Introduction
Titan, the largest moon of Saturn, is the only moon in the solar system to have a significant atmosphere (Kuiper, Reference Kuiper1944). While composed mostly of nitrogen (Niemann et al., Reference Niemann2010), the atmosphere also possesses 2–5% methane (CH4), which participates in a meteorological cycle of evaporation, cloud formation and rainfall, in a similar manner to the hydrological cycle on Earth (Figure 1). This occurs because methane at Titan’s surface conditions (90–93 K, 1.467 bar) is already close to its triple point (90.67 K, 0.117 bar). Therefore, surface methane reservoirs are readily evaporated by sunlight, humidifying the lower atmosphere, where methane can form clouds at altitudes ∼10–30 km above the surface (Lorenz, Reference Lorenz1993; Mitchell et al., Reference Mitchell, Lora, Jeanloz and Freeman2016). These clouds lead to infrequent but possibly severe methane rainstorms leading to wetting of the surface (Turtle et al. Reference Turtle, Perry, Hayes, Lorenz, Barnes, McEwen, West, Del Genio, Barbara, Lunine and Schaller2011; Dhingra et al. Reference Dhingra, Barnes, Brown, Burrati, Sotin, Nicholson, Baines, Clark, Soderblom, Jauman and Rodriguez2019) and creation of erosion channels (Perron et al. Reference Perron, Lamb, Koven, Fung, Yager and Ádámkovics2006; Jaumann et al. Reference Jaumann, Brown, Stephan, Barnes, Soderblom, Sotin, Le Mouélic, Clark, Soderblom, Buratti and Wagner2008). Seasonal migration of clouds have been tracked by imaging (Rodriguez et al. Reference Rodriguez, Le Mouélic, Rannou, Sotin, Brown, Barnes, Griffith, Burgalat, Baines, Buratti and Clark2011; Turtle et al. Reference Turtle, Perry, Barbara, Del Genio, Rodriguez, Le Mouélic, Sotin, Lora, Faulk, Corlies and Kelland2018), mostly arising in the summer hemisphere where insolation is strongest, leading to the concept of ‘methane monsoons’ (Faulk et al., Reference Faulk, Mitchell, Moon and Lora2017; Charnay et al., Reference Charnay, Barth, Rafkin, Narteau, Lebonnois, Rodriguez, Courrech Du Pont and Lucas2015). Titan is therefore the only world other than Earth known to have a meteorological cycle in contact with a solid surface at the present day.
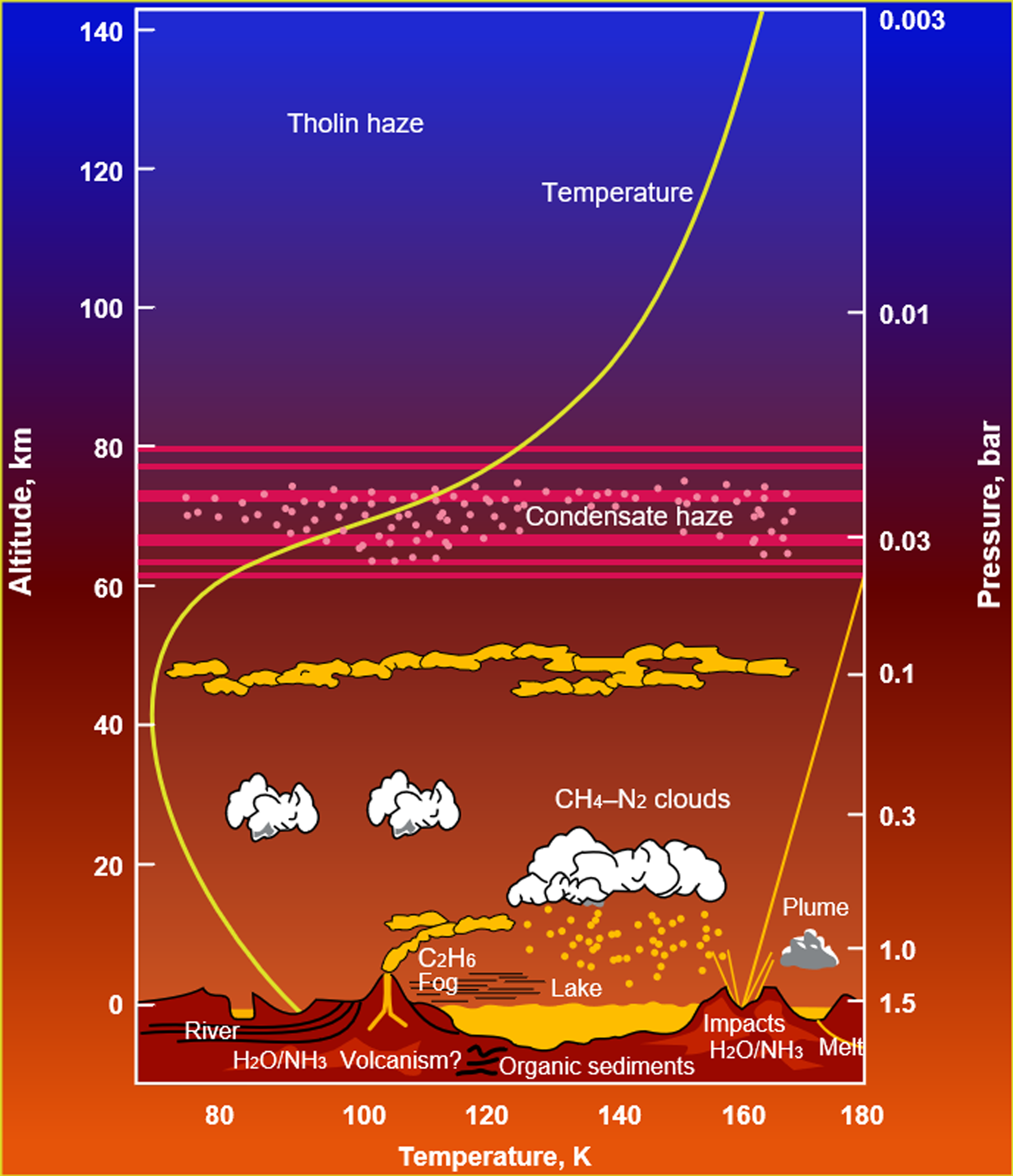
Figure 1. Atmospheric phenomena together with the temperature and pressure profile of Saturn’s moon Titan (Image credit: NASA/ESA).
Methane serves many roles in Titan’s atmosphere, including regulating the energy balance via a ‘greenhouse effect’ (McKay et al., Reference McKay, Pollack and Courtin1991), but also importantly by serving a feedstock for organic photochemistry (Yung et al., Reference Yung, Allen and Pinto1984; Dobrijevic et al., Reference Dobrijevic, Loison, Hickson and Gronoff2016; Vuitton et al., Reference Vuitton, Yelle, Klippenstein, Hörst and Lavvas2019; Willacy et al., Reference Willacy, Chen, Adams and Yung2022). Methane – along with nitrogen – is dissociated in Titan’s upper atmosphere by solar UV and energetic particles, reforming into complex organic molecules (Figure 2) (Loison et al., Reference Loison, Dobrijevic and Hickson2019; Vuitton et al., Reference Vuitton, Yelle, Klippenstein, Hörst and Lavvas2019; Waite et al., 2007) at the expense of H2 lost to space (Strobel, 2012). Currently, there are 24 known molecules in Titan’s atmosphere (Nixon, Reference Nixon2024), with many more complex types lurking in the rich mass spectrum detected by instruments on the Cassini-Huygens spacecraft (Vuitton et al., 2007; Waite et al., 2005, 2007) that remain largely unidentified.
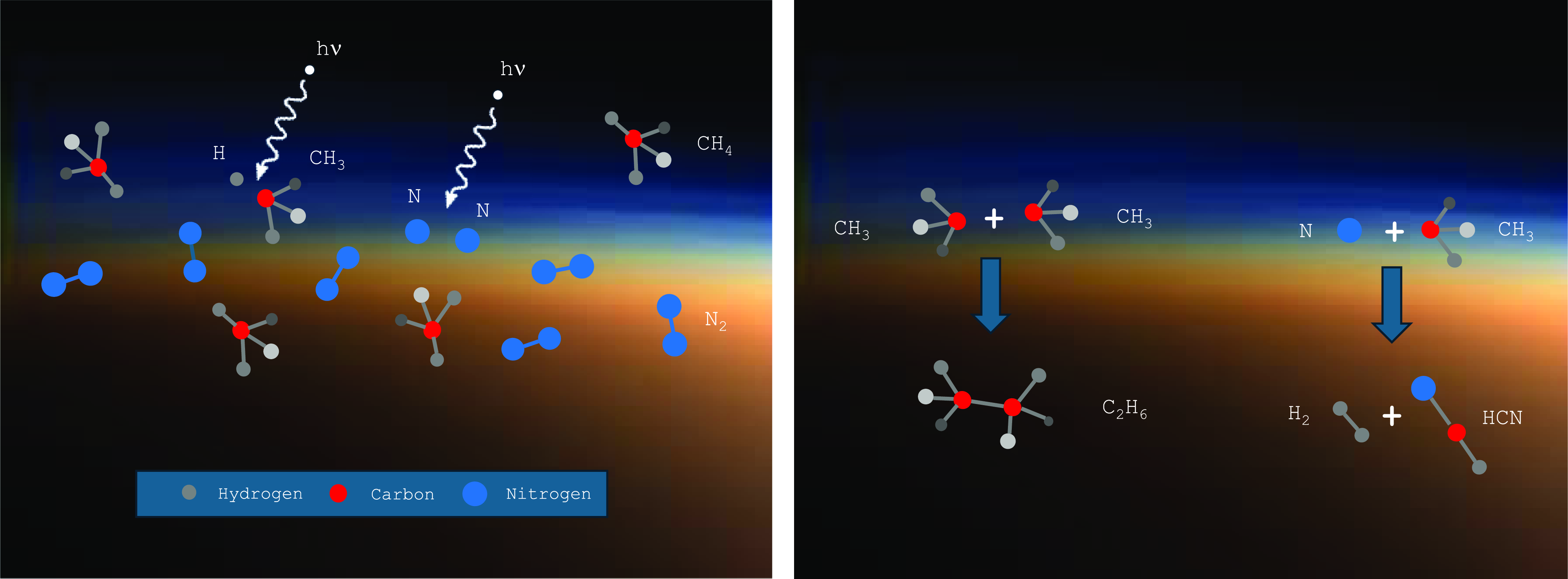
Figure 2. Left: UV radiation leads to partial fragmentation of atmospheric molecules into radicals. Right: Radicals recombine to make new, more complex species.
With such an active production of organic molecules, questions have naturally arisen about the extent reached by the chemical complexity (Raulin, 1992, 2008), and whether Titan can serve as a present-day laboratory for studying processes that may have led to the origin of life on Earth. Such speculation has been lent additional credence by laboratory simulation experiments, similar to the famous Miller-Urey experiment (Miller and Urey, Reference Miller and Urey1959), demonstrating that amino acids can indeed be formed from simple ingredients found on Titan (Khare et al., Reference Khare, Sagan, Ogino, Nagy, Er, Schram and Arakawa1986; Neish et al. Reference Neish, Somogyi and Smith2010; Poch et al., Reference Poch, Coll, Buch, Ramírez and Raulin2012; Farnsworth et al., Reference Farnsworth, McLain, Chung and Trainer2024).
Most experiments simulating atmospheric reaction conditions begin with N2 and CH4 reagents, and by means of energetic bond breaking, show that refractory organic residues formed by UV radiation in the atmosphere and dubbed ‘tholins’ (Khare et al., Reference Khare, Sagan, Zumberge, Sklarew and Nagy1981; Imanaka et al., Reference Imanaka, Khare, Elsila, Bakes, McKay, Cruikshank, Sugita, Matsui and Zare2004; Carrasco et al., Reference Carrasco, Schmitz-Afonso, Bonnet, Quirico, Thissen, Dutuit, Bagag, Laprévote, Buch, Giulani and Adandé2009; Cable et al., Reference Cable, Hörst, Hodyss, Beauchamp, Smith and Willis2012; Hörst and Tolbert, Reference Hörst and Tolbert2013) result, which tend to form aggregates due to the polarity of their head groups (Figure 3). Tholins are typically lacking in oxygen necessary to form nucleobases, but hydrolysis in the presence of water that may be periodically present on Titan surface can remedy this problem (Khare et al., Reference Khare2009, Reference Khare, Sagan, Ogino, Nagy, Er, Schram and Arakawa1986). Another route of possible reaction pathways has recently been demonstrated by adding CO into the original gaseous mixture (Hörst et al., Reference Hörst, Yelle, Buch, Carrasco, Cernogora, Dutuit, Quirico, Sciamma-O’Brien, Smith, Somogyi and Szopa2012).
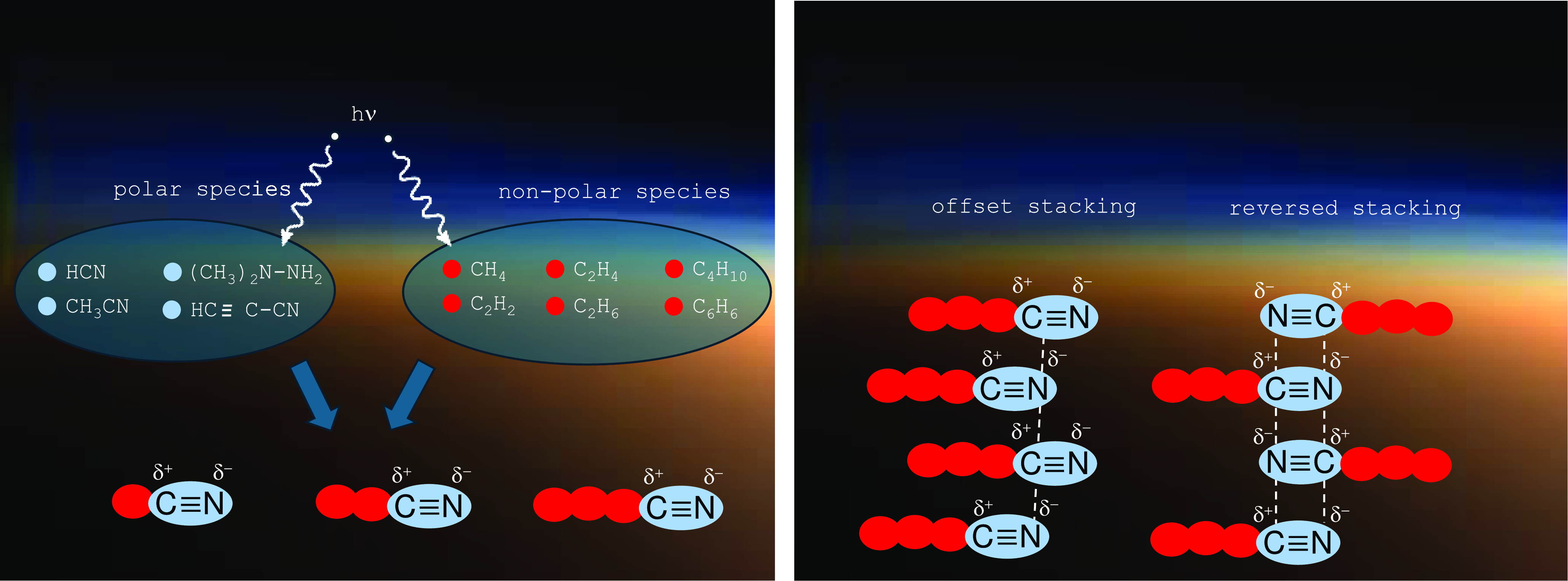
Figure 3. Left: Radical reactions lead to the formation of amphiphilic “tholins.” Right: Due to the dipolar nature of the polar head groups, those amphiphilic molecules self-aggregate in specific structures. In case of “offset stacking,” the shift between adjacent molecules leads to attractive electrostatic interactions.
A key step towards the origin of life on Earth was likely the formation of vesicles, spherical structures formed by bilayer membranes that resemble the structural boundaries of living cells (Deamer and Barchfeld, Reference Deamer and Barchfeld1982; Oró et al., Reference Oró, Miller and Lazcano1990; Luisi, Reference Luisi2006; Pross, Reference Pross2016; Lingam and Loeb, Reference Lingam and Loeb2021; Fahrenbach and Cleaves, Reference Fahrenbach and Cleaves2024). The main question in the context of origin of life on Titan is, whether such a vesicle formation might be possible under the given conditions. Even though the conditions on Titan are very different from those on early Earth, it may also serve as a present-day laboratory for studying fundamental processes that may be relevant for the origin of life on Earth. In the following, we want to demonstrate that a principal mechanism known for terrestrial vesicle formation is also relevant under the conditions of Titan and that it could lead to cell-like compartments and eventually to the evolution of protocells over long periods of time on its surface. We suggest that compartments such as vesicles can, in principle, form under extreme conditions, even in absence of water, in a hydrophobic environment, without terminal end groups containing oxygen and under very low temperatures. Considering that the formation of compartments is the key step for the development of a protocell, the breadth of external conditions under which life could form is considerably increased. In any case, the proposed vesicle formation would represent a possible first step in a development towards increasing order and complexity (Mayer, Reference Mayer2020) as key requirements for the origin of life.
Vesicle formation on early Earth
It is generally accepted that the formation of cell-like compartments is a key step in the development of terrestrial life (Morowitz, Reference Morowitz1992; Segré et al., Reference Segré, Ben-Eli, Deamer and Lancet2001; Szostak et al., Reference Szostak, Bartel and Luisi2001; Rasmussen et al., 2009; Deamer et al., Reference Deamer2010). Among all possible varieties of such compartments, membrane vesicles or liposomes seem to be the most likely alternative. Vesicles with bilayer membranes very similar to actual biomembranes easily form by self-aggregation of amphiphilic compounds in an aqueous environment. Among all structural components of living cells, membrane compartments are the ones that are most easily accessible in a prebiotic context. Therefore, it seems natural that their formation is a valid first step in the origin of life (Segré et al., Reference Segré, Ben-Eli, Deamer and Lancet2001).
This given, it is tempting to search for local conditions and planetary environments that lead to the formation of membrane compartments. Vesicles are known to form by current-induced shear acting on multilayer structures (Zipfel et al., Reference Zipfel, Lindner, Tsianou, Alexandridis and Richtering1999; Courbin and Panizza, Reference Courbin and Panizza2004; Has and Pan, Reference Has and Pan2020). This type of vesicle formation is similar to the formation of amphiphilic multilayers during wet-dry cycling as most prominently proposed in the hot springs theory for the origin of life (Damer and Deamer, Reference Damer and Deamer2015, Reference Damer and Deamer2020). Another practical approach for vesicle formation is solvent transfer and solvent evaporation (Kim et al., Reference Kim, Jacobs and White1985; Pidgeon et al., Reference Pidgeon, McNeely, Schmidt and Johnson1987; Has and Pan, Reference Has and Pan2020). On the terrestrial surface, this alternative is less likely to occur, mainly due to the lack of hydrophobic solvents. Instead, early Earth conditions offer a third, much more probable pathway: vesicle formation via aerosol droplets (Dobson et al., Reference Dobson, Allison, Tuck and Vaida2000, Tuck, Reference Tuck2002, Donaldson et al., Reference Donaldson, Tervahattu, Tuck and Vaida2004). In the presence of amphiphiles, aerosol droplets are very likely to carry a monolayer of amphiphilic compounds. If these aerosol droplets settle onto the surface of a pond, a lake or an ocean, which again carries an amphiphile monolayer, those two monolayers combine and form a bilayer with a spherical shape corresponding to the original droplet. Hereby, vesicles are generated that contain the liquid phase of the aerosol and that are dispersed in the liquid medium of the water body. Since both liquid media do not necessarily coincide in their composition, osmotic pressure may develop across the membrane structure.
Lately, it was proposed that a similar mechanism could occur under hydrothermal conditions in tectonic fault zones (Mayer et al., Reference Mayer, Schreiber and Dávila2015). Tectonic fault zones are systems of interconnected cracks and cavities that reach from the surface down to the Earth’s mantle (Schreiber et al., Reference Schreiber, Locker-Grütjen and Mayer2012). They are filled with water and carbon dioxide as bulk solvents together with all types of hydrothermal chemistry. Amphiphilic compounds form hydrothermally, e.g. by Fischer-Tropsch chemistry (Berndt et al., Reference Berndt, Allen and Seyfried1996; Schreiber et al., Reference Schreiber, Mayer, Schmitz, Rosendahl, Bronja, Greule, Keppler, Mulder, Sattler and Schöler2017). Near a depth around 1 km, the carbon dioxide phase undergoes a transition from the supercritical to the gaseous phase, leading to an accumulation of amphiphilic compounds and other organic constituents (Mayer et al., Reference Mayer, Schreiber and Dávila2017). The periodic formation of vesicles induced by pressure variations (e.g. caused by tidal phenomena or geyser eruptions) could even start a structural evolution process potentially leading to primitive protocells (Mayer et al., Reference Mayer, Schreiber, Dávila, Schmitz, Bronja, Meyer, Klein and Meckelmann2018). Thus, this environment not only offers the pathway to vesicles but also allows for more complex structures to evolve.
Potential vesicle formation on Titan
Compared to the early Earth, conditions on Titan are vastly different, especially regarding the low temperatures and the absence of water (Hörst, Reference Hörst2017). The surface temperature varies between 89–93 K (small equator to winter pole variation, Jennings et al., Reference Jennings, Flasar, Kunde, Samuelson, Pearl, Nixon, Carlson, Mamoutkine, Brasunas, Guandique and Achterberg2009 and Reference Jennings, Tokano, Cottini, Nixon, Achterberg, Flasar, Kunde, Romani, Samuelson, Segura and Gorius2019; Cottini et al. Reference Cottini, Nixon, Jennings, de Kok, Teanby, Irwin and Flasar2012), falling through the troposphere to a low of around 70 K at approximately 45 km. This cold trap at the tropopause ensures that almost all volatiles are condensed out of the atmosphere below 45 km, leaving only N2 (∼95%), CH4 (∼5%), H2 (∼0.1%), and CO (50 ppm) as significant components. On the other hand, there are lakes and there is regular precipitation (Lunine and Lorenz, Reference Lunine and Lorenz2009). With the low surface tension of methane/nitrogen mixtures (Wohlfarth, Reference Wohlfarth and Lechner2016), droplets tend to be very small such that the formation of aerosols is likely. Therefore, among all possible pathways towards vesicles, the one linked to aerosol droplets appears to be favored.
Regarding amphiphilic compounds, the recent Cassini mission revealed the presence of organic nitriles (Müller-Wodarg et al., Reference Müller-Wodarg, Griffith, Lellouch and Cravens2014, Stevenson et al., Reference Stevenson, Lunine and Clancy2015, Nixon, Reference Nixon2024). Such compounds with the general structure R-CN are basically amphiphilic and have the capability to self-aggregate in non-polar environments (Ruckenstein and Nagarajan, Reference Ruckenstein and Nagarajan1980). In case of organic nitriles R-CN, these aggregates would lead to close contacts between the partially positively charged carbon atoms and the partially negatively charged nitrogen atoms. This could result in stacks of R-CN molecules with alternating orientations (Stevenson et al., Reference Stevenson, Lunine and Clancy2015), or in an arrangement of molecules with common orientation being alternatingly shifted by the CN bond length (Figure 3, right). Obviously, the resulting aggregates have the potential to form membranes in non-polar media. Moreover, vesicle structures may form that, due to their liposome-like structure, are being referred to as azotosomes (Stevenson et al., Reference Stevenson, Lunine and Clancy2015). In other words, they represent vesicles with non-polar content dispersed in a non-polar environment. Their membranes consist of R-CN molecules with an inner layer of CN residues and two outer layers of organic chains R.
Even if such vesicles are just kinetically stable and may not form spontaneously (Sandström and Rahm, Reference Sandström and Rahm2020), their formation via the aerosol droplet mechanism could lead to their temporary existence. Once being dispersed in a liquid environment on Titan, they could collect other amphiphilic components and hereby gain stabilization. Another result of the Cassini mission was the detection of carbon chain anions, which points to the possible formation of more complex organic molecules (Desai et al., Reference Desai, Coates, Wellbrock, Vuitton, Crary, González-Caniulef, Shebanits, Jones, Lewis and Waite2017). Such complex organic components could integrate into the existing R-CN membranes, hereby lowering their free enthalpy. This could eventually result in thermodynamically stable vesicles (azotosomes). In the following, we propose a step-by-step procedure that could lead to the continuous formation of vesicles, to their further development and to their evolution towards stable and functional protocell-like structures. It starts with kinetically driven monolayer and vesicle formation, proceeds via a thermodynamically driven stabilization process and leads to a periodicity-driven evolution process. These steps are visualized in Figures 4–8.
-
(A) Formation of amphiphilic monolayers on methane-nitrogen surfaces (Figure 4)
-
Amphiphilic compounds that have formed in the atmosphere will be carried into the lakes by condensation or rainfall and will preferentially accumulate in the top layer of the lake (Farnsworth, et al., Reference Farnsworth, Soto, Chevrier, Steckloff and Soderblom2023). Driven by their amphiphilicity and by dipolar interaction (that is predominantly attractive due to the intermolecular shift that leads to a direct contact between a partially negative and a partially positive residue), they form a stacked monolayer on the surface of the lake. Due to the effect of the amphiphile on the surface tension, this layer is thermodynamically stable and will regenerate rapidly even if the surface is disturbed (Figure 4).
-
(B) Formation of secondary droplets (Figure 5)
-
During rainstorms, droplets of liquid methane fall onto the lake’s surface. In addition, the occurrence of hail has been postulated (Graves et al., Reference Graves, McKay, Groffith, Ferri and Fulchignoni2007). The impact of rain droplets or solid particles causes local splashing, resulting in the separation of small secondary droplets from the coated surface (Figure 5, left). This separation process is supported by the reduced surface tension due to the action of the amphiphile. Consequently, each secondary droplet will carry a thermodynamically stable amphiphilic monolayer. This results in an aerosol of coated droplets right above the lake’s surface (Figure 5, right).
-
(C) Vesicle formation (Figure 6)
-
Driven by gravitation, the coated secondary droplets settle onto the surface of the lake. Their monolayer interacts with the corresponding monolayer of the bulk surface by forming a double layer. On further integration into the bulk phase, this double layer fully encloses the original content of the secondary droplet, resulting in a vesicle structure (Figure 6, left). This vesicle will not be thermodynamically stable, however it will show enough kinetic stability to survive temporarily, especially under the given low-temperature conditions. Even if the double-layer membranes cannot self-assemble otherwise (Sandström and Rahm, Reference Sandström and Rahm2020), this pathway of formation leads to kinetically stable vesicles in liquid dispersion (Figure 6, right). This pathway of vesicle formation overcomes the barrier of a positive free enthalpy change that completely prevents spontaneous self-assembly of vesicles in the continuous phase. Hence, it may allow for the temporary presence of kinetically stable vesicles from acrylonitrile or even from acetonitrile, propionitrile, butanenitrile, pentanenitrile, or hexanenitrile (as well as corresponding amines such as aminopropane, aminobutane, aminopentane or aminohexane), which altogether are less favorable for spontaneous formation. The postulated mechanism is completely analogous to the one proposed for hydrothermal environments in tectonic fault zones that have been experimentally reproduced (Mayer et al., Reference Mayer, Schreiber and Dávila2015, Reference Mayer, Schreiber, Dávila, Schmitz, Bronja, Meyer, Klein and Meckelmann2018).
-
In liquid dispersion, the vesicles collect those (potentially rare) amphiphiles that integrate well into the existing double layer. The higher the driving force for integration, the higher the decrease in the free energy of the membrane, leading to growing thermal stability of the membrane. This way, those stabilizing amphiphiles will be selected and will accumulate in the vesicles, which in turn gain stability (Figure 7, left). The vesicles themselves will be selected for their stability, such that the stabilizing amphiphiles will undergo an overall accumulation in the lake, with a level of concentration growing beyond the one of the original chemical equilibrium (Figure 7, right).
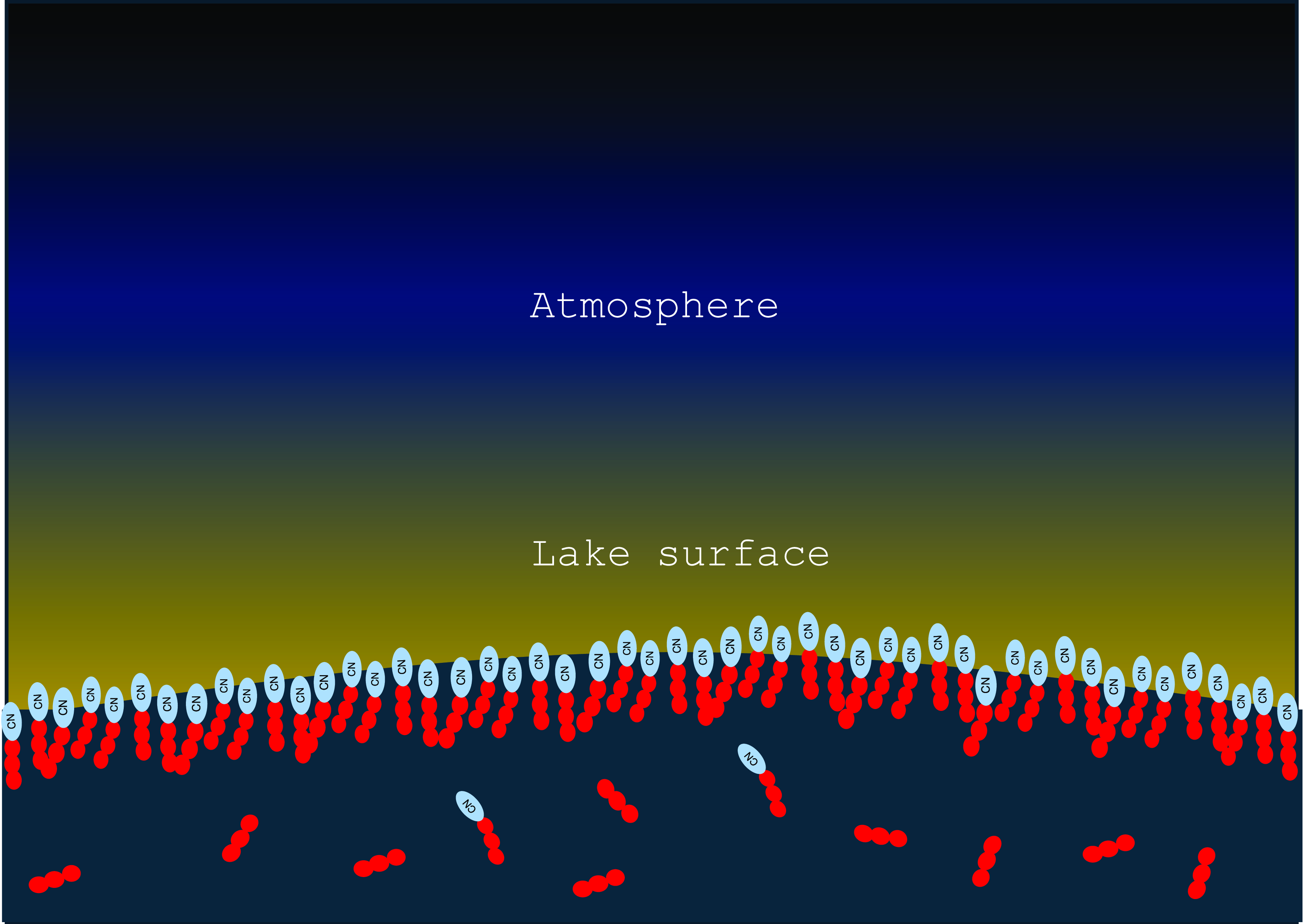
Figure 4. Condensation and rainfall bring a mixture of polar and non-polar molecules to Titan lakes. Due to the interfacial tension, larger aggregates have the tendency to accumulate on the surface of lakes.
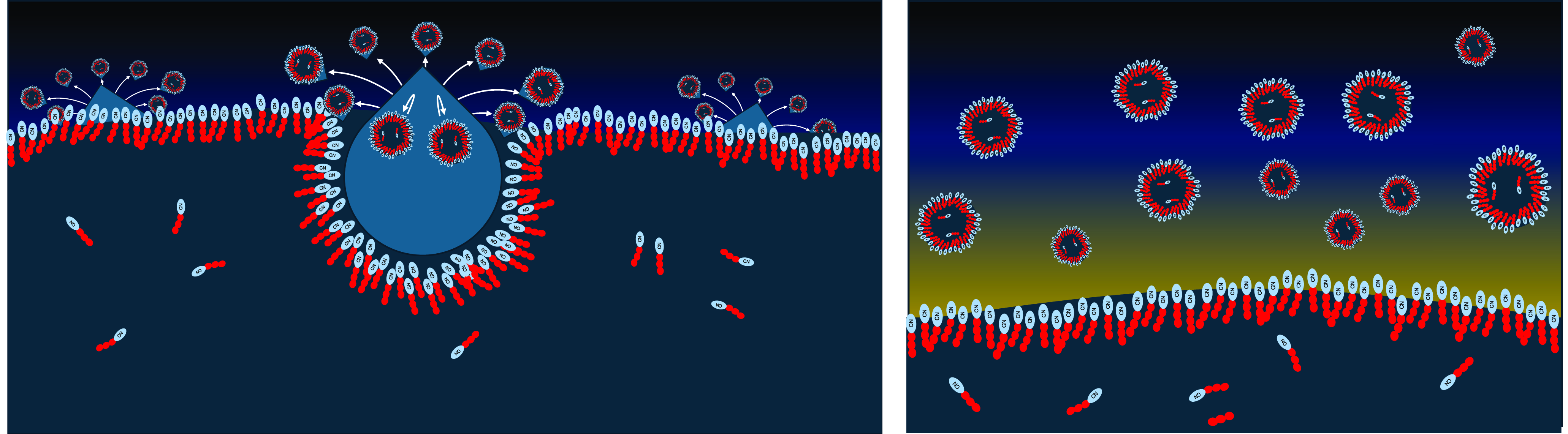
Figure 5. Left: Methane droplets or hail particles can splash the lake surface, throwing up a spray of small lake droplets that retain the surface monolayer. Right: A mist of coated methane droplets forms above the lakes in the wake of passing rain storms.
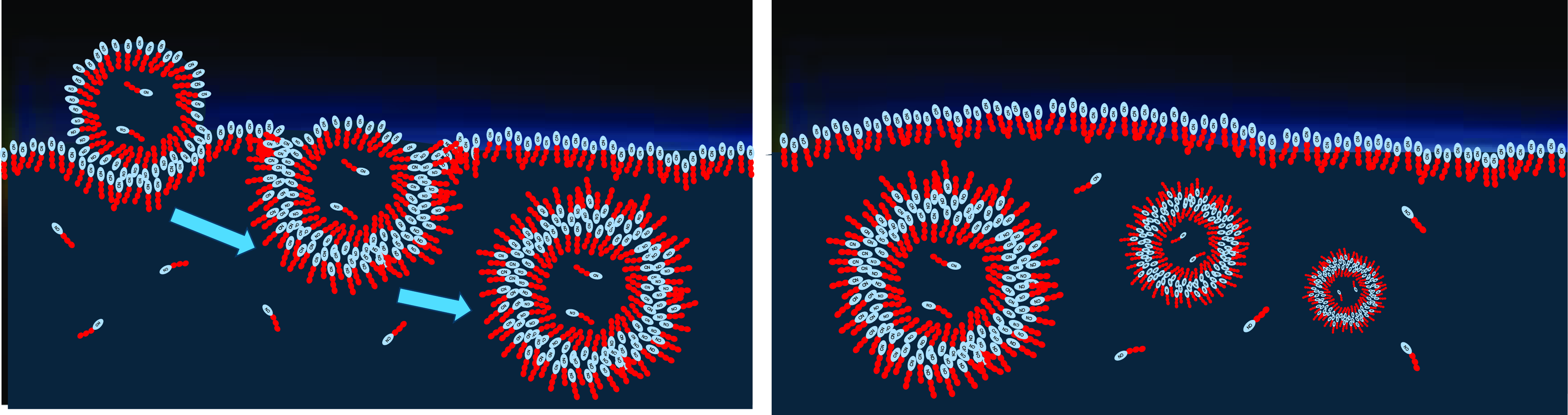
Figure 6. Left: When the methane droplets come into contact with the lake surface, the monolayers combine to bilayers and form vesicles. Right: Initially, the freshly formed vesicles are just kinetically stable and therefore prone to slow thermal decomposition.
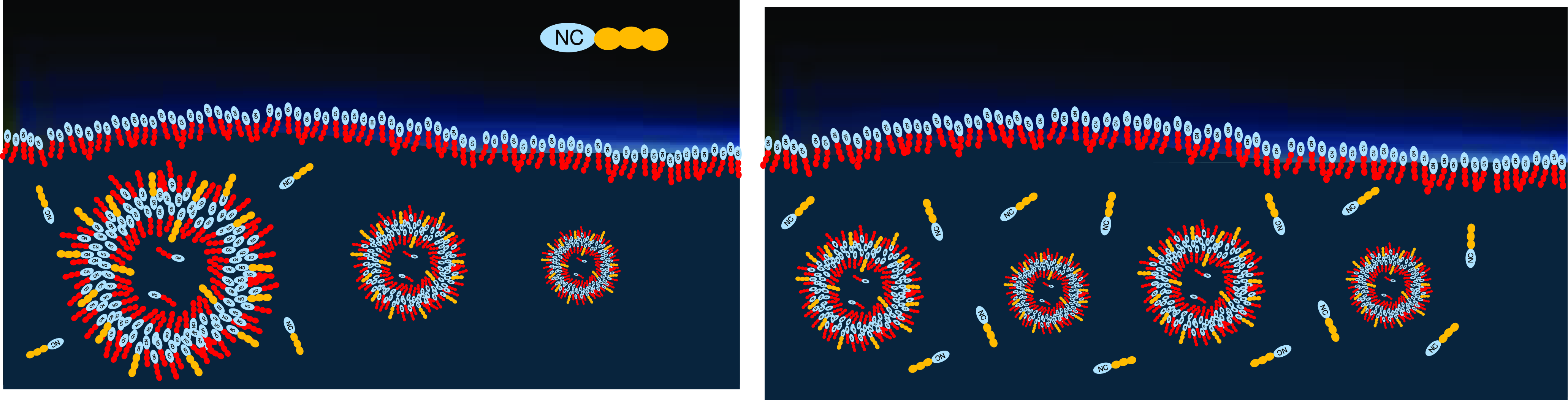
Figure 7. Left: Vesicles gain thermodynamic stability by collecting other, energetically favored amphiphiles. Right: Stable vesicles will accumulate over time, and so will the corresponding stabilizing amphiphiles that are temporarily protected from decomposition.
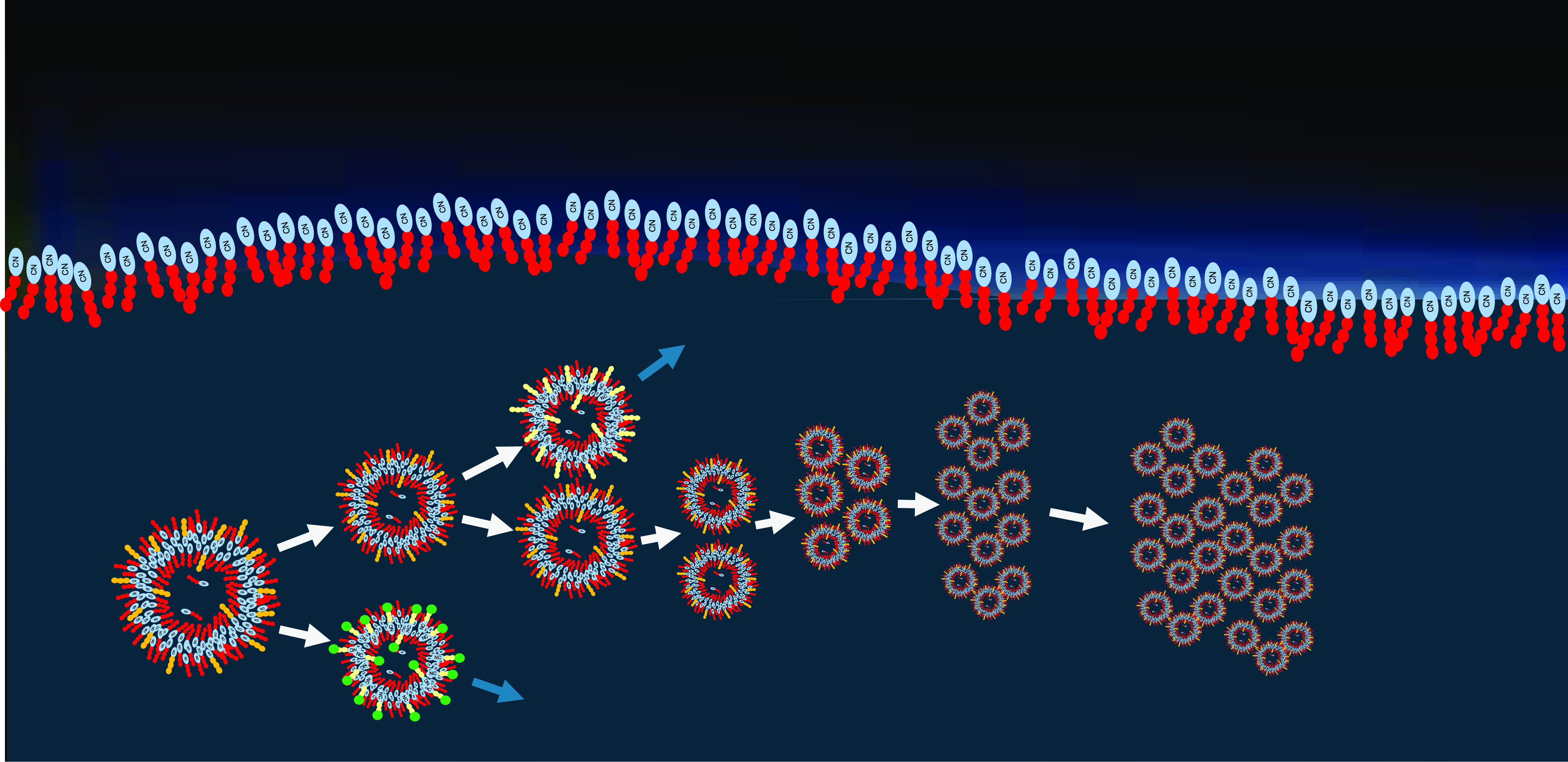
Figure 8. In a long-term compositional selection process, the most stable vesicles will proliferate, while less stable ones form dead ends (blue arrows). In consequence, this leads to an evolution process leading to increasing complexity and functionality.
Different stabilizing amphiphiles and different populations of vesicles may develop in separate locations of the liquid content of the lake. Their molecular memory (somewhat equivalent to a compositional genome) consists in the local composition of accumulated amphiphiles. This way, different populations of vesicles could coexist and start to compete as soon as they would mix in the liquid on longer timescales. Resulting from this competition, the most stable compositions would survive and proliferate while less stable ones form dead ends in the overall development (Figure 8). Such steps could lead to a primitive form of evolution process, potentially resulting in early protocells. This phenomenon of primitive evolution among amphiphilic structures has been postulated as the GARD model (Lancet et al., Reference Lancet, Zidovetzki and Markovitch2018; Kahana and Lancet, Reference Kahana and Lancet2021; Kahana et al., Reference Kahana, Segev and Lancet2023; Segré et al., Reference Segré, Ben-Eli and Lancet2000; Markovitch and Lancet, Reference Markovitch and Lancet2012; Markovitch and Krasnogor, 2028; Mayer et al., Reference Mayer, Lancet and Markovich2024), a computational treatment on evolving micelles and vesicles. Following this model, the formation of vesicle membranes can be described by a reaction network. Within this network, attractor dynamics between various types of amphiphiles lead to the formation of so-called composomes (Kahana et al., Reference Kahana, Segev and Lancet2023). Similar to a genome, composomes have the capability to carry and multiply coded information (Segré et al., Reference Segré, Ben-Eli and Lancet2000), hereby creating a compositional genome. Such a development leads to membrane structures of increasing order and complexity and, eventually, to functional vesicles (Mayer et al., Reference Mayer, Lancet and Markovich2024).
Possible laboratory experiments
In principle, all postulated phenomena are accessible by laboratory experiments. In the first place, it would be desirable to verify the mechanism of vesicle formation. This experiment could be performed in an artificial low-temperature environment (a cryostat connected to a Dewar reaction container) with liquid methane solution as a continuous liquid phase at the bottom and methane forming the gaseous phase. The liquid methane would contain the amphiphiles, either a single tholin or a multi-component mixture thereof. The device should allow a computer-controlled pressure variation (such as the nitrogen-operated piston used in Mayer et al., Reference Mayer, Schreiber, Dávila, Schmitz, Bronja, Meyer, Klein and Meckelmann2018) in order to induce the periodic formation of precipitating methane droplets. Alternatively, a temperature gradient could be generated that could lead to continuous reflux conditions, with methane droplets falling onto the surface of a liquid methane phase on the bottom. In any case, droplets should fall from a considerable height (2–3 m) in order to create the level of spray that is necessary for the secondary droplet formation. In a first step, the liquid methane phase could be analyzed for vesicles. The simplest approach would be light scattering, possibly combined with sedimentation. If vesicles are being formed via the proposed mechanism, the intensity of scattered light should continuously increase until a steady state that is given by an equilibrium between vesicle generation and natural vesicle decomposition. In an isolated sample, no or very little sedimentation should be observed, as expected from the small difference in density between vesicles and the surrounding continuous phase.
In a second, more challenging experiment starting with a mixture of amphiphiles, the composition of amphiphiles in vesicles could be studied over time. This would require a partial separation of the vesicles, for example by centrifugation or by chromatography. Subsequently, the composition of amphiphiles in the separated (vesicle-rich) samples would be compared to the composition in the bulk phase. This comparison would yield data on the amphiphile composition in the vesicle membranes.
Finally, possible vesicle evolution could be followed over extended periods of time. In analogy to experiments simulating the conditions in the Earth’s crust (Mayer et al., Reference Mayer, Schreiber, Dávila, Schmitz, Bronja, Meyer, Klein and Meckelmann2018), a periodic vesicle formation at, say, minute-scale intervals could be run over a time frame of weeks, allowing for thousands of consecutive vesicle generations to form. Under these conditions, we would expect a compositional selection towards an optimized stability of the vesicle membranes. The composition of the resulting membrane would then represent the result of an evolution process and could be analyzed in detail. This analysis would involve calculation of molecular dynamics simulating the given membrane composition.
Relevant detection techniques for space missions
Is there a feasible approach to verify these postulated developments? For ultimate verification of the existence of Titan vesicles, the focus must eventually be on probing the continuous liquid phase of the lakes of Titan. Missions to the polar regions and the lakes of Titan have been proposed, including the use of a boat and of a submarine (Stofan et al., Reference Stofan, Lorenz, Lunine, Bierhaus, Clark, Mahaffy and Ravine2013; Mitri et al., Reference Mitri, Coustenis, Fanchini, Hayes, Iess, Khurana, Lebreton, Lopes, Lorenz, Meriggiola and Moriconi2014; Oleson et al., Reference Oleson, Lorenz, Paul, Hartwig and Walsh2018). Based on the given hypothesis, we argue that, for a plausible scenario, dispersed vesicles exist in the lakes, albeit perhaps at a low concentrations. If detected, the occurrence of such vesicles could be an indicator for prebiology – early developments towards the evolution of self-reproducing cellular life. Hence, the capability to search for such vesicles should be an task of key importance for any Titan lake-sea mission.
Due to their extremely delicate structure and the small variation of refractive index, vesicles are not easy to detect. Optical observation and tracking under dark-field illumination is an option (Finder et al., Reference Finder, Wohlgemuth and Mayer2004), but requires sample preparation and tends to be time consuming. Detection by field-gradient supported nuclear magnetic resonance (PFG-NMR) yields rich information (Mayer et al., Reference Mayer, Schreiber, Dávila, Schmitz, Bronja, Meyer, Klein and Meckelmann2018), but is out of the question because NMR equipment is too bulky for spaceflight deployment. The same is true for analytical centrifugation, electron microscopy, chromatography, or field flow fractionation. Nanoparticle tracking analysis or flow cytometry may be possible but require suitable flow cell devices or bulky optical microscopy (Erdbrügger and Lannigan, Reference Erdbrügger and Lannigan2016). Resistive pulse sensing that is commonly applied to extracellular vesicles (Erdbrügger and Lannigan, Reference Erdbrügger and Lannigan2016) is hampered by the non-aqueous medium with its extremely low conductivity.
Instead, the most promising approach for vesicle detection on Titan may be the local application of laser light sources. Laser diodes are compact, light and relatively low in energy consumption. A very versatile and straightforward use of a laser source is to produce light scattering (Figure 9, bottom left), either in its static or its dynamic version (Milton, Reference Milton1996). Both methods are primarily useful when applied to dispersed particles but also allow for the detection of large molecules (Bohren and Huffman, Reference Bohren and Huffman1998). Every particle larger than a few nm will be a source of scattered light that can be detected at a variable angle with the original laser beam. Overall, the intensity of the scattered light will depend on the particle’s refractive index (as compared to the refractive index of the medium), on the particle concentration and on the size of the particles (Kerker, Reference Kerker1969; Barber and Hill, Reference Barber and Hill1990). More specific information about the nature of the particles is obtained by the angular dependence of the scattered light intensity. For spherical particles in the characteristic size range of vesicles (100 nm–50 µm), the angular dependence pattern is described by the Mie theory (Mie, Reference Mie1908; Bohren and Huffman, Reference Bohren and Huffman1998). With polydisperse vesicles, a corresponding superposition of Mie patterns is expected.
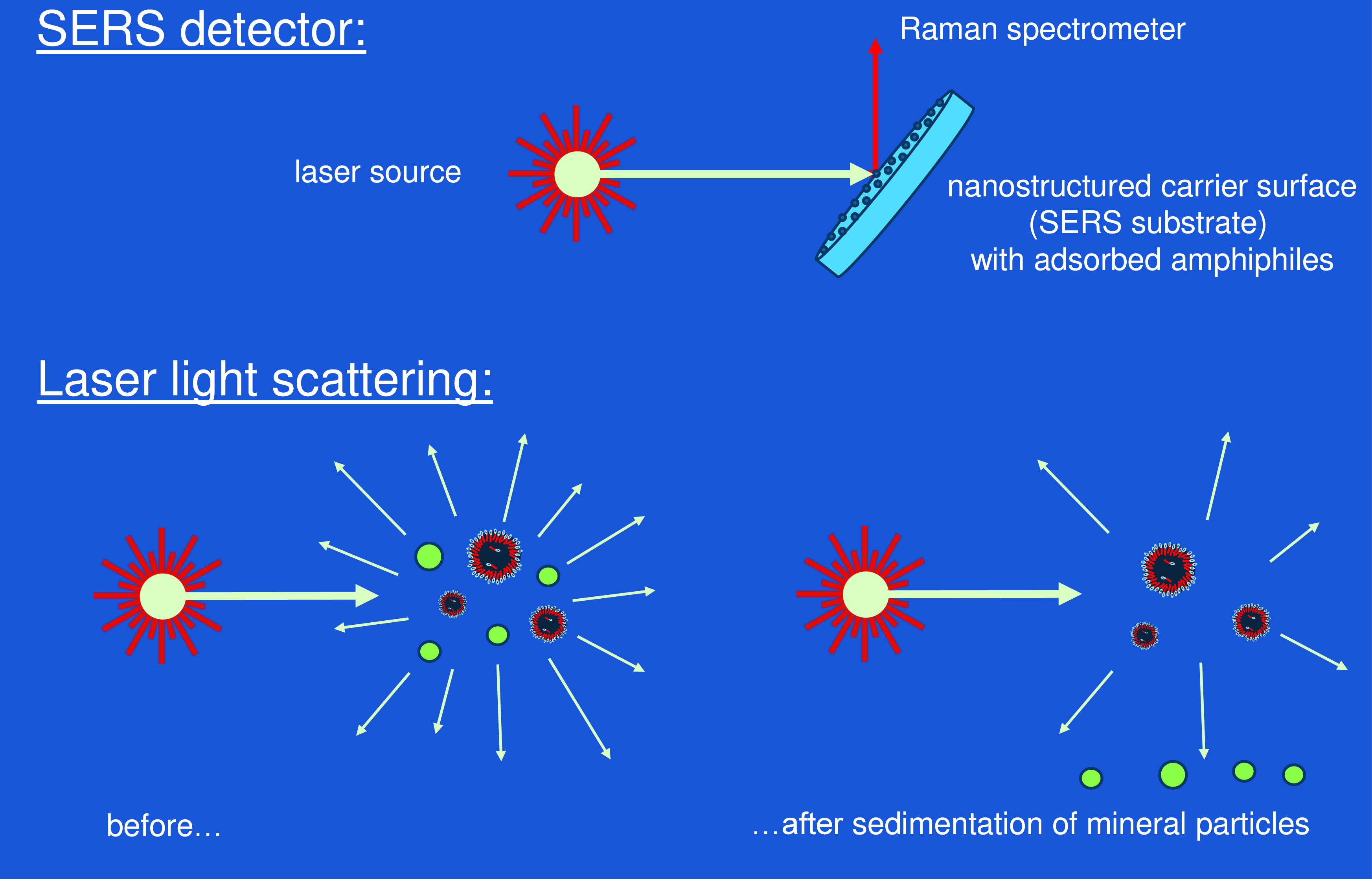
Figure 9. Detection of amphiphilic components and vesicles using a laser light source. Top: a SERS substrate (flat carrier surface with metallic nanoparticles) is immersed into the fluid phase containing amphiphilic components. Raman spectra of amphiphiles adsorbing to the nanoparticles can be detected by the SERS effect with extremely high sensitivity. Bottom: Principal setup for laser light scattering. Vesicles can be discriminated against mineral nanoparticles by a time-dependent observation, allowing for the sedimentation of mineral particles (green).
In this case, the polydispersity can be studied by dynamic light scattering (Berne and Pecora, Reference Berne and Pecora2000). This approach makes use of the temporal fluctuations of the scattered light caused by the Brownian motion of the particles. It would require a probe of a few centimeters in size that is immersed into the continuous liquid phase of the Titan’s lake somewhere along the shoreline. It would contain a laser source and a polarizer. The laser beam would pass through a few centimeters of the liquid phase, while the temperature of the medium is determined. The dispersed light is collected by a photo multiplier and a so-called speckle pattern is produced (Goodman, Reference Goodman1976). The decay of the autocorrelation function of the speckle pattern reveals the size of the particles because large particles diffuse more slowly (and the speckle pattern remains autocorrelated longer) whereas small particles diffuse more quickly (and the speckle pattern remains autocorrelated for a shorter time). The angle of detection could be variable in order to optimize the result. The time dependent data are used to yield an autocorrelation function that is directly linked to the Brownian motion spectrum of the particles. With known temperature and viscosity (or by calibration with particles of a known size), this results in a reliable size distribution of the particles.
At this point, the dynamic light scattering probe will detect all kinds of particles, including mineral dust or ice crystals. In order to differentiate vesicles against such compact particles, the sedimentation behavior should be studied in combination with light scattering (Figure 9, bottom). For this purpose, the probe is shielded from convective motion of the surrounding fluid phase, e.g. by a tube with small openings. Under these conditions, all particles with a density significantly above the liquid medium (e.g. mineral particles) will undergo a sedimentation process (Figure 9, bottom right). The expected vesicles with a density very near to the liquid phase will remain in dispersion over extended periods of time. Hence, the simplest approach for vesicle identification consists in repeated measurements over several hours while detecting scattering intensities and resulting size distribution functions. All particles that still contribute to static or dynamic scattering after such a waiting period can be safely identified as vesicles.
For additional identification of the vesicle’s chemistry, laser light scattering could be combined with Raman spectroscopy. For that purpose, the scattered light could be analyzed for signals in the infrared regime, using the technology of hand-held Raman spectrometers. The sensitivity of this analysis could be drastically enhanced by further use of metallic nanoparticles on a flat substrate (Figure 9, top) using the surface enhanced Raman spectroscopy effect known as SERS (Blackie et al., 2009). Such an approach would open a realistic chance to actually identify the amphiphilic molecules that form the vesicle membranes, even if they occur in very low concentrations. For that purpose, a small quantity of the liquid phase of Titan’s lake would be deposited onto a nanostructured noble metal surface, e.g. using silver nanoparticles (Mock et al., Reference Mock, Barbic, Smith, Schultz and Schultz2002). All amphiphilic components will readily adsorb to the nanoparticles due to their surface activity. At this point, the theoretical amplification of their Raman signal amounts to eleven orders of magnitude (Blackie et al., 2009). In practical use of a mobile Raman device, the amplification will still be strong enough to allow for sensitive in-field detection of trace molecules (Hermsen et al., Reference Hermsen, Lamers, Schoettl, Mayer and Jaeger2021).
All in all, a detector using a Laser device for dynamic light scattering (for particle size distribution and sedimentation measurements) combined with SERS would be a very powerful analytical instrumentation. It would allow the identification of possible amphiphilic molecules and the observation the proposed mechanism of vesicle formation. Furthermore, it could approach a multitude of other questions, such as for other possible organic molecules or organic as well as inorganic particles in liquid dispersion. As an equipment for the Dragonfly project, it would open the pathway for most exciting discoveries.
Conclusions and further directions
Based on the known composition of the atmosphere and the lakes on Titan, it is possible that vesicles will form in the liquid phase. The mechanism of vesicle formation involves spray droplets that are coated by a monolayer of amphiphiles. On interaction with another monolayer on the lake’s surface, bilayer membranes may form that encapsulate the liquid phase of the original droplet. The resulting vesicles would be kinetically stable but may develop thermodynamic stability by continuous compositional selection of amphiphiles. Different populations of stable vesicles (developing due to local accumulations of amphiphiles as caused by concentration gradients, topographic conditions, local turbulences, and mixing zones) may compete, leading to a long-term evolution process that could eventually result in primitive protocells.
No matter how far such a process may have actually evolved on Titan, the existence of any type of vesicles would be a very important discovery. It would prove that early steps towards increasing order and complexity have taken place on Titan, which represent the necessary precondition for abiogenesis.
An ideal means of detection for any of these developments could include a laser device with combined light scattering analysis and SERS. It would allow for very sensitive detection of amphiphiles as well as for the observation of dispersed vesicles.
Funding statement
CAN was supported for his part in the research by funding from the NASA Astrobiology Institute grant “Habitability of Hydrocarbon Worlds.”
Competing interests
We declare that we have no conflicts of interested related to our study.











