In the aftermath of a radiological or nuclear accident or of a malicious incident involving release or use of radionuclides, workers, first responders, or members of the public may become internally contaminated with the radionuclides through various pathways, such as breathing in contaminated air, eating or drinking contaminated food or water, or through absorption of the radioactive material in wounds. This potential internal contamination incident needs to be rapidly detected and assessed for the level of contamination to determine the need for treatment. If contamination is deemed clinically significant, patients should receive prompt treatment with decorporation drugs. A previous review summarizing the experiences gained and lessons learned from the management of past incidents and accidents identified significant gaps in both contamination assessment methods and in the evidence-base of the medical management of internal contamination.Reference Li, Alves dos Reis and Ansari 1
Transuranic actinides, such as the isotopes of plutonium (238Pu, 239Pu, 240Pu), americium (241Am), and curium (242Cm, 244Cm), are frequently mentioned accident reports involving internal contamination of workers.Reference Klumpp, Bertelli and Dumit 2 These radionuclides are typically generated in nuclear power production or nuclear weapon programs. Internal contamination with these radionuclides can lead to an increased risk of late pulmonary fibrosis and cancer (lung, bone, liver). 3 The health effects asociated with the exposure depend on the level of contamination and the route of exposure.
Assessment of internal contamination with transuranic actinides is typically time consuming, especially when the contamination level is low. However, some rapid techniques/methods have been developed and implemented for quick screening and assessment, which provide information on the magnitude of contamination in minutes to hours instead of days to weeks.Reference Guilmette, Bertelli and Miller 4
Current clinical management of internal contamination with these radionuclides involves the use of a chelating/decorporation agent, Ca-DTPA or Zn-DTPA, which binds and removes the radionuclides from the blood stream and internal organs.Reference Stradling, Taylor and Henge-Napoli 5 WHO’s “National Stockpiles for Radiological and Nuclear Emergencies: Policy Advice” (2023) recommends including Ca-DTPA and Zn-DTPA in national stockpiles. 6 However, currently there are no evidence-based clinical guidelines available. Moreover, some of the case studies included in the reviews for the purpose of this guideline project indicated that DTPA was generally not availalble in the hospital.
In 2023, the WHO Radiation Emergency Medical Preparedness and Assistance Network (REMPAN) program initiated a project aimed at developing evidence-based guidelines for the assessment and management of internal contamination with transuranic actinides, including the above-listed isotopes of plutonium, americium, and curium, but excluding those of neptunium (e.g., 237Np) that have different physical and chemical properties and for which DTPA decorporation is not effective.Reference Ansoborlo, Amekraz and Moulin 7 The working title of the project is “Internal Contamination Assessment and Management,” abbreviated as iCAM.
WHO has developed a comprehensive protocol for evidence-based guidelines development, covering the methodology, the process, and the roles of the project team, consisting of: WHO Internal Steering Group (ISG), Guideline Development Group (GDG), External Review Group (ERG), methodologist, systematic review experts, and observers. 8 The iCAM project followed the official WHO protocol during its planning, design, and execution. Since the initiation of the project in mid-2023, the project team has conducted 3 in-person and several online meetings and has completed the definition of research questions with the outcomes of interest, conducted a systematic review of the available scientific evidence, and evaluated the contextual factors to be considered, with the last step remaining to make recommendations planned for early 2025. This short paper reports on the project progress and results achieved to date and shares plans for the next steps.
Methods
GDG and Management of Conflicts of Interest
The Guideline Development Group (GDG) was established by the WHO Secretariat based on the WHO global expert network membership – REMPAN, that brings together the world’s subject matter experts in the field of radiation emergency preparedness and medical response.Reference Carr 9 The GDG members come with diverse areas of relevant expertise including radiation dosimetry, radiation biology, health physics, public health, emergency response, clinical and radiation toxicology, radiation emergency medicine, and occupational medicine. 10 The geographic distribution of the group has over-representation by the experts from North America and Europe, which is related to the historical context of the required expertise evolution and their availability being intrinsically linked to the development of nuclear power and nuclear weapons industry and research in those parts of the world predominantly. That is why achieving a homogenous geographic distribution of relevant expertise has been challenging. To compensate for this shortcoming, the External Review Group (ERG) is drawing on the widely distributed representation of general specialists working in the field of radiation emergency preparedness and response capitalizing mostly on the membership of WHO REMPAN. In addition, a smaller group of topical experts (TEG) was set up to address some specific technical aspects of radiation dose assessment, bioassays, radiotoxicity of actinides, and pharmaco-kinetics of decorporating therapy agents.
Efforts were made to engage individuals who may be affected by any future guidelines, including the end users of the future guidelines – occupational medicine practitioners and a patient representative, who were invited to attend the GDG meetings as observers.
During the period of 2023-2024 the Secretariat organized 3 in-person working meetings of the GDG, including meetings in September 2023 (Seoul, South Korea), December 2023 (Madrid, Spain), and July 2024 (Orlando, USA), as well as regular on-line meetings to review the progress and discuss various issues.
All GDG participants’ conflicts of interest were handled in accordance with WHO rules, which were based on recommendations from the Guidelines International Network and the Institute of Medicine.Reference Schunemann, Al-Ansary and Forland 11 Individuals were requested to declare any potential financial and scientific interests prior to their appoint to the panel. After reviewing the disclosures, the WHO Internal Steering Group (ISG) members determined that members of the guideline panel, including the co-chairs, had no conflicts of interest at the time of appointment.
The GRADE® Approach
For the development of evidence-based guidelines, WHO requires the use of approved methodologies to systematically develop evidence-based statements that assist providers, recipients, and other stakeholders to make informed decisions regarding appropriate health interventions, 8 including the GRADE® approach (Grading of Recommendation Assessment, Development, and Evaluation), which is broadly applied in the assessment of certainty of evidence about selected health outcomes and in the assessment of strength of recommendations for action.Reference Schünemann, Brożek and Guyatt 12 The GRADE® approach, applied in this project, involves 5 major steps to arrive at recommendations: specifying health care questions, choosing outcomes of interest, rating the importance of the outcomes, evaluating the available evidence, and integrating this evidence with consideration of the values and preferences of patients and society.
Formulating Research Questions
The GRADE® approach uses PICO to formulate research questions:Reference Neumann, Mustafa, Morgan, Neumann and Schünemann 13
P: Population
I: Intervention
C: Comparator
O: Outcome
The first step in developing the research question about the assessment and management of internal contamination with transuranic actinides is to identify the population (P) where the intervention is a sensible and reasonable management approach. Next, the intervention of interest (I) is carefully selected. Equally important is the selection of an appropriate comparator (C).
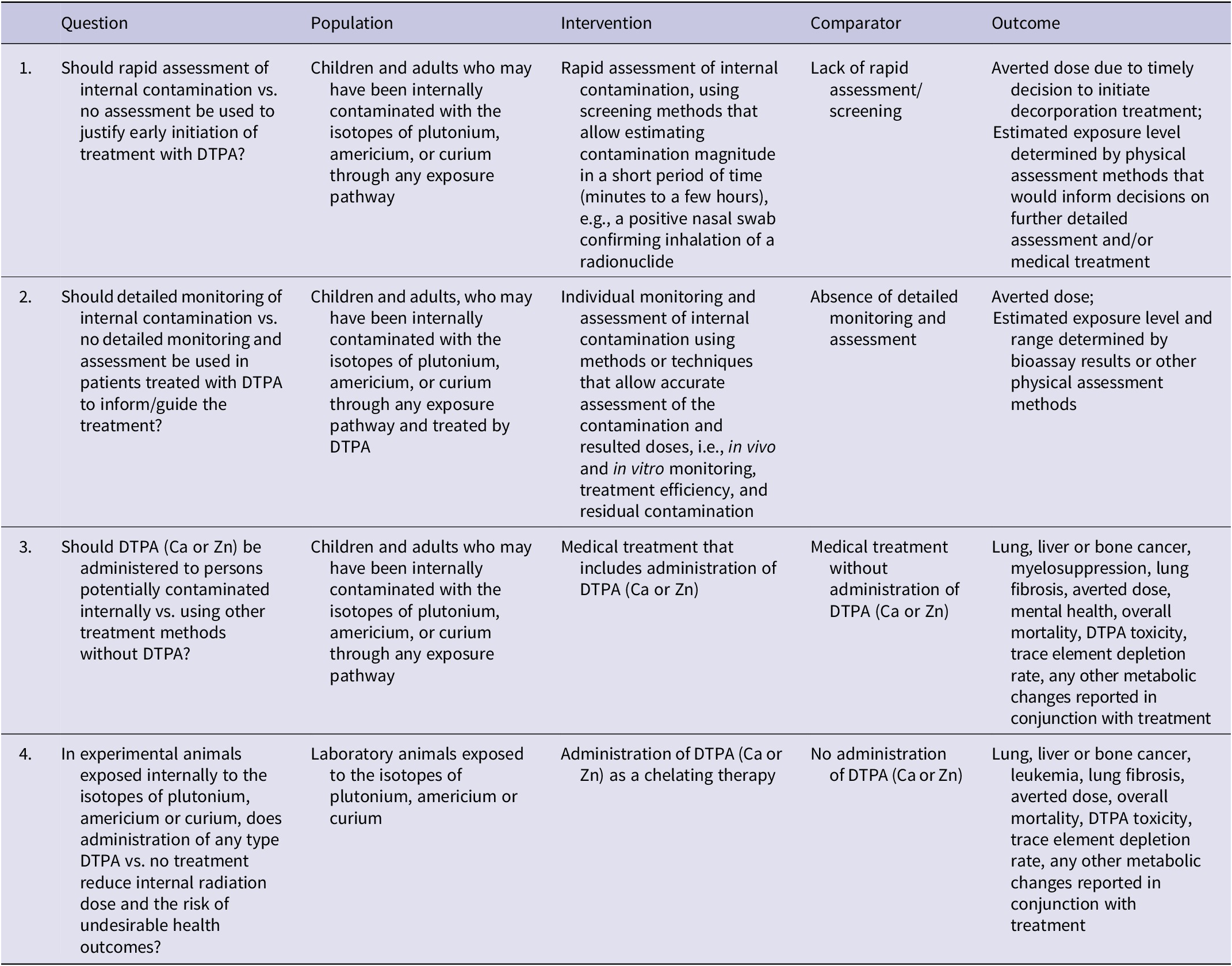
Under the guidance of the methodologist and with inputs from the TEG, the GDG formulated 4 PICO questions, 2 focused on the assessment and 2 on the management of internal contamination (Table 1). These research questions serve as essential guidance for searching and grading evidence and form the basis for conducting systematic reviews.
Table 1. The PICO questions formulated for assessment and management of internal contamination with transuranic actinides
Rating the Importance of Outcomes
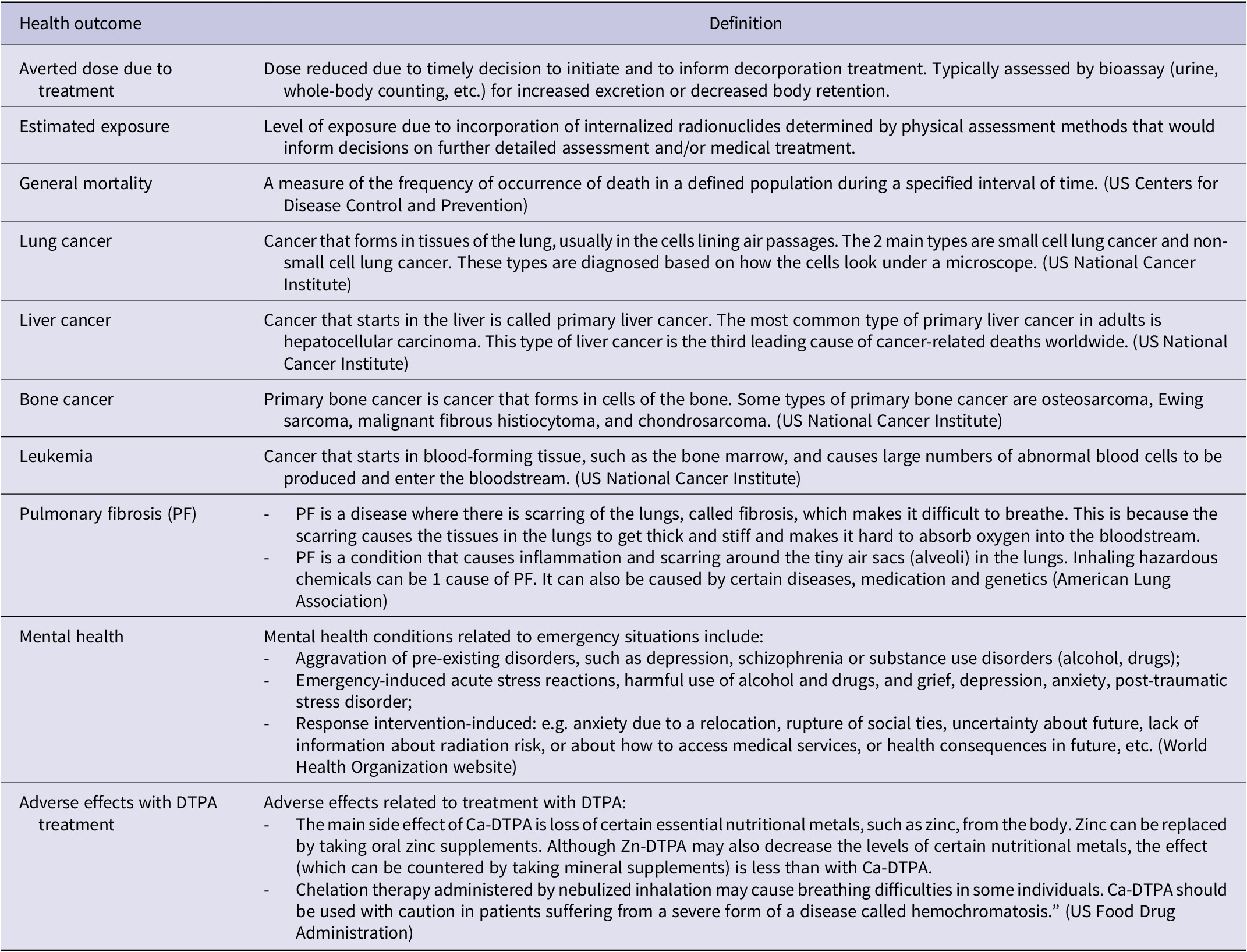
Recommendations cannot be based solely on information about individual outcomes; so, decision-making always requires a balance between health benefits and harms. Guideline panels must consider all outcomes that are critical or important to patients to make well-informed recommendations. Guideline developers must base the choice of outcomes not on what outcomes are measured and for which data are available, but rather on what is deemed important. Developers of guidelines will also initially classify the importance of the outcomes. GRADE® specifies 3 categories of outcomes according to their significance for decision-making: (1) critical; (2) important but not critical; and (3) of limited importance. Critical and important outcomes will have a bearing on guideline recommendations; however, the third will, in most situations, not. In this project, the GDG identified 10 health outcomes associated either with internal contamination with transuranic actinides or with the associated interventions (assessment or management) (Table 2). The relative importance of the health outcomes will be discussed and rated by the GDG members on a 1-9 scale (7-9: critical for decision making; 4-6 important but not critical for decision making; and 1-3 of limited importance for decision-making).
Table 2. Definition of the health outcomes for internal contamination with transuranic actinides
Searching and Grading Evidence
The systematic search of evidence was conducted based on a protocol developed by the systematic review team, in consultation with the GDG. The following electronic databases were searched: MEDLINE (1946-present), EMBASE (1947-present), and the Cochrane Library (from inception to present). Additionally, the websites of major national or international organizations involved in radiation emergency management were searched for relevant technical reports pertaining to the defined PICOs. The GDG will assess the identified reports for their significance and relevance in relation to the PICOs. All search strategies for the databases were developed by an experienced research librarian and fully documented. The search of the electronic databases was completed on June 12, 2023. The systematic review aimed to address research questions that are not typically evaluated in randomized clinical trials (RCTs), given the unique circumstances surrounding the accidental exposures that necessitate treatment. Therefore, for humans, the review primarily included non-randomized studies, while animal studies covered both experimental and observational study designs. Studies were excluded if they did not align with the components of the PICO questions, such as those not targeting DTPA treatment or 1 of the defined outcomes.
The risk of bias (RoB) assessment for animal studies was performed with the National Toxicology Program - Office of Health Assessment and Translation, 14 , 15 while human studies were assessed with the JBI critical appraisal tool for case reports.Reference Moola, Munn, Tufanaru, Aromataris and Munn 16 The aforementioned tool is a deviation from the initial protocol, which was necessary because the National Toxicology Program – Office of Health Assessment and Translation (NTP-OHAT) RoB tool showed no sufficient fit for the assessment in human studies. Due to substantial heterogeneity in the included studies, both in terms of exposure assessment and treatments, as well as for reported outcomes, no meta-analysis was feasible. Instead, a narrative review and synthesis was conducted instead, following the methodology outlined in the Cochrane Handbook (Chapter 12)Reference McKenzie, Brennan, Higgins, Thomas, Chandler, Cumpston, Li, Page and Welch 17 along with the summary in a harvest plot table.Reference Ogilvie, Fayter and Petticrew 18 Two researchers independently performed a screening of the identified evidence, data extraction, and RoB assessment. Further details regarding the methods used for the systematic review are provided in the protocol published elsewhere.Reference Gill, Dreger and Zeeb 19
Assessing Certainty of the Evidence
It is planned that for each effect estimate of the outcomes of interest, the GRADE approach will be used to evaluate the certainty in the body of evidence (also referred to as quality of the evidence or confidence in the estimated effects). This evaluation will consider the following domains: risk of bias, precision, consistency, directness of the evidence, risk of publication bias, presence of large effects, dose-response relationship, and an evaluation of the effect of residual, opposing confounding factors. Four levels of certainty will be distinguished, ranging from very low-high.Reference Guyatt, Oxman and Vist 20
Formulating Recommendations
For each research question, a GRADE Evidence-to-Decision (EtD) framework table will be used, using the GRADEpro Guideline Development Tool (www.gradepro.org).Reference Alonso-Coello, Schunemann and Moberg 21 , Reference Alonso-Coello, Oxman and Moberg 22 The EtD table covers the following contextual factors: resource utilization (cost-effectiveness), values and preferences (relative importance of outcomes), equity, acceptability, and feasibility. Before, during, or after the GDG meetings, members of the GDG panel will provide suggestions and identify any missing evidence in the draft EtD tables.
Formulating recommendations involves evaluation of the balance between benefits and harms from the intervention, as well as the certainty of evidence. The larger the difference between benefit and harm and the higher the certainty of evidence, the more likely it is for the recommendation to be strong; otherwise, it may be only conditional or even weak. For a recommendation, the lower the variability and uncertainty in values, the more likely it is for the recommendation to be strong; similarly, the lower the amounts of resources required or the more feasible it is to implement, the more likely it is for the recommendation to be strong.
Results
Identified Health Outcomes
Table 2 summarizes the identified health outcomes for internal contamination with transuranic actinides or related intervention, together with their definition in the context of this project. The most important outcomes were prioritized during the evidence gathering and summarization. Following an internal contamination incident, the individuals involved will be assessed for the level of contamination (estimated exposure) that will inform decisions on treatment to decorporate the internalized radionuclides and reduce radiation dose to the individuals (averted dose due to treatment), ultimately leading to reduced incidence and mortality from all diseases induced by or associated with such internal contamination (general mortality).
Internal contamination with transuranic actinides could lead to deposition in and irradiation of organs and tissues. This can potentially cause cancers primarily affecting lungs, liver, bones, or blood-forming tissues (lung cancer, liver cancer, bone cancer, leukemia). In cases of contamination through inhalation, damage to lung tissues and functions resulting in pulmonary fibrosis, have been reported.Reference Azizova, Moseeva and Grigoryeva 23
Decorporation treatment with DTPA (Ca or Zn) may cause adverse health effects (adverse effects with DTPA treatment), such as the depletion of certain essential elements for healthReference Taylor, Williams and Roberts 24 , Reference Tominaga, Shimomura and Tanosaki 25 or causing bronchospasm in asthmatic individuals when delivered in nebulized forms. Like other emergency situations, following internal contamination with transuranic actinides, the affected individuals and family members may express concerns about the health consequences from the contamination itself and/or may experience anxiety during the assessment and treatment process (mental health).Reference Klumpp, Bertelli and Hoffman 26
PICO Questions
Table 1 presents the PICO questions formulated for the assessment and management of internal contamination with transuranic actinides. PICO1 is on the use of rapid assessment to inform the early initiation of treatment. Following internal contamination, a fraction of the intake of transuranic actinides enters the systemic circulation. Once deposited in tissues/organs, decorporation treatment becomes less effective. Therefore, if decorporation treatment is necessary, it should be initiated/administered as early as possible after contamination. However, an accurate and detailed intake assessment requires time, as it typically involves laborious sample preparation and measurement using complex instrumentation. Thus, rapid assessment using faster screening methods, such as nasal swabs that allow estimating intake magnitude in a short period of time (minutes to hours), may be used to justify early start of the decorporation treatment, which, in turn, can help avert the dose that the patient would otherwise receive. Note that sometimes environmental measurements and/or model predictions provide useful information for potential internal contamination, which complements rapid assessment results.
PICO2 is on continuing and detailed monitoring and assessment for decisions on termination of treatment. With Ca- or Zn-DTPA treatment, the activity of the radionuclides retained in the systemic circulation and tissues/organs decreases over time. When it reaches a certain level, further treatment becomes less effective and could be discontinued. Retention and/or excretion of the radionuclides during the treatment period are monitored and assessed using detailed bioassay methods/techniques, either in vivo (e.g., whole body counting) or in vitro (e.g. urine bioassay).
PICO3 is on decorporation treatment using DTPA (Ca or Zn). Internal contamination with plutonium, americium, or curium can potentially occur in an occupational or environmental settings due to accidental or intentional release of these radionuclides. DTPA is a medication that binds with these transuranic elements and facilitates their elimination from the body, thus decreasing the radiation dose delivered to target organs as well as the risk of adverse health outcomes, including cancer.
PICO4 is on the effect of DTPA treatment in experimental animals internally contaminated with the isotopes of plutonium, americium, or curium. While human case reports present the effects of treatment directly on humans, animal studies provide valuable data regarding treatment efficacy, often expressed as reduced contamination levels (body burden) following treatment, resulting in a reduction in the risk of adverse health outcomes.
Evidence Profiles
Overall, 7 human studies (case reports or case series) and 30 animal studies were identified among more than 7000 records originally screened. The cases reported for human studies were all from workplace accidents. Of the 37 included studies, the distribution in DTPA types was as follows: (1) no information on DTPA-type (n = 4), (2) Zn-DTPA (n = 7), (3) Ca-DTPA (n = 14), (4) C2E2 (DTPA ester drug) (n = 1), (5) Ca-or Zn-DTPA (n = 6), and (6) combined Ca- and Zn-DTPA (n = 5). Two of the studies relevant for PICOs 3 and 4 were also applicable to PICOs 1 and 2. These 2 studies described rapid and detailed measurement methods for internal contamination with transuranic radionuclides.
The search of additional sources, primarily from the webpages of national and international organizations involved in radiation protection, resulted in 1 additional report to be included from initially 10 reports identified by screening. This included report described side effects in humans treated with DTPA.
Only 2 included studies reported a treatment effect with the outcome averted dose,Reference Bertelli, Waters and Miller 27 , Reference Sugarman, Findley and Toohey 28 while 2 other studies reported on DTPA toxicity by describing side effects.Reference Taylor, Williams and Roberts 24 , Reference Tominaga, Shimomura and Tanosaki 25 These outcomes were included in the PICO questions 3 and 4. After discussion with the GDG, the review team decided to use exposure level as a proxy surrogate outcome for averted dose. The outcomes related to treatment effect (other than averted dose) in the included studies were diverse and heterogeneous. Most of the included studies presented their results with exposure levels (after DTPA administration and in controls) as an outcome (28 animal studies).
Most studies showed significant risk of bias, primarily due to major imprecision stemming from a small number of treatment recipients in human case reports or the inability to assess imprecision in animal studies. There was inconsistency arising from varying magnitudes of the outcome. Publication bias could not be formally assessed. Furthermore, an initial assessment of the certainty of evidence conducted by the systematic review team indicated a very low certainty of evidence.
Contextual factors
Priority of the problem being addressed. The guidelines address both assessment and medical management of internal contamination with certain transuranic radionuclides; they are urgent problems recognized by occupational and public health communities and the interventions bear important consequences. Rapid assessment allows for timely estimation on the contamination level that informs the decisions on medical treatment. Continuing and detailed monitoring and assessment informs the treatment efficacy and the time to terminate treatment, while treatment itself helps avert the radiation dose to the patients and consequently reduces the risk of adverse health effects.
Balance of benefits and harms. Compared to the benefits from assessment and treatment mentioned above, harms associated with or resulting from these interventions are acceptable. Both rapid and detailed assessment use non-invasive sampling and measurement methods, and the anticipated undesirable effects are minimal. Decorporation treatment using DTPA is associated with some undesirable effects, such as depleting essential elements (e.g., Zn, Mn), especially when Ca-DTPA is used, or causing bronchospasm in asthmatic patients when the drug is administered via a nebulizer. However, such undesirable effects are generally tolerable, and the benefits received from the treatment outweigh these effects.
Certainty of evidence. Rapid assessment has frequently been used in the management of contamination incidents to inform decisions on medical management, with high level of certainty that it is helpful in facilitating timely decisions when accurate contamination information is not yet available. Methods and techniques for continuing monitoring and detailed assessment have been developed and successfully applied in the management of practical contamination cases; such monitoring and assessment provide high certainty of evidence for decisions regarding the termination of treatment when the benefits for continued treatment cannot be justified. The treatments using DTPA (Ca, Zn) help decorporate internalized transuranic actinides and avert the radiation dose to the patients, as demonstrated in the management of practical cases, offering a high certainty of evidence of its efficacy.
Values and preferences related to the outcomes of an intervention. Patients and family members, along with radiation experts and the medical professionals involved in the management of the contamination, would value the availability of information about the initial level of contamination obtained from rapid assessment and the activities of the radionuclides removed by the treatment and retained in the body obtained by continuing and detailed assessment. This information is essential for decisions on initiation and termination of the treatment. The benefits resulting from decorporation treatment, particularly in the reduction of risks of adverse health effects from radiation exposure, are clearly recognized by all stakeholders.
Resource implications. Rapid assessment based on individual measurements using simple sampling methods/techniques and existing measurement methods and instrumentation does not require many resources. Detailed assessment involves the application of advanced methods and instrumentation for monitoring and measurement, requiring relatively more resources, especially when the continuing monitoring and assessment lasts for a long period of time. The decorporation drugs used for treatment are relatively affordable but their availability may become an issue when large quantities are needed. Costs associated with the decorporation treatment in comparison with the treatment of the potential adverse health outcomes, including cancer resulting from the contamination, are relatively low.
Equity. For both rapid and detailed assessments, in most cases it is not anticipated that there are individuals or groups that may experience a less advantageous impact. However, in rare cases where a mass population need to be screened and assessed, it is possible that inequities will be experienced by different groups. For example, there may be marginalized members of the affected population. This concern also extends to treatment. For treatment, there are different considerations due to the age of the patient, pregnancy status, and comorbidities, such as renal failure and asthma.
Acceptability. It is with high certainty that the affected population would accept rapid assessment and detailed assessment, provided they are well informed about the benefits of such assessments, including the involved methods/procedures in sampling and measurement. However, some individuals who are asked to provide a large number of urine samples become non-compliant, despite being informed of the benefits; they would not consistently submit samples in a timely manner. For treatment, people would accept that the resulting benefits outweigh the harms, but in some cases where protracted/prolonged treatments are necessary, the acceptability may decrease.
Feasibility. Rapid assessment requires minimal resources and efforts for implementation. Detailed assessment requires more resources, which may impact on the sustainability of resources required for implementation, especially when the number of contaminated individuals is large. Availability of the methods and instrumentation and trained personnel in laboratories managing contamination cases may be limited, posing significant barriers. Treatment may be sustained for a relevant duration of time, and issues, such as cost and availability of the drugs, may arise. Potential access to stockpiles of necessary medications is crucial for effective management. DTPA must be administered intravenously or by a nebulizer, not orally, which may impact the ability to start treatment soon after contamination.
Discussion
Throughout the project, experts in the GDG and TEG worked closely with the methodologist and the systematic review experts. Additionally, 1 patient representative and 2 medical practitioners were invited to join the discussions; their perspectives and inputs greatly helped the project team in defining the concerned health outcomes, the research questions, and the contextual factors, which will contribute to the final recommendations to be developed in early 2025, following the EtD process. Once the guidelines draft is completed, it will be circulated for external review by REMPAN network members, and experts in other national or international organizations. Feedback received will be incorporated in the final version of the guidelines.
The project team recognized that there are some limitations in the evidence profile and the applicability of the recommendations to be developed: (1) The included evidence showed a positive indication of treatment efficacy, but initial assessment on the certainty of evidence showed very low certainty; (2) The experimental design and outcome measurement/reporting of animal studies varies across the studies reviewed, which made it difficult to compare results from different studies; (3) In human case reports, health follow-ups after decorporation treatment were usually lacking so assessment of the long-term health effects could not be easily made; (4) The decorporation treatment using DTPA (Ca or Zn) is not applicable to some other transuranic actinides such as neptunium (Np), as they exhibit different physical/chemical properties than the actinides addressed in this project; and (5) Decorporation treatment is not applicable to the inhalation of insoluble actinide-bearing particles, which requires different medical management approaches.
The project team also identified some areas where further research is needed, including: (1) Rapid methods for intake and dose assessment: current bioassay methods take days to weeks, affecting the decisions on treatment; (2) Characterization of the physical/chemical properties of the radionuclides, for example, solubility, as they may lead to significant overestimation or underestimation of the intake and dose; (3) A new chelating agent, HOPO, which is currently in phase I clinical trial, is promising for actinide decorporation; the pharmacokinetics of actinides treated with HOPO need to be studied as this determines the dosage, frequency and duration of the treatment; (4) The psychological impact of contamination and treatment using DTPA decorporation on patients and family members needs to be addressed.
Acknowledgements
WHO thanks the generous funding supports from Swiss Federal Office of Public Health, Switzerland and Federal Ministry of the Environment, Nature Conservation and Nuclear Safety, Germany, the guidance and support from the WHO Guidelines Review Committee and the valuable contributions from Drs. Laurent Bodin, Cornelius Hermann, Qingjie Liu, Jeanne Loyen, Inmaculada Sierra, Matthew Simpson, Takako Tominaga, Sara Dumit, Hubert Peiffer, and Dominique Leray.
Author contribution
Zhanat Kenbayeva is the project lead; Nicolas Dainiak and Chunsheng Li are the co-chairs of the guideline development group; Juan Jose Yepes-Nuñez is the methodologist; Hajo Zeeb and Sehajpreet Gill are the systematic reviewers; all others are members of the guideline development group. Chunsheng Li, Juan Jose Yepes-Nuñez, Hajo Zeeb, Sehajpreet Gill, and Zhanat Kenbayeva prepared the first draft of the manuscript; all others reviewed and provided inputs for improvements and finalization.
Funding statement
The project is financially supported by Swiss Federal Office of Public Health, Switzerland, and Federal Ministry of the Environment, Nature Conservation and Nuclear Safety, Germany.
Competing interests
The authors declare none.



