Introduction
Many multivoltine migratory insects expand their ranges annually to cover large breeding distributions (Chapman et al. Reference Chapman, Reynolds and Wilson2015; Stefanescu et al. Reference Stefanescu, Soto, Talavera, Vila and Hobson2016). Mass-movements of butterflies (Lepidoptera), moths (Lepidoptera), locusts (Orthoptera: Acrididae), and other insects are often aided by weather systems that promote long-distance movement (Chapman et al. Reference Chapman, Reynolds and Wilson2015; Hu et al. Reference Hu, Lim, Horvitz, Clark, Reynolds, Sapir and Chapman2016) and facilitate successful breeding in distant breeding habitats (Chapman et al. Reference Chapman, Bell, Burgin, Reynolds, Pettersson and Hill2012). Global climate change could result in changes to host-plant distributions (e.g., Lemoine Reference Lemoine2015), which might negatively impact less-mobile habitat specialists (Warren et al. Reference Warren, Hill, Thomas, Asher, Fox and Huntley2001) but benefit mobile specialists such as migratory butterflies (Batalden et al. Reference Batalden, Oberhauser and Peterson2007). To track such movements, it is essential to determine the origins of immigrant individuals (Hobson et al. Reference Hobson, Doward, Kardynal and McNeil2018) and whether they successfully recruit offspring into the population once they have arrived in a new area (McNeil Reference McNeil1978; Chapman et al. Reference Chapman, Bell, Burgin, Reynolds, Pettersson and Hill2012).
Determining the area of origin of individual butterflies can be accomplished using intrinsic markers such as stable isotopes (Rubenstein and Hobson Reference Rubenstein and Hobson2004). Stable isotopes are powerful tools for assigning natal origins for monarch butterflies (Danaus plexippus (Linnaeus) (Lepidoptera: Nymphalidae)) because isotopic ratios are typically transferred in a predictable fashion trophically in food webs and are ultimately retained in wing tissue of insects (Hobson et al. Reference Hobson, Wassenaar and Taylor1999; Flockhart et al. Reference Flockhart, Kyser, Chipley, Miller and Norris2015). In North America, stable-hydrogen isotopes in precipitation vary predictably across latitudinal gradients (Hobson et al. Reference Hobson, Wassenaar and Taylor1999; Terzer et al. Reference Terzer, Wasenaar, Araguás-Araguás and Aggarwal2013), whereas stable-carbon isotopes in milkweed (Asclepias Linnaeus; Apocynaceae) vary dependent upon latitude, elevation, year, and temperature (Miller et al. Reference Miller, Wassenaar, Hobson and Norris2011; Flockhart et al. Reference Flockhart, Brower, Ramirez, Hobson, Wassenaar, Altizer and Norris2017a). Stable isotope ratios are fixed in metabolically inactive tissue once grown, and they can therefore provide information on the natal origin of an individual no matter how far that individual has migrated (Wassenaar and Hobson Reference Wassenaar and Hobson1998; Hobson et al. Reference Hobson, Wassenaar and Taylor1999). Thus, natal location can be linked to capture location (Yang et al. Reference Yang, Ostrovsky, Rogers and Welker2016; Flockhart et al. Reference Flockhart, Dabydeen, Satterfield, Hobson, Wassenaar and Norris2018). Isotope measurements in wing tissue provide a means to differentiate immigrants from local recruits in areas where both might occur (Malcolm et al. Reference Malcolm, Cockrell and Brower1993; Miller et al. Reference Miller, Wassenaar, Hobson and Norris2012).
Monarch butterflies conduct long-distance movements between breeding locations in the northern United States of America and southern Canada, to overwintering areas in the highlands of central Mexico (Urquhart and Urquhart Reference Urquhart and Urquhart1978; Brower Reference Brower1995). The main breeding areas of the monarch butterfly in eastern Canada lie in southern Ontario, Québec, and Manitoba (D.T.T.F., unpublished data). Under global climate change conditions, the breeding season length (Batalden et al. Reference Batalden, Oberhauser and Peterson2007) and the distribution of the milkweed host plants of the monarch butterfly (Lemoine Reference Lemoine2015) are expected to change. A major expansion of monarch butterflies into western Canada occurred in 2012 (Acorn Reference Acorn2012) that resulted in a breeding range in Canada three times larger than a typical year (D.T.T.F., unpublished data). This atypical migratory movement and range expansion provided an opportunity to test recruitment at northern edge of their range (Committee on the Status of Endangered Wildlife in Canada 2016). In this study, we used stable isotope (δ2H, δ13C) measurements to assign the natal origins of monarch butterflies collected well beyond their normal breeding distribution to document patterns of immigration and test for successful local reproduction in western Canada during the summer of 2012. We were interested in where these colonising individuals originated and whether these colonising individuals were successful breeders.
Methods
Monarch butterfly collections
The unprecedented movement of monarch butterflies into western Canada in 2012 (Acorn Reference Acorn2012) provided an opportunity to determine the natal origins of immigrants and test for successful local reproduction. Butterfly specimens (n=34) were collected in western Canada over five months during 2012 (Table 1). Butterflies were collected haphazardly by citizen scientists in Manitoba, Saskatchewan, and Alberta during the breeding season and participants were instructed to record the date the butterfly was captured as well as the capture location (e.g., latitude and longitude). For each specimen we measured the wing length and scored the wing condition on a six-point scale where a score of zero was pristine condition and five was extremely worn and frayed (Flockhart et al. Reference Flockhart, Wassenaar, Martin, Hobson, Wunder and Norris2013). Specimens were stored in glassine or paper envelopes and placed in a freezer or stored at room temperature until prepared for isotope analysis.
Table 1 Data of individual monarch butterflies collected and analysed in this paper including the capture latitude and longitude, date, and stable-hydrogen (δ2H) and stable-carbon (δ13C) isotope values.
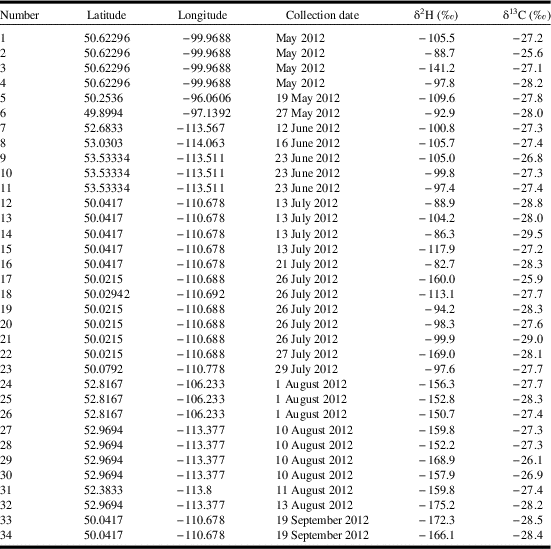
Note: A lack of collection day means the day of the month was unknown.
Stable isotope analysis
Stable isotope preparation and analysis for δ13C and δ2H followed standard procedures as described in Flockhart et al. (Reference Flockhart, Brower, Ramirez, Hobson, Wassenaar, Altizer and Norris2017a). Wings were first cleaned in a 2:1 chloroform:methanol solvent and chitin subsamples (1.0±0.1 mg) for δ13C were analysed using continuous-flow isotope-ratio mass spectrometry and subsamples (0.35±0.02 mg) for δ2H isotopes were analysed using flash pyrolysis using continuous-flow isotope-ratio mass spectrometry. Nonexchangeable δ2H values were obtained using the comparative equilibrium procedure (Wassenaar and Hobson Reference Wassenaar and Hobson2003) and normalised to the Vienna standard mean ocean water-standard light Antarctic precipitation (VSMOW-SLAP) scales. Precision of laboratory keratin control standards based on within-run replicates (n=5) was better than ± 1.6‰ for δ2H. Laboratory standards and their assigned values for hydrogen isotopes were EC1 (Caribou hoof standard, CBS) and EC2 (Kudu horn standard, KHS) with δ2H values of –197‰ and –54‰, respectively. Laboratory standards for stable carbon isotopes were bowhead whale baleen (BWBII) and bovine gelatin (PUGEL) with assigned δ13C values of −18.5‰ and −13.6‰ versus the Vienna Pee Dee Belemnite standard. Precision of laboratory keratin control standards was better than ±0.2‰ for δ13C. All analyses were conducted at the Stable Isotope Hydrology and Ecology Research Laboratory of Environment and Climate Change Canada, Saskatoon, Saskatchewan, Canada. Stable isotope values are presented in Table 1.
Assignment of natal origins
For each butterfly we calculated a probability of natal origin based on correspondence between the measured δ2H and δ13C values in the wing tissue and the predicted values of monarch butterfly δ2H and δ13C wing tissue for each location, termed an isoscape. Following standard approaches (Wunder Reference Wunder2010; Flockhart et al. Reference Flockhart, Wassenaar, Martin, Hobson, Wunder and Norris2013), we assumed a bivariate normal distribution for the error term to assign a probability of natal origin (Royle and Rubenstein Reference Royle and Rubenstein2004). The probability density of individual i having location j as the natal origin is
![]() $Y_{i} \,\sim\,N(\mu _{j} ,\,\Sigma )$
where Y
i
is a vector of measured δ2H and δ13C values, μ
j
a vector of the mean predicted δ2H and year-specified δ13C values (here designated as 2012) derived from the calibrated isoscapes with a spatial resolution of 0.167° (Flockhart et al. Reference Flockhart, Brower, Ramirez, Hobson, Wassenaar, Altizer and Norris2017a), and Σ the positive-definite variance–covariance matrix of δ2H and δ13C values of monarch butterflies raised at known locations in Hobson et al. (Reference Hobson, Wassenaar and Taylor1999). We applied Bayes’ rule to invert the conditional probabilities of natal origin to produce a spatially explicit posterior probability density function. The posterior probability surface considers that the eastern monarch butterfly breeding distribution outline from Flockhart et al. (Reference Flockhart, Brower, Ramirez, Hobson, Wassenaar, Altizer and Norris2017a), which serves as a binomial probability [0,1] where the multiplier of zero applies to all areas outside the breeding distribution while the areas within the breeding distribution are assigned a multiplier of one (Wunder Reference Wunder2010).
$Y_{i} \,\sim\,N(\mu _{j} ,\,\Sigma )$
where Y
i
is a vector of measured δ2H and δ13C values, μ
j
a vector of the mean predicted δ2H and year-specified δ13C values (here designated as 2012) derived from the calibrated isoscapes with a spatial resolution of 0.167° (Flockhart et al. Reference Flockhart, Brower, Ramirez, Hobson, Wassenaar, Altizer and Norris2017a), and Σ the positive-definite variance–covariance matrix of δ2H and δ13C values of monarch butterflies raised at known locations in Hobson et al. (Reference Hobson, Wassenaar and Taylor1999). We applied Bayes’ rule to invert the conditional probabilities of natal origin to produce a spatially explicit posterior probability density function. The posterior probability surface considers that the eastern monarch butterfly breeding distribution outline from Flockhart et al. (Reference Flockhart, Brower, Ramirez, Hobson, Wassenaar, Altizer and Norris2017a), which serves as a binomial probability [0,1] where the multiplier of zero applies to all areas outside the breeding distribution while the areas within the breeding distribution are assigned a multiplier of one (Wunder Reference Wunder2010).
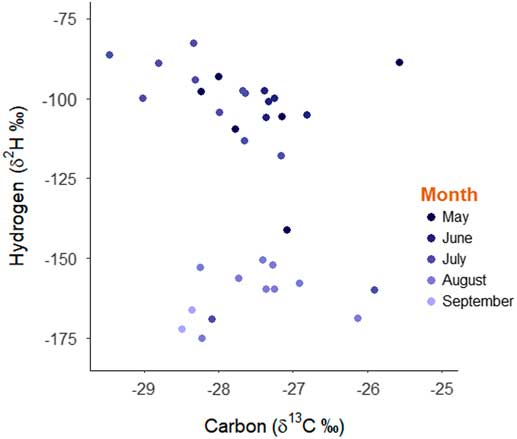
The locations with the highest probability values are the more likely locations of natal origin (Royle and Rubenstein Reference Royle and Rubenstein2004; Wunder Reference Wunder2010). To transform a probability surface into a designated area of natal origin, we reclassified those pixels with greater than a 2:1 odds ratio as one and all other locations as zero (Hobson et al. Reference Hobson, Wunder, van Wilgenburg, Clark and Wassenaar2009). We then classified monarch butterflies into two groups based on whether individuals were captured between May and July or during August and September (Fig. 1). We summed the natal origin surfaces for these groups and present maps of natal origin based on these time periods to identify individuals that were immigrants that colonised western Canada to breed from individuals that were born locally in western Canada. Finally, for each butterfly we calculated the distance between the centroid of the natal origin of the binary surface using the pointDistance function and the bearing between natal origin and capture location using the bearingRhumb functions in the geosphere package (Flockhart et al. Reference Flockhart, Dabydeen, Satterfield, Hobson, Wassenaar and Norris2018). We used the raster package (Hijmans Reference Hijmans2015) in programme R (R Core Team 2014) to conduct all spatial interpretations and natal origin assignments.
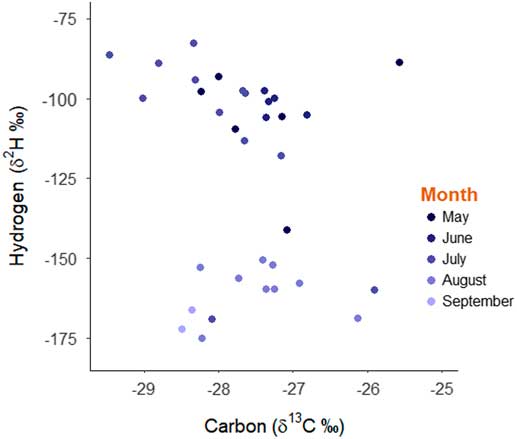
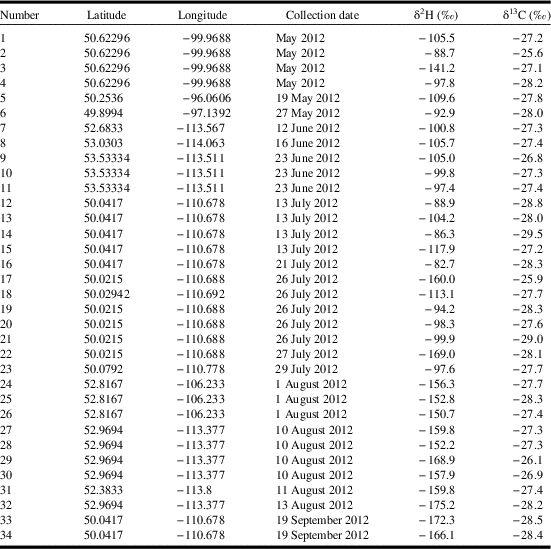
Fig. 1 Scatterplot of stable-hydrogen (δ2H) and stable-carbon (δ13C) isotope values of monarch butterflies and the month of collection in western Canada in 2012.
Statistical analysis
For early and late butterflies, we used t-tests to test for differences in distances between the centroid of natal origin and the capture location. We used aov.circular function in the circular package to test for differences in bearing between the centroid of natal origin and capture location (Agostinelli and Lund Reference Agostinelli and Lund2013). We compared wing length between early and late monarch butterflies by using a generalised linear model that included sex to control for differences in wing length between males and females (Altizer and Davis Reference Altizer and Davis2010; Flockhart et al. Reference Flockhart, Fitz-gerald, Brower, Derbyshire, Altizer and Hobson2017b). To compare wing wear score between early and late monarch butterflies we used a Mann–Whitney U-test.
Results
The distribution of stable-hydrogen isotope values of collected monarch butterflies was bimodal with respect to capture date whereas the distribution of stable-carbon isotope values was unimodal (Fig. 1). Using the known distribution of isotope values for milkweed foodplants, it is apparent that monarch butterflies captured between May and July 2012 had natal origins in the Midwest of the United States of America, including Minnesota, Iowa, Missouri, Wisconsin, Illinois, Indiana, Michigan, Ohio, and Pennsylvania (Fig. 2A). In contrast, monarch butterflies captured in western Canada in August and September had natal origins in western Canada indicating successful recruitment of monarch butterflies within the expanded geographic range (Fig. 2B). Monarch butterflies collected between May and July travelled farther (mean=1913 km, standard deviation=696 km) than monarch butterflies collected in August and September (mean=372 km, SD=198 km; t=9.82, df=28.27, P<0.001) but there were no differences in the estimated bearings between natal origin and capture location between these two groups of butterflies (monarch butterflies collected between May and July: mean=299°, SD=31.5°; Monarch butterflies collected August and September: mean=222°, SD=99.4°; χ2=0.96, df=1, P=0.33). After controlling for sex, monarch butterflies collected in May through July had shorter wings (mean=50.2 mm, SD=2.51 mm) compared to monarch butterflies collected in August and September (mean=52.5 mm, SD=1.67 mm; β=−2.321, SE=0.85, t=−2.74, P=0.01). Monarch butterflies collected in May through July had more heavily worn wings (median score=4) compared to monarch butterflies collected in August and September (median score=1; W=238, P<0.001).
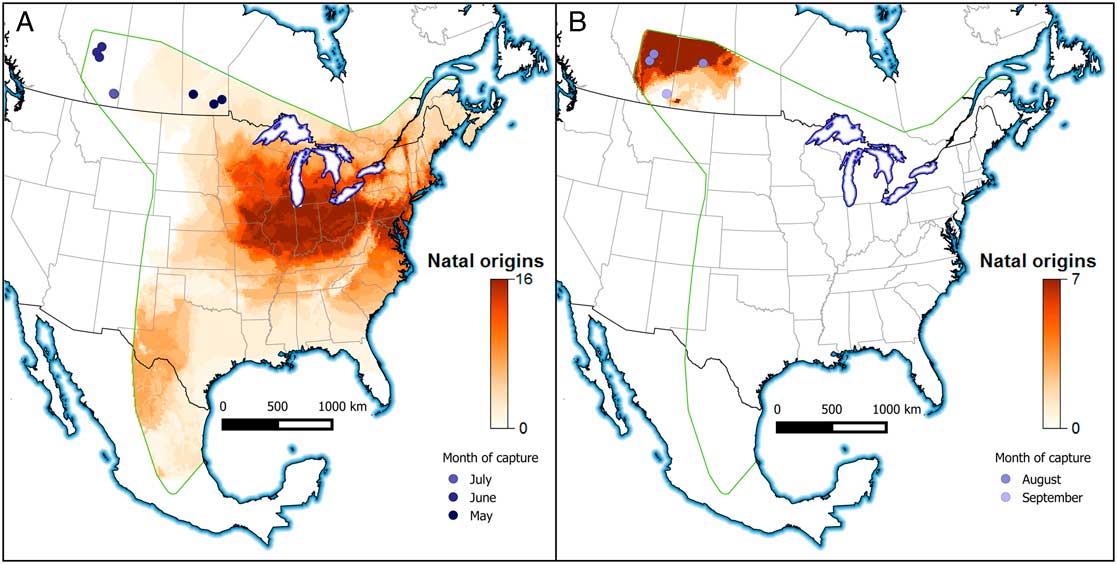
Fig. 2 Assigned natal origins of monarch butterflies using stable-hydrogen (δ2H) and stable-carbon (δ13C) isotopes of monarch butterflies captured in (A) May (darkest blue points), June and July (n=23 butterflies) and (B) in August and September (lightest blue points) (n=11 butterflies) in 2012 in western Canada. Cell values are indicated with the scale is the sum of the binary maps of all individuals using a 2:1 odds ratio for early and late portions of the breeding season. For example, a value of 16 represents that cell being consistent with representing the natal origins of 16 of the 23 butterflies captured in May to July. Capture locations are indicated with the coloured points and the green outline indicates the extent of the breeding distribution considered in the natal origin assignments.
Discussion
Monarch butterflies that migrated to western Canada in 2012 originated from breeding areas in the United States of America, principally in the Midwest, in agreement with previous studies (Miller et al. Reference Miller, Wassenaar, Hobson and Norris2012; Flockhart et al. Reference Flockhart, Wassenaar, Martin, Hobson, Wunder and Norris2013). However, we show that these colonising monarch butterflies successfully recruited offspring into the western Canadian population, and these individuals emerged as adults in August and September. Monarch butterflies were, therefore, able to detect host plants for oviposition despite these areas having few milkweeds (Woodson Reference Woodson1954; Moss and Packer Reference Moss and Packer1983; Lavin and Seibert Reference Lavin and Seibert2011). In southern Manitoba, six species of Asclepias are known, while to the west in the aspen parklands and southern boreal forests of Alberta, only Asclepias ovalifolia Decaisne occurs, the latter as a cryptic understorey plant (Duffey Reference Duffey1970; Budd and Best Reference Budd and Best1976; Lavin and Seibert Reference Lavin and Seibert2011). In addition, some horticultural milkweeds are present but uncommon in gardens, often with the intent of promoting monarch butterfly breeding.
The core distribution of monarch butterflies in Canada is centred on southern Ontario, Québec, and Manitoba (D.T.T.F, unpublished data) and, therefore, the colonisation in 2012 extended well beyond their normal breeding distribution (Acorn Reference Acorn2012). In any given year, the first observations of monarch butterflies in southern Ontario are of individuals moving north through Michigan and Ohio. In some years, first observations may occur in late April and the number of observations increase over the next two months suggesting a colonisation from breeding areas to the south, until approximately July, when monarch butterflies have reached the breeding distribution limits (Flockhart et al. Reference Flockhart, Wassenaar, Martin, Hobson, Wunder and Norris2013). These initial sightings include both individuals that may have overwintered and first generation individuals that originate from the central and southern United States of America (Malcolm et al. Reference Malcolm, Cockrell and Brower1993; Miller et al. Reference Miller, Wassenaar, Hobson and Norris2012). In typical years, first observations in southern Manitoba occur in June, and these butterflies are also thought to have originated in the Midwest via Minnesota and North Dakota (D.T.T.F, unpublished data). We were therefore curious whether the monarch butterflies that colonised western Canada in 2012 came from eastern Canada, north of the Great Lakes or, as is apparent in Figure 2, from the American Midwest to the south.
Monarch butterflies that recruit into the population at northern latitudes were predicted to have more negative hydrogen isotope values but we did not expect seasonal changes in precipitation to result in later-emerging monarch butterflies to have more negative stable-hydrogen isotopes values than earlier monarch butterflies (Miller et al. Reference Miller, Wassenaar, Hobson and Norris2011). Additionally, above-average temperatures in the United States of America in June likely aided the migration of monarch butterflies north beyond their normal distribution but these extreme temperatures also were unlikely to result in more depleted isotope values of monarch butterflies from southern areas. However, monarch butterflies raised on milkweed further south and irrigated with water from the Rocky Mountains (e.g., in areas of Colorado) could result in lower δ2H values similar to those from monarch butterflies raised in western Canada. Monarch butterflies occasionally occur in areas like Colorado during the later summer but rarely in the spring. Although our sample size was limited for late-season monarch butterflies from western Canada, the evidence does support that monarch butterflies indeed recruited from these areas.
Consistent with the evidence that monarch butterfly migrating longer distances have longer wings (Altizer and Davis Reference Altizer and Davis2010; Flockhart et al. Reference Flockhart, Fitz-gerald, Brower, Derbyshire, Altizer and Hobson2017b), local recruits in our study (those presumably migrating to Mexico) were larger than immigrants (those that originated from the Midwest). Larger wing size could result from local climate or host-plant chemistry (Malcolm et al. Reference Malcolm, Cockrell and Brower1989). Longer wings may enhance survival during long, arduous migrations such that individuals with shorter wings are selectively removed (Johansson et al. Reference Johansson, Söderquist and Bokma2009; Flockhart et al. Reference Flockhart, Fitz-gerald, Brower, Derbyshire, Altizer and Hobson2017b).
Monarch butterflies that successfully immigrated to western Canada in 2012 produced offspring that recruited into the local population despite low diversity and abundance of suitable host plants in these locations (Woodson Reference Woodson1954). However, monarch butterflies often locate milkweed plants in landscapes with few existing host plants. For example, monarch butterflies may lay more eggs on isolated plants in agricultural landscapes (Oberhauser et al. Reference Oberhauser, Prysby, Mattila, Stanley-Horn, Sears and Dively2001; Pleasants and Oberhauser Reference Pleasants and Oberhauser2013; Pitman et al. Reference Pitman, Flockhart and Norris2018) or have lower lifetime fecundity (Zalucki and Lammers Reference Zalucki and Lammers2010) due to search effort when host plants occur at low density (Jaenike Reference Jaenike1978; Wiklund Reference Wiklund1981). Our results support the idea that monarch butterflies will be able to find milkweed and successfully reproduce in novel environments (Batalden et al. Reference Batalden, Oberhauser and Peterson2007) such as those predicted to occur from the expansion of host plants under global climate change (Lemoine Reference Lemoine2015). Under these scenarios, northern breeding areas may indeed contribute to monarch butterfly population dynamics.
Monarch butterflies are likely not true navigators but rather use an adjustable sun-compass to orient during migration (Mouritsen and Frost Reference Mouritsen and Frost2002; Mouritsen et al. Reference Mouritsen, Derbyshire, Stalleicken, Mouritsen, Frost and Norris2013). Monarch butterflies produced in western Canada could conceivably migrate to overwintering areas in Mexico, migrate to overwintering sites in California, United States of America, or fail to migrate to either location. Thus, it is unclear whether monarch butterflies breeding in western Canada act as a source or sink for monarch butterfly populations in North America (Mouritsen et al. Reference Mouritsen, Derbyshire, Stalleicken, Mouritsen, Frost and Norris2013). Flockhart et al. (Reference Flockhart, Brower, Ramirez, Hobson, Wassenaar, Altizer and Norris2017a), using some data from 2012, failed to detect western Canadian monarch butterflies in the overwintering areas in Mexico. However, this study was based on a sample of 40 monarch butterflies and a much larger sample size is needed before western Canadian contributions to the Mexican roosts can be confirmed or refuted (D.T.T.F., K.A.H., and D.R.N., unpublished data). Monarch butterflies collected in California in 2009 came from a wide area including Montana, Idaho, and Wyoming (Yang et al. Reference Yang, Ostrovsky, Rogers and Welker2016), with δ2H values overlapping with the western Canadian values in the present study, again preventing any firm conclusions about the contributions of western Canadian populations. These uncertainties highlight an important knowledge gap that could be filled with additional sampling of monarch butterflies, as well as refinement of modelling approaches for delineation of natal origin. Such developments could contribute significantly to management plans for monarch butterflies in North America.
Acknowledgements
The authors thank B. Fitz-gerald for preparing isotope samples and K. Kardynal for analytical advice on the carbon isoscape. Many people deserve thanks for contributing monarch butterflies to this study: M. Benson (Manitoba), D. Dilay (Manitoba), A. Leighton (Saskatchewan), J. Scott (Alberta), S. Barkley (Alberta), F. Sperling (Alberta), and B. Acorn (Alberta). D.T.T.F. was supported by a Liber Ero Postdoctoral Fellowship. Funding was provided by the Natural Sciences and Engineering Research Council of Canada (D.R.N.).