other tick species were more likely to be present on medium-sized hosts compared to small mammals suggesting that managing these hosts may reduce tick populations on a farm. Raccoons had the highest abundance of H. longicornis, the greatest number of cofeeding tick species (6 species total) and the second highest tick Shannon diversity index. The next most heavily parasitized mammal after the raccoon was the Virginia opossum, which had the fourth highest tick Shannon diversity index. In previous studies in Tennessee, >90% of the ticks were collected from these 2 species and raccoons similarly had the highest tick species diversity (Kollars, Reference Kollars1993). The high abundances of H. longicornis on these 2 species are concerning because they could serve as reservoir hosts for some Anaplasma and Rickettsia species (Boostrom et al., Reference Boostrom, Beier, Macaluso, Macaluso, Sprenger, Hayes, Radulovic and Azad2002; Levin et al., Reference Levin, Nicholson, Massung, Sumner and Fish2002). Raccoons are known to have large variable home ranges in the Southeast (Hill et al., Reference Hill, Miller, Helton, Chipman, Gilbert, Beasley, Dharmarajan and Rhodes2023) and females often seek out dens in forested or edge environments near agricultural areas when rearing young (Henner et al., Reference Henner, Chamberlain, Leopold and Burger2004). The Virginia opossum is also known to use edge environments and have large home ranges for its medium size (Hill et al., Reference Hill, Miller, Helton, Chipman, Gilbert, Beasley, Dharmarajan and Rhodes2023). These selection behaviours by raccoon and Virginia opossum coincide with H. longicornis habitat preferences (Trout Fryxell et al., Reference Trout Fryxell, Chavez-Lindell, Butler and Odoi2023), suggesting that managing these animals may help prevent the spread and/or establishment of H. longicornis on a farm. Differences in the abundances of H. longicornis life stages, in which raccoon had more larvae but Virginia opossum had more females, could be due to adult life stages selecting less immature tick-infested hosts (Bloemer et al., Reference Bloemer, Zimmerman and Fairbanks1988; Estrada-Peña et al., Reference Estrada-Peña, Nava, Tarragona, de la Fuente and Guglielmone2020; Tiffin et al., Reference Tiffin, Skvarla and Machtinger2021).
Eastern chipmunks are known to exist in relatively small home ranges outside of their burrows (Yahner, Reference Yahner1978); therefore, decreased H. longicornis tick numbers could be due to fewer eastern chipmunk encounters. Eastern chipmunks also had the highest tick Shannon diversity index; decreased abundances could be due to negative interactions between tick species (Krasnov et al., Reference Krasnov, Stanko and Morand2007). Although the lowest number of H. longicornis was found infesting eastern chipmunks, they can serve as a potential reservoir host for some Borrelia and Rickettsiales-related tickborne pathogens (Mclean et al., Reference Mclean, Ubico and Cooksey1993; Keesing et al., Reference Keesing, McHenry, Hersh, Tibbetts, Brunner, Killilea, LoGiudice, Schmidt and Ostfeld2014; Siy et al., Reference Siy, Larson, Zembsch, Lee and Paskewitz2021). Despite collecting I. scapularis, D. variabilis, and A. americanum from rodents, H. longicornis were not found on these hosts. Peromyscus leucopus had the third highest tick Shannon diversity index, O. nuttalli and P. maniculatus had the fifth highest, and P. gossypinus had the sixth highest. Although H. longicornis is known to occasionally feed on rodents, they are not the primary host of this tick species and are not a major concern for transmission of rodent-associated pathogens such as B. burgdorferi (Breuner et al., Reference Breuner, Ford, Hojgaard, Osikowicz, Parise, Rosales Rizzo, Bai, Levin, Eisen and Eisen2020; Ronai et al., Reference Ronai, Tufts and Diuk-Wasser2020). Additionally, this study found I. scapularis to be more abundant on medium-size mammals compared to small mammals.
Overall, raccoons and opossums, had the highest likelihood of having invasive ticks present at the wildlife–livestock interface, which can be isolated for tick management in large abundances, were identified. Baiting regimes focused on these host species could be evaluated for acaracide applications to target H. longicornis and other important tick species on Tennessee farm settings since they feed and distribute a number of species. Additionally, raccoons and opossums are also likely to take advantage of livestock operations, by exploiting feed and watering systems, and come into frequent contact with cattle (Atwood et al., Reference Atwood, Deliberto, Smith, Stevenson and Vercauteren2009). Medium-size wildlife could be targeted for on-animal acaracide application by using methods modified from the 4 deer’4-poster’ station (Solberg et al., Reference Solberg, Miller, Hadfield, Burge, Schech and Pound2003) or systemically with acaracide-treated feed (Pound et al., Reference Pound, Miller, George, Oehler and Harmel1996). These methods for controlling ticks on meso mammals should be further studied for tick control. Additionally, culling animals with extremely high population densities could also reduce the number of ticks on sites by decreasing tick host populations (Martin et al., Reference Martin, Buttke, Raphael, Taylor, Maes, Parise, Ginsberg and Cross2023). However, for this method, population density counts would need to be well maintained to ensure that a species' populations are well managed.
Different tick species are known to parasitize certain regions of hosts' bodies, including the cervical spine, head, pinna, tail, legs, scrotum, and anus (Koch, Reference Koch1982; Hart et al., Reference Hart, Schad, Bhaskar, Reynolds, Morley and Thangamani2022). The inclination to parasitize different body regions could be a result of accessing host-seeking/attachment site, attraction to pheromones or host excessive heat, humidity, or protection from grooming (Hair et al., Reference Hair, Sauer and Durham1975; Cardé and Baker, Reference Cardé, Baker, Bell and Carde1984; Shaw et al., Reference Shaw, Keesing, Mcgrail and Ostfeld2003; Carr and Salgado, Reference Carr and Salgado2019; Lydecker et al., Reference Lydecker, Etheridge, Price, Banks and Hochuli2019). In this study, A. americanum, H. longicornis, D. variabilis, or I. scapularis interacted together equally on raccoon, Virginia opossum, and eastern chipmunk host's facial regions which also had the second highest average Shannon index of species diversity. Host pinna regions had the highest abundance and greatest tick diversity overall, although A. americanum presence outnumbered all other tick species. These results could be due to ticks using negative geotaxis, a mechanism in which ticks walk upwards to find available attachment sites upon encountering a host (Lees, Reference Lees1948; Kröber et al., Reference Kröber, Bourquin and Guerin2013). Others have noted that tick selection of host pinna regions could be due to inaccessibility of hosts at grooming these areas (Guerin et al., Reference Guerin, Kro, Ber, Mcmahon, Guerenstein, Grenacher, Vlimant, Diehl, Steullet and Syed2000). Previous research has identified various volatiles on the host's head, used during mating season, could attract ticks to this body region (Gassett et al., Reference Gassett, Wiesler, Baker, Osborn, Miller, Marchinton and Novotny1997). Carr and Salgado (Reference Carr and Salgado2019) found that ticks use their Haller's organ to detect radiant heat emitted from hosts which may explain the number and diversity of ticks removed from the host's head and pinna regions (Vianna and Carrive, Reference Vianna and Carrive2005). Some tick species are highly attracted to host body regions that source CO2 (Norval et al., Reference Norval, Peter and Meltzer1992), which could contribute to our outcome in which a greater abundance and diversity of tick species congregate on the head and pinna regions. Kaur et al. (Reference Kaur, Jaiswal and Mishra2017) reported that ticks generally chose highly vascular areas on host body regions with thinner skin, ticks infesting hosts head and pinna regions in our study could be due to similar results. Ticks could also be attracted to certain regions of hosts due to females emitting assembly pheromones, which signal clustering of ticks to enhance mating opportunities (Sonenshine et al., Reference Sonenshine, Taylor’ and Corrigan1985). Additional research on interactions between native US tick species and H. longicornis pheromones should be further investigated.
In our study, the presence of A. americanum on a host increased the likelihood that H. longicornis was also present. Haemaphysalis longicornis followed similar activity and host selection patterns as A. americanum, in which all life stages of both species were found predominantly on medium-size mammals but were almost non-existent on small mammals. Other studies investigating H. longicornis host use also found similar results in the US (Thompson et al., Reference Thompson, White, Shaw, Garrett, Wyckoff, Doub, Ruder and Yabsley2021; Tufts et al., Reference Tufts, Goodman, Benedict, Davis, VanAcker and Diuk-Wasser2021; White et al., Reference White, Bevins, Ruder, Shaw, Vigil, Randall, Deliberto, Dominguez, Thompson, Mertins, Alfred and Yabsley2021). Here, A. americanum and H. longicornis were found to equally occupy and interact on raccoon and Virginia opossum host's ventrum. Jang et al. (Reference Jang, Kim, Kim, Yoon, Han, Kim, Oh, Yoon, Wie and Hur2016) also noted the primary attachment sites for H. longicornis were the extremities, abdomen, and inguinal region. Cooccurrence of these 2 tick species on these regions may also be related to reduced competition or based on density dependence in available feeding space (Anderson et al., Reference Anderson, Ezenwa and Jolles2013). Since these 2 tick species were readily present together on the ventrum and pinna regions had the highest abundance of both tick species, targeting these regions for tick control with an acaracide application could be important for the management of both species. Importantly, these two tick species are also found to have predicted geographic distributions which overlap one another in the US (Raghavan et al., Reference Raghavan, Townsend Peterson, Cobos, Ganta and Foley2019; Namgyal et al., Reference Namgyal, Couloigner, Lysyk, Dergousoff and Cork2020). Investigations of questing ticks from the environment found a contrasting result where H. longicornis larvae and nymphs decreased when A. americanum larvae and nymphs increased (Trout Fryxell et al., Reference Trout Fryxell, Chavez-Lindell, Butler and Odoi2023); perhaps suggesting competition at questing sites and not necessarily on the hosts. Future studies are necessary to understand interactions between environmental and host cues associated with tick-feeding patterns.
Contrastingly, the presence of tick species D. variabilis decreased the likelihood of A. americanum or H. longicornis also being present. Dermacentor variabilis had a lower presence on the ventrum, pinna, and appendage regions compared to A. americanum or H. longicornis, but a higher presence on the dorsum compared to H. longicornis. Haemaphysalis longicornis is found to interact in similar space with A. americanum but there appears to be possible competition between D. variabilis, which could have serious implications for the spread of tickborne diseases via cofeeding (Randolph et al., Reference Randolph, Gem and Nuttall1996). For example, pathogens associated with A. americanum in the US which can be potentially transmitted to H. longicornis due to cofeeding include Bourbon and Heartland viruses and Ehrlichia, Anaplasma, and Rickettsia bacteria (Ewing et al., Reference Ewing, Dawson, Mathew, Barker, Pratt and Telford1997; Lee et al., Reference Lee, Na, Kim, Li, Cho, Park, Lee, Eo, Klein and Chae2005; Wright et al., Reference Wright, Sonenshine, Gaff and Hynes2015; Godsey et al., Reference Godsey, Savage, Burkhalter, Bosco-Lauth and Delorey2016, Reference Godsey, Rose, Burkhalter, Breuner, Bosco-Lauth, Kosoy and Savage2021; Beard et al., Reference Beard, Occi, Bonilla, Egizi, Fonseca, Mertins, Backenson, Bajwa, Barbarin, Bertone, Brown, Connally, Connell, Eisen, Falco, James, Krell, Lahmers, Lewis, Little, Neault, Pérez de León, Randall, Ruder, Saleh, Schappach, Schroeder, Seraphin, Wehtje, Wormser, Yabsley and Halperin2018; Cumbie et al., Reference Cumbie, Trimble and Eastwood2022). Future studies which investigate host attributes associated with H. longicornis and established tick species on-animal aggregations will be important for understanding tick community structure and pathogen transmission.
Haemaphysalis longicornis were more likely to be present on a host as the number of tick species on that host increased; overall, 1–5 tick species cofeeding on hosts were found. Competition between species is often the result of limited shared resources (Mirrahimi et al., Reference Mirrahimi, Perthame and Wakano2014). In this study, H. longicornis presence was predicted to occur when a greater number of tick species were present, suggesting a neutral relationship. In addition, H. longicornis was less likely to be present whenever it occurred alone with either tick species A. americanum, I. scapularis, or D. variabilis. Cofeeding ticks can transmit pathogens to one another (Randolph et al., Reference Randolph, Gem and Nuttall1996). Although a diverse tickborne pathogen community infesting hosts and tick vectors could promote competition between pathogens which ultimately reduces their abundance (Genne et al., Reference Genne, Sarr, Gomez-Chamorro, Durand, Cayol, Rais and Voordouw2018), tick–pathogen community structure is less studied for H. longicornis in the US. Presently, established tick species which can be used to identify the presence of an H. longicornis on wildlife hosts were identified. The increased likelihood for H. longicornis to be present feeding on a host when multiple tick species are also present at the same time is a concern because the transmission risk is unknown for the cofeeding behaviour.
Importantly, significant IPM strategy effects on the host level; specifically, ticks were less likely to be present on wildlife sampled from farm 1 compared to wildlife sampled from farm 2 or 3, were observed. Previously the management decisions used by producers effectively reduced H. longicornis populations from the environment were documented (Butler and Trout Fryxell, Reference Butler and Trout Fryxell2023), and here, those management effects were seen on wildlife as well, fewer H. longicornis ticks were on wildlife from farm 1. The percentages for raccoon, opossum, and eastern chipmunk caught at each farm were also similar although the total number of wildlife species varied. Understanding interactions between the environment, hosts, tick population dynamics, and human exposure is important for creating a tick IPM strategy (Stafford et al., Reference Stafford, Williams and Molaei2017). Mechanical, cultural, and on-animal chemical control can reduce H. longicornis in the environment and on wildlife in a farm setting.
Different mammal host and tick species characteristics which can be targeted for control of an invasive tick at the wildlife–livestock interface were identified. Future studies may investigate on-host management regimes in the field to reduce H. longicornis. These could include culling or acaracide-baiting regimes to target medium-size hosts (raccoons and opossum), or behaviours associated with these hosts during periods such as mating season using attractants; however, these methods need to be evaluated. These medium-size hosts could also be targeted by directing management towards farm practices that allow access to livestock food. The likelihood that H. longicornis was present on a host was associated with the presence of multiple species feeding concurrently. Tick control that can target a greater number of tick species may help simultaneously reduce both invasive and established tick species. Identifying activity periods for when established tick species are feeding simultaneously could be important for future H. longicornis management. Subsequent research could focus on how semiochemicals on hosts affect tick-feeding strategies. This study identified host and tick variables which were associated with H. longicornis at the wildlife–livestock interface. In addition, previous research including producer led IPM strategies in the environment was identified that reduced the likelihood that ticks would be present on wildlife hosts. Although evaluation before culling animals is recommended, future studies should investigate H. longicornis management by targeting these variables to reduce tick presence.
Data availability statement
Data will be made available upon request from corresponding author.
Acknowledgements
We thank the farm owners for allowing Butler to sample wildlife to collect ticks on their properties.
Author contributions
R. A. B., L. I. M., D. G. and R. T. T. F. conceived research ideas. R. A. B. and R. T. T. F. designed the study. R. A. B. conducted data gathering, performed statistical analyses and wrote the initial draft of the manuscript. All authors participated in manuscript review and editing.
Financial support
Funding for R. A. B. was provided by the Department of Entomology and Plant Pathology at the University of Tennessee. Additional support was provided by the medical and veterinary research fund. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
None.
Ethical standards
All ethical standards were approved by the University of Tennessee IACUC under protocol #2774-0620.












