The Adoption of Agropastoral Lifestyles in the Southern Andes
Around 200 BC, human groups living in the southeastern tip of northwestern Argentina (NWA) underwent significant transformations in their lifeways and their relationships with the environment, animals, plants, and materiality (Núñez Regueiro Reference Núñez Regueiro1974; Olivera Reference Olivera, Berberián and Nielsen2001; Tarragó Reference Tarragó and Ledergerber-Crespo1999). During the Formative period (200 BC–AD 900), people settled in high-altitude and intermontane valleys and ravines and developed different ways of building, making vessels, and carrying out ritual practices, generating a complex picture of ways of being in the world anchored in the domestic realm (Aschero and Ribotta Reference Aschero, Ribotta, Arenas, Manasse and Noli2007; Oliszewski et al. Reference Oliszewski, Jorge, Guillermo, Matías Gramajo Bühler and Eugenia Naharro2017; Salazar et al. Reference Salazar, Montegú, Molar, Fiorani, Franco, Moyano and Franco Salvi2022; Scattolin et al. Reference Scattolin, Inés Cortés, María Fabiana Bugliani, Pereyra Domingorena, Izeta and Lazzari2009). All these changes in interrelationality, cosmologies, and material expressions occurred concurrently with a more profound transformation of human–environment and human–nonhuman relationships, resulting in the adoption of food production economies.
By the first centuries of the first millennium AD, people living across NWA villages and hamlets had fully committed to the farming of domesticated crops such as maize, quinoa, gourds, and beans without abandoning gathering practices (Franco Salvi, Salazar, and Berberián Reference Franco Salvi, Salazar and Berberián2014; Korstanje et al. Reference Korstanje, Quesada, Franco Salvi, Lema, Maloberti, Korstanje, Lazzari, Basile, Bugliani, Lema and Domingorena2015; Oliszewski et al. Reference Oliszewski, Molar, Arreguez, Carrizo and Martínez2019). In addition to the evidence of plant management and exploitation, archaeological remains also indicate the herding of Andean camelids (i.e., Lama glama), showing the dynamic configuration of subsistence practices among early villagers in NWA (Calo et al. Reference Calo, Bugliani, Scattolin, del Pilar Babot, Marchoff and Pazzarelli2012; Oliszewski et al. Reference Oliszewski, Jorge, Guillermo, Matías Gramajo Bühler and Eugenia Naharro2017).
Most research about farming practices in NWA has been restricted to archaeobotanical and faunal remains, specialized architecture such as field systems, and soil analyses (Capparelli et al. Reference Capparelli, Oliszewski, Leila Pochettino, Oliva, de Grandis and Rodríguez2007; Korstanje et al. Reference Korstanje, Quesada, Franco Salvi, Lema, Maloberti, Korstanje, Lazzari, Basile, Bugliani, Lema and Domingorena2015; Oliszewski and Arreguez Reference Oliszewski and Arreguez2015; Oliszewski et al. Reference Oliszewski, Molar, Arreguez, Carrizo and Martínez2019; Quesada Reference Quesada2006). This approach has helped us gain a better understanding of village lifeways. However, it tends to overlook overall site-formation processes, such as what organic materials are more likely to decay in certain soil conditions and their impact on interpreting the archaeological record. Furthermore, the long-lasting influence of ecological-based explanations of socioeconomic organization, such as Murra’s (Reference Murra1975) verticality model or Nuñez and Dillehay’s (Reference Núñez and Dillehay1995) movilidad giratoria (circular mobility) schemes in NWA archaeology, contributes to biasing the reconstruction of ancient subsistence economies.
Recent archaeometric developments can improve archaeological inference, illuminate anthropological questions about the past, and address biases in academic production. For instance, stable isotope analysis of human remains (Calo and Cortés Reference Calo and Leticia2009; Killian Galván et al. Reference Killian Galván, Cortés and Rabuffetti2021; Neveu Collado et al. Reference Neveu Collado, Killian Galván, Mondini and Alejandra Korstanje2024; Oliszewski et al. Reference Oliszewski, Killian Galván, Srur, Olivera and Martínez2020), phytoliths and starch analyses (Grill et al. Reference Grill, Franco Salvi and Salazar2013; Longo Reference Longo2021; Molar Reference Molar2022), and dental calculus (Molar Reference Molar2022) has provided a fine-grained understanding of the nature and complexity of subsistence strategies in the intermontane valleys. In Quebrada de los Corrales, a high-altitude ravine above 3,000 m asl, isotopic and archaeobotanical studies show the contribution of meat products and C3 plants (quinoa, chanar, and algarroba) to the overall diet, expanding our understanding of the role of plant foods within early farming societies in NWA (Martínez et al. Reference Martínez, Oliszewski, Arreguez, Backwell, Luna, Molar and Eugenia Naharro2020; Oliszewski and Arreguez Reference Oliszewski and Arreguez2015; Oliszewski et al. Reference Oliszewski, Molar, Arreguez, Carrizo and Martínez2019, Reference Oliszewski, Killian Galván, Srur, Olivera and Martínez2020). In contrast, in El Cajón Valley, stable isotope analysis of human remains indicates the prevailing exploitation and consumption of meat resources, with a minor intake of vegetable foods during a broad period from 3600 to 1300 BP (Killian Galván et al. Reference Killian Galván, Cortés and Rabuffetti2021). Moreover, in El Cajón, scholars have noted an increasing contribution of C4 plants toward the end of the Formative period, suggesting the progressive intensification of maize agriculture (Calo and Cortés Reference Calo and Leticia2009).
These findings exemplify the flexibility and variability of subsistence practices in NWA's intermontane valleys and ravines, as well as the complementarity of standard and newer lines of evidence. In particular, organic residue analysis (ORA) provides direct insights into past food production and local economies, revealing hidden aspects of materiality not otherwise preserved (Roffet-Salque et al. Reference Roffet-Salque, Dunne, Altoft, Casanova, Cramp, Smyth, Whelton and Evershed2017:621). In our case, obtaining direct chemical evidence of animal and plant foodstuffs processing in ceramic containers can help evaluate the food resources exploited by Formative period villager groups in NWA and overcome the constraints of previous archaeological approaches.
Here, we apply chemical techniques to cast light on the local economy of La Ciénega (Tucuman, Argentina), a small high-altitude valley with intense human occupation. Previous archaeological research characterized the area as a specialized herding settlement that was subsidiary to other agricultural villages in NWA, especifically the Tafí Valley (González and Nuñez Regueiro Reference González and Núñez Regueiro1960; Salazar and Kuijt Reference Salazar and Kuijt2016); this characterization was based on a combination of historical, ethnographic, and environmental data that attempted to explain the lack of visible archaeological remains that could be associated with agricultural production (Cremonte Reference Cremonte2003).
In this article, we argue that the absence of material indicators of agriculture is insufficient to sustain the characterization of La Ciénega as a specialized pastoral village. To support this argument, we present preliminary evidence from the analysis of 13 potsherds by gas chromatography-mass spectrometry (GC-MS). We conclude that biomolecular analysis is a valuable methodology that can provide insights into specific daily practices at the intra-site and household levels. This approach paves the way for a more comprehensive understanding of village lifeways and food production in La Ciénega and other archaeological regions in Argentina. Additionally, we believe that systematically exploring this line of research in the future can yield important opportunities to test archaeological models of food production and local economies.
Organic Residue Analysis (ORA) as a Tool for the Study of Local Economies
When applied to specific archaeological problems, information derived from organic residues is useful for reconstructing ancient economic practices and systems, foodways, and food habits (Dunne et al. Reference Dunne, Grillo, Casanova, Whelton and Evershed2019; Roffet-Salqué et al. Reference Roffet-Salque, Dunne, Altoft, Casanova, Cramp, Smyth, Whelton and Evershed2017). ORA is based on the concept of an “archaeological biomarker,” which relies on matching the chemical fingerprint of absorbed residues trapped in a pot’s porous wall and their distribution to the chemical structure of modern organisms, as well as identifying the degradation/alteration products more likely to be involved (Evershed Reference Evershed2008a). Because of their chemical properties, such as hydrophobia and their widespread occurrence in living organisms, lipids can provide an integrated record of the lifetime use of the vessel (Evershed Reference Evershed2008b; Miller et al. Reference Miller, Whelton, Swift, Maline, Hammann, Cramp and McCleary2020; Reber Reference Reber2022). Analysis of lipids can give firsthand evidence of food processing and consumption, whereas other (and complementary) material remains indicate indirect or successive instances of the food web, such as farming, grinding, and disposal (Dunne et al. Reference Dunne, Grillo, Casanova, Whelton and Evershed2019). Among early farming societies, ORA has proven a valuable proxy in shedding light on the transition of Mesolithic–Neolithic foodways and in mapping the adoption of dairying during the Pre-Pottery Neolithic / Neolithic periods in Euro-Asian contexts (e.g., Copley et al. Reference Copley, Berstan, Dudd, Docherty, Mukherjee, Straker, Payne and Evershed2003; Cramp et al. Reference Cramp, Ethier, Urem-Kotsou, Bonsall, Borić, Boroneanţ and Evershed2019; Cramp and Evershed Reference Cramp, Evershed, Spataro and Villing2015; Debono Spiteri et al. Reference Debono Spiteri, Gillis, Roffet-Salque, Castells Navarro, Guilaine, Manen and Muntoni2016; Evershed et al. Reference Evershed, Payne, Sherratt, Copley, Coolidge, Urem-Kotsu and Kotsakis2008; Smyth and Evershed Reference Smyth and Evershed2015).
To retrieve and characterize absorbed lipid residues, archaeologists need to use specific techniques with biomolecular resolution. High-resolution techniques, such as GC-MS and gas chromatography combustion isotope ratio mass spectrometry (GC-C-IRMS), can identify the chemical fingerprints of products processed or consumed within a pot in the past. Yet these compounds have different levels of specificity. Certain classes of biomarkers allow chemical identification at the species level because they are only synthesized by one or a small group of plant or animal species; for example, erucic acid produced by Brassicaceae vegetables (Colombini et al. Reference Colombini, Modugno and Ribechini2005; Evershed et al. Reference Evershed, Heron and Goad1991). Yet, in most cases, related organisms share metabolic pathways and biological processes. Therefore, many compounds cannot be assigned to a particular product (Eerkens Reference Eerkens2005). Despite this lack of specificity at the species level, ORA can be highly informative in distinguishing animal (carcass and dairy) from vegetable sources (oils, waxes, and resins; Evershed Reference Evershed2008b) and thus is very useful in reconstructing some dimensions of past food economies.
Large amounts of free fatty acids (saturated and monounsaturated), triacylglycerols (usually degraded as di- and monoacylglycerols), and cholesterol (when found together with its degradation products 7-keto-cholesterol, 5α-cholestanol, campestanol, and cholesta-4-ene-3-6-diol) indicate the presence of degraded animal fats (Whelton et al. Reference Whelton, Hammann, Cramp, Dunne, Roffet-Salque and Evershed2021). Plant materials produce large amounts of phytosterols, higher amounts of C16:0, unsaturated fatty acids, and their oxidation derivatives (i.e., hydroxy fatty acids and dicarboxylic acids; Dunne et al. Reference Dunne, Maria Mercuri, Evershed, Bruni and Di Lernia2016; Dunne Reference Dunne2022). Specifically, saturated fatty acids (C16:0 and C18:0), hydroxy fatty acids, and dicarboxylic acids are abundant in plant oils. Wax esters, long-chain n-alkanols, and even-numbered long-chain fatty acids indicate plant waxes (Dunne et al. Reference Dunne, Richard and Lucy2017). The use of compound-specific stable carbon analysis of fatty acids can achieve a high level of resolution.
In Argentina, the recent application of organic residue analysis has contributed to our knowledge of pottery use, ritual practices, and regional sociopolitical transformations, such as the role of feasting during the Inca conquest (e.g., Lantos et al. Reference Lantos, Orgaz, Panarello and Maier2017, Reference Lantos, Careaga, Palamarczuk, Aversente, Bonifazi, Petrucci and Maier2020; Ortiz and Heit Reference Ortiz and Heit2014; Pérez et al. Reference Pérez, Acosta, Naranjo and Malec2015). Foodways and subsistence strategies have received less attention, because research has generally been focused on the study of hunter-gatherer societies and the exploitation of marine resources in the Patagonia and Chaco regions (Chaile et al. Reference Chaile, Lantos, Maier, Cassiodoro and Tessone2018; Frère et al. Reference Frère, Isabel González, Constenla, Bayón, Berón, Luna, Bonomo, Montalvo, Aranda and Aizpitarte2010; Gómez Otero et al. Reference Gómez Otero, Constenla and Schuster2014; Pérez et al. Reference Pérez, Acosta, Naranjo and Malec2013; Stoessel et al. Reference Stoessel, Martínez and Constenla2015). A long-term study of maize consumption by Lantos and others (Reference Lantos, Spangenberg, Giovannetti, Ratto and Maier2015) did consider a few samples taken from an early context, but no extensive lipid studies have been conducted to investigate early village societies in NWA.
This article provides the first results of the application of organic residue analysis for the Formative period in the intermontane valleys of Argentina. We argue that biomolecular research and analysis constitute an important proxy for casting light on ancient food practices in La Ciénega Valley. Moreover, we hold that biomolecular research provides nuanced models of the adoption of food economies and subsistence, especially in cases where other lines of evidence—macro- and micro-botanic remains, farming agriculture, and zooarchaeological remains—are absent or inconclusive.
La Ciénega Valley: Ecological Possibilities and the Archaeological Data
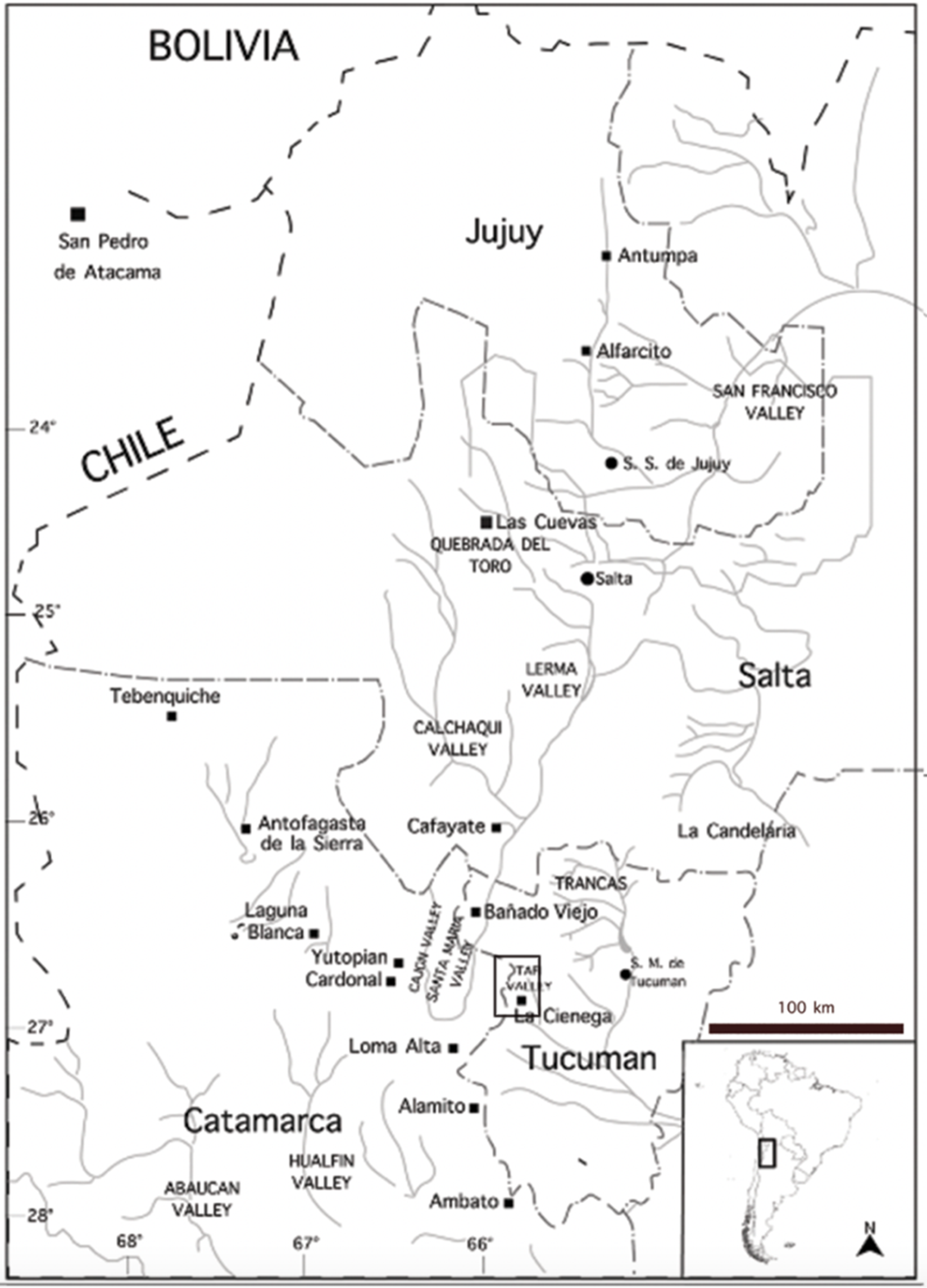
Located among the mid- and high-altitude valleys in the southeast end of NWA, La Ciénega (Figure 1) has an altitude that ranges between 2,400 and 2,800 m asl: it has a semiarid climate and is part of the foggy grasslands, a transitional ecological niche between the rainforest (yungas) and the dry plateau (Puna; Cabrera Reference Cabrera1976).

Figure 1. Map of northwestern Argentina. The black square demarks the study area. Map by Agustina Vazquez Fiorani.
Local vegetation in La Ciénega is well suited to cold and wet weather and is resistant to the cloud covers, mists, and rains that affect the valley (Cremonte Reference Cremonte1996). The area is covered by moorland vegetation, with grasslands, shrublands, and other low-growing plants that can prosper in acidic soils (Figure 2). Zooarchaeological records include wild camelid species, domesticated Lama glama, and cervids (e.g., Mazama americana). However, there are no modern camelid herds in La Ciénega, and farming activities are limited to sheep herding—even though the cold and wet climate offers good opportunities for the grazing of camelids, with several dense pastures and marshes that could have provided enough fodder for big llama herds in the past (Cremonte Reference Cremonte1996).

Figure 2. La Ciénega Valley landscape (figure prepared by Agustina Vazquez Fiorani). (Color online)
Cremonte (Reference Cremonte1996) mentions isolated cases of horticulture by some inhabitants, mainly vegetables and potatoes grown in stone enclosures. From interviews with the local population conducted in the late 1980s, we know that they complemented their subsistence basis by exchanging mutton for crops and wood with other groups in the eastern montane forest, such as the Anfama Valley (Cremonte Reference Cremonte1996). Despite its apparent absence in the archaeological record, soil studies in La Ciénega show optimal conditions for agriculture; therefore, it is possible that there was large-scale agriculture in the past that was likely interrupted during Spanish rule, as has already been observed in other sectors of NWA (Cremonte Reference Cremonte1996).
Cremonte (Reference Cremonte2003) argued that La Ciénega was associated with the nearby Tafí Valley villages because of similarities in settlement patterns, constructive techniques, and material culture. She proposed, however, that La Ciénega had a “peripheral and frontier nature” compared to the cultural center of Tafí, which was situated in an ecological zone that allowed the configuration of a seasonal pastoralism circuit in which a herding economy was connected through exchange routes to the plains and rainforests (Cremonte Reference Cremonte2003:58).
This interpretation was inspired by a zonal complementarity model in which Andean societies share an ideal of self-sufficiency anchored at the household and community level (Aldenderfer and Stanish Reference Aldenderfer, Stanish and Aldenderfer1993; Murra Reference Murra1975; Nielsen Reference Nielsen, Hirth and Pillsbury2015). In summary, because Andean geography imposes great ecological constraints on human economies and their subsistence within relatively short distances, Andean groups seem to have developed a novel use of space, economic exploitation, and social organization founded on a local ideal of self-sufficiency. Through relocated households’ effective colonization of different ecological niches, an ethnic group could secure communal access to complementary resources without the consolidation of specialized traders or proto-market mechanisms (Murra Reference Murra1975). Therefore, the apparent cultural homogeneity observed in prehispanic occupations placed in different ecological zones should be explained by this political, cultural, and economic strategy. Recent applications from archaeological science add nuance to the link between environmental affordances and socioeconomic organization, pointing to the coexistence of agricultural activities and herding (Killian Galván et al. Reference Killian Galván, Cortés and Rabuffetti2021; Molar Reference Molar2022; Neveu Collado et al. Reference Neveu Collado, Killian Galván, Mondini and Alejandra Korstanje2024; Oliszewski et al. Reference Oliszewski, Killian Galván, Srur, Olivera and Martínez2020). Still, the zonal complementary model remains highly influential in the region’s archaeology, especially in La Ciénega Valley.
In recent years, an archaeology team from the University of Córdoba (Equipo de Arqueología del Sur de las Cumbres Calchaquíes) and the Argentinian National Research Council (CONICET), in collaboration with the local Indigenous community of the Tafí Valley, have resumed archaeological investigations (Franco Salvi et al. Reference Franco Salvi, Salazar, López Lillo, Vázquez Fiorani and Montegú2023; Moyano et al. Reference Moyano, Franco, López Lillo, Vázquez Fiorani, Manuel Montegú, Chiavassa-Arias, Justiniano, Etchegoin Tonello, Franco Salvi and Salazar2023). Although Cremonte’s previous work in the region remains a groundbreaking contribution, new architectural, material, and contextual evidence suggest a strong reliance on plant resources at La Ciénega and casts doubt on the traditional characterization of the valley as a herding spot.
Materials and Methods
Archaeological Background
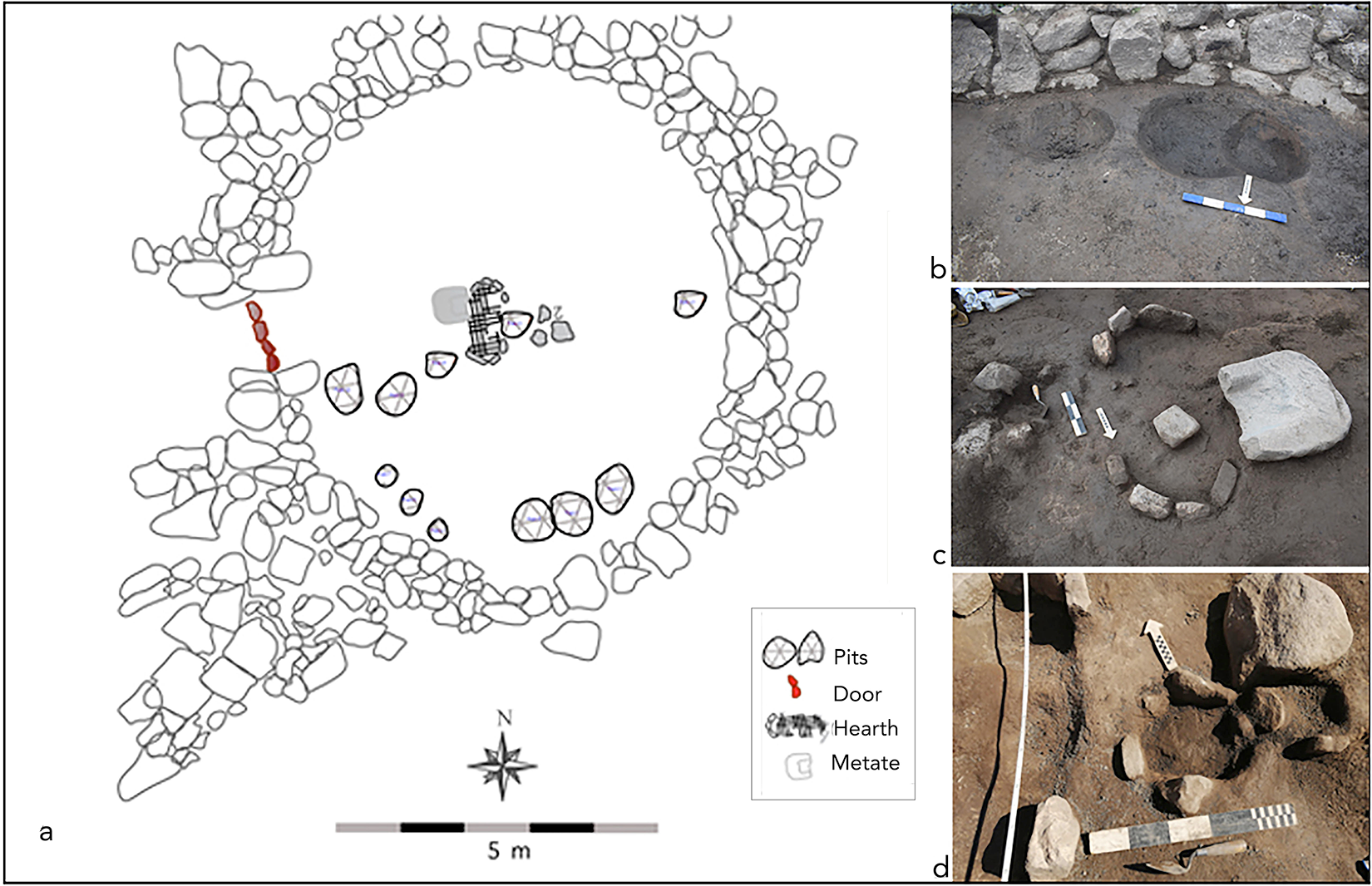
Archaeological occupations at La Ciénega Valley include 129 household compounds clustered in three sites scattered in the landscape. Fieldwork at the site Lomita del Medio, located in the middle section of La Ciénega Valley, provides the opportunity to investigate the material practices and daily life within early villagers in the south Calchaquí valleys. The site is made up of a household complex formed by a series of circular enclosures with three patios placed in a north–south orientation (n = 18 rooms). We excavated five structures—R89, R90, R91, R93, and R94—in full (Figure 3a), which provided insights into the intensive use of the domestic cluster during the first millennium AD (Franco Salvi et al. Reference Franco Salvi, Salazar, López Lillo, Vázquez Fiorani and Montegú2023; Moyano et al. Reference Moyano, Franco, López Lillo, Vázquez Fiorani, Manuel Montegú, Chiavassa-Arias, Justiniano, Etchegoin Tonello, Franco Salvi and Salazar2023). Radiocarbon samples of charred wood recovered from the central hearth of R94 (Figure 3b,c,d) date to between cal AD 435 and cal AD 640 (Franco Salvi et al. Reference Franco Salvi, Salazar, López Lillo, Vázquez Fiorani and Montegú2023:5).

Figure 3. Lomita del Medio household complex: (a) R94 enclosure (kitchen) floor plan with principal features; (b) pits; (c) hearth and grinding stone; (d) secondary heart (figure prepared by Valeria Franco Salvi and Julián Salazar). (Color online)
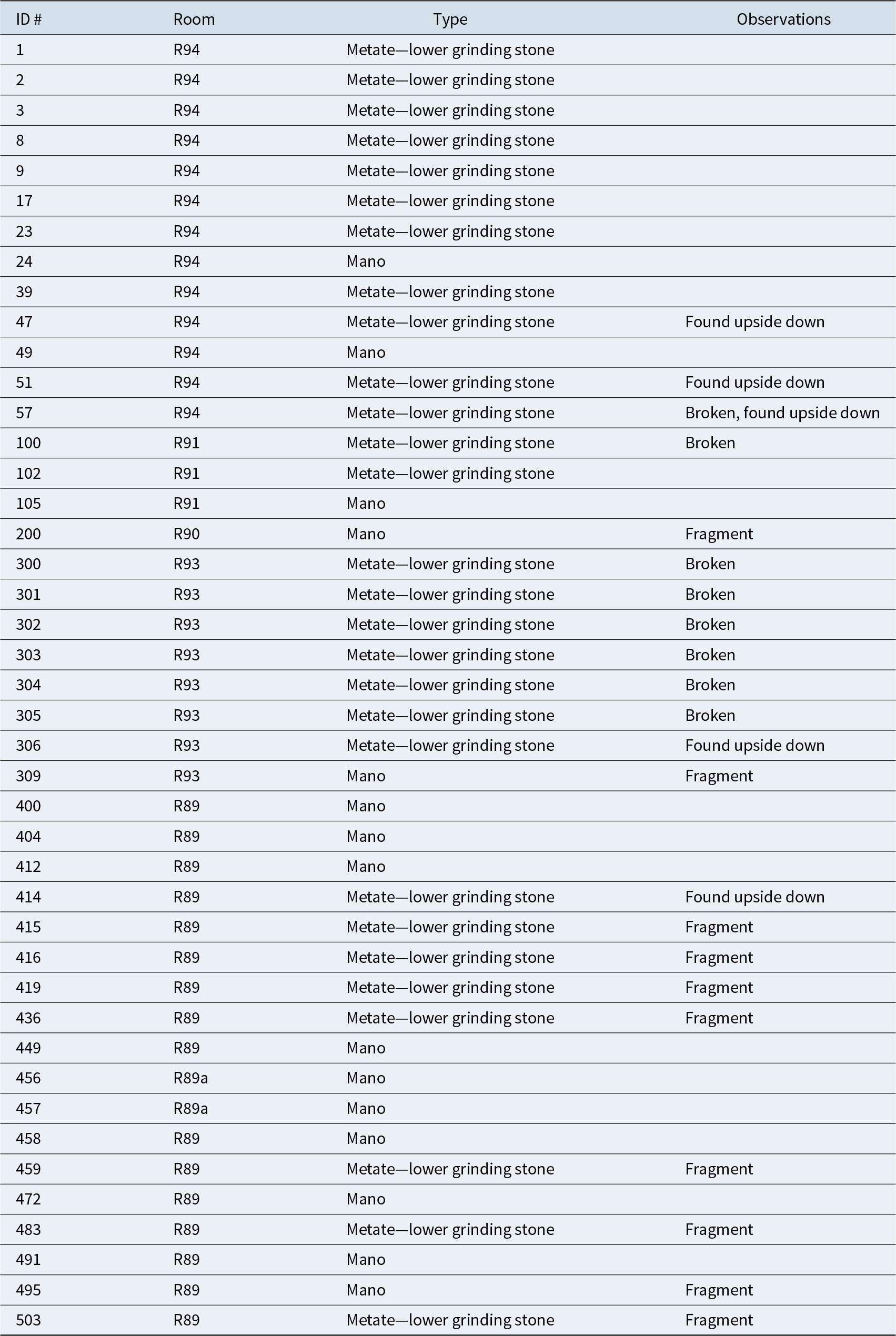
The R94 enclosure is associated with a kitchen space dedicated to the processing and cooking of foods. The presence of a formal hearth, several broken cooking pots, lithic artifacts (knives, hammers) and knapped flakes, grinding stones (manos and metates), and botanic macro- and micro-remains sustain this interpretation. In Table 1 we summarize all the grinding artifacts recovered from Lomita del Medio and their provenance within the household cluster. Within the archaeobotanical assemblage, we identified specimens of Geoffroea decorticans (n = 6) and the presence of meat fibers, maize, squash, and quinoa phytoliths on the edges of lithic knives.
Table 1. Description of Grinding Stones Recovered from Lomita del Medio Unit.

We sorted the ceramic assemblage recovered from the occupation floor of R94 by typological group and then grouped it into 110 families of fragments. These fragments were then analyzed with a petrographic microscope and further classified into three petrographic groups: coarse granitic, fine granitic, and metamorphic (Vazquez Fiorani et al. Reference Vazquez Fiorani, Tsoupra, Salazar, Mirão and Beltrame2024). Because of the technological and morphological suitability of ceramic pastes for specific activities (temper, porosity, wall thickness, vessel shape, and surface treatments), we were able to determine the following functional differences within the groups:
• Coarse granitic samples are characterized by porous and thick brownish pastes without surface treatments. Technological choices regarding raw material selection and paste recipes—for example, the use of high amounts of quartz as a temper—suggest they were manufactured with local clays to endure thermal shock. The vessels are associated with shapes such as open globular pots and bowls with mouths larger than 20 cm in diameter.
• Fine granitic samples do not exhibit compositional differences with coarse granitic groups, indicating that they were crafted using very similar raw materials. Fine wares are made of dense and thinner brownish-to-grayish pastes that are easily distinguishable by the naked eye and optical petrography. Morphological reconstruction of fine granitic samples allows the identification of service ware, such as small open bowls (<20 cm) and constricted neck jars.
• Metamorphic samples are vessels with coarse pastes decorated with incised dots made with rolling clay pits. Their metamorphic origin indicates that they are nonlocal. It is hard to ascribe them to cooking activities; rather, they might have been used as containers for prepared foods or drinks used on special occasions (due to their limited co-occurrence within the ceramic assemblage).
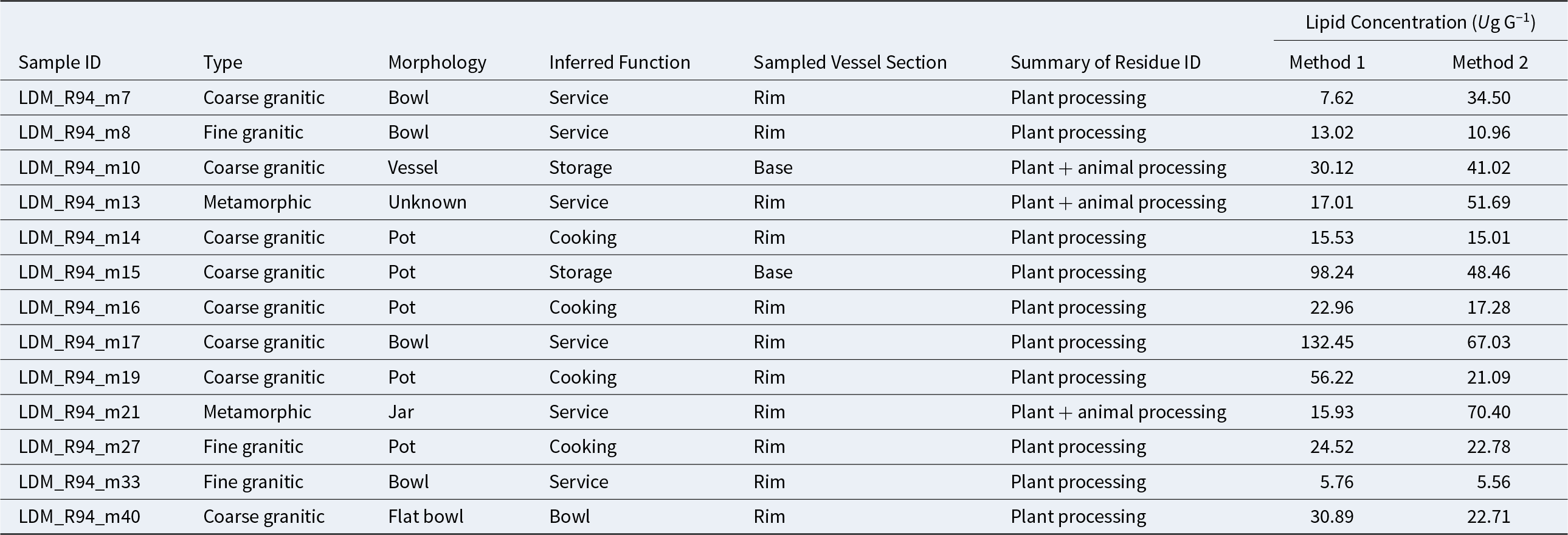
To better understand the local economy and daily food production, we selected 13 sherd samples for lipid analysis by GC-MS (Table 2). We selected samples considering size (larger than 4 cm), morphology type / petrographic group (representing different types of wares and functionality), and storage conditions in the field and laboratory (Evershed Reference Evershed2008a). Indeed, all samples were unwashed, did not exhibit evidence of handling or contamination agents, and were stored in aluminum foil without contact with plastics.
Table 2. Comparison of Extracted Odd-Chain Fatty Acids and Sterols with Method 1 and Method 2.

Sample Preparation and Lipid Extraction
We cleaned the ceramic sherds using a Dremel 3000 multi-tool to remove any contamination from soil sediment or due to handling or storage. After cleaning, we ground the sherds to powder with a mortar and pestle made of agate until obtaining a fine powder of approximately less than 10 µm.
We subjected the powdered samples to two types of extractions to retrieve absorbed lipids. Method 1 is an acidic extraction using H2SO4/MeOH, as described by Correa-Ascencio and Evershed (Reference Correa-Ascencio and Evershed2014), and Method 2 is a chloroform-methanol extraction using CHCl3/MeOH (2:1, v/v; Evershed et al. Reference Evershed, Heron and Goad1991). We decided to use two extraction methods because of the absence of previous work on similar samples, which made it impossible to determine beforehand the best procedure for our samples and research questions (Correa-Ascencio and Evershed Reference Correa-Ascencio and Evershed2014; Papakosta et al. Reference Papakosta, Smittenberg, Gibbs, Jordan and Isaksson2015; Reber Reference Reber2021). Furthermore, we thought that two different extraction methods (even if destructive) could be complementary, because the solvent extraction could recover acyl lipids saponified during acidic extraction and help maximize the recovered lipid yields to understand subsistence patterns (Reber Reference Reber2021).
Method 1. We added 20 µL of n-tetratriacontane (1 mg/mL; standard) to a test tube with 2 g of powdered sample. Next, we added 5 ml of H2SO4/MeOH (2% v/v) to the tube and heated it at 70ºC for two hours. We checked the pH <3 with a pH paper. Afterward, we centrifuged the samples for 10 minutes at 2,500 rpm. We decanted the clear supernatant in a second tube for each sample and added 2 mL of ultrapure H2O. Next, we added 3 mL of hexane to the potsherd residue and vortexed it for two minutes at 2,500 rpm. We incorporated the hexane supernatants from tube 1 to tube 2 and vortexed again. We repeated the operation three times, and then we removed the hexane fractions from tube 2 to a clean vial 3 with a pipette. We added hexane to the remaining H2SO4/MeOH/H2O solution in tube 2 and vortexed it twice. Again, we added the hexane fractions to the clean vial 3. Then, we evaporated the extract in vial 3 under N2 until dry.
Method 2. We added 20 µL of n-tetratriacontane (1 mg/mL; standard) and 2 mL of CHCl3/MeOH (2:1, v/v) solution to a test tube with 2 g of powdered sample. We ultrasonicated the samples for 15 minutes and then centrifuged it for 15 minutes at 2,500 rpm. We removed the solvent by decanting it to a vial. We repeated ultrasonication and centrifugation with the remaining solid in the tube after adding the solvent again. Afterward, we dried the extract using a water bath at 40ºC under a stream of N2.
In the two methods, to obtain the total lipid extract (TLE), we added 250 μl of hexane to the dry extract. We separated 50 μL of the redissolved extract in a 1.5 mL glass vial. We dried the sample in the vial with N2. Finally, we redissolved each sample in 50 μl of hexane and derivatized it with 50 μl of BSFTA/TMCS inside a microwave for 30 seconds at 700 W. We dried the extract under a stream of N2. Before GC-MS analysis, we added 100 μL of hexane to the lipid extract.
We used this formula to calculate the yield of recovered lipids in each sample. Following Reber (Reference Reber2021:5), quantification only considered the integrated areas of compounds that did not derive from possible contamination.
 \begin{equation*}\frac{{\left[ {Integrated\,area\,of\,peaks\,of\,interest} \right]}}{{\left[ {Integrated\,area\,of\,internal\,standard\,peak} \right]}}*\left[ {amount\,of\,internal\,standard} \right]\end{equation*}
\begin{equation*}\frac{{\left[ {Integrated\,area\,of\,peaks\,of\,interest} \right]}}{{\left[ {Integrated\,area\,of\,internal\,standard\,peak} \right]}}*\left[ {amount\,of\,internal\,standard} \right]\end{equation*}GC-MS Conditions and Organic Compounds Identification
The organic extracts were analyzed by Shimadzu GC2010 GC coupled to a Shimadzu GCMS-QP2010 ultra mass spectrometry. The capillary column used for separation was a Phenomenex Zebron-ZB-5HT (15 m length, 0.25 mm internal diameter, 0.10 µm film thickness). The conditions of method use for GC were the column oven temperature (50°C), injection volume of 1 µL, temperature of 250°C in a split-less injection mode, and a sampling time of one minute. The carrier gas was He (prim. Press. 300–500) with a linear velocity of 62.4 (cm/sec). The pressure was set at 31.5 kPa with a total flow of 152.5 mL/min and a column flow of 1.48 mL/min. Purge flow was 3 mL/min in the split-less mode.
The gas chromatography temperature program started at 50°C for 2 min, ramped up to 150°C, then to 250°C, and finally increased to 350°C, at which point it was held for two minutes. The total program time was 67.33 minutes. The MS ion source temperature was placed at 240°C, and the interface temperature was maintained at 280°C. The mass spectrometer was programmed to acquire data between 40 and 850 m/z. Identification and peak assignment were done using the National Institute of Standards and Technology (NIST) and Wiley mass spectra libraries through the Automated Mass Spectral Deconvolution and Identification System (AMDIS).
Results and Discussion
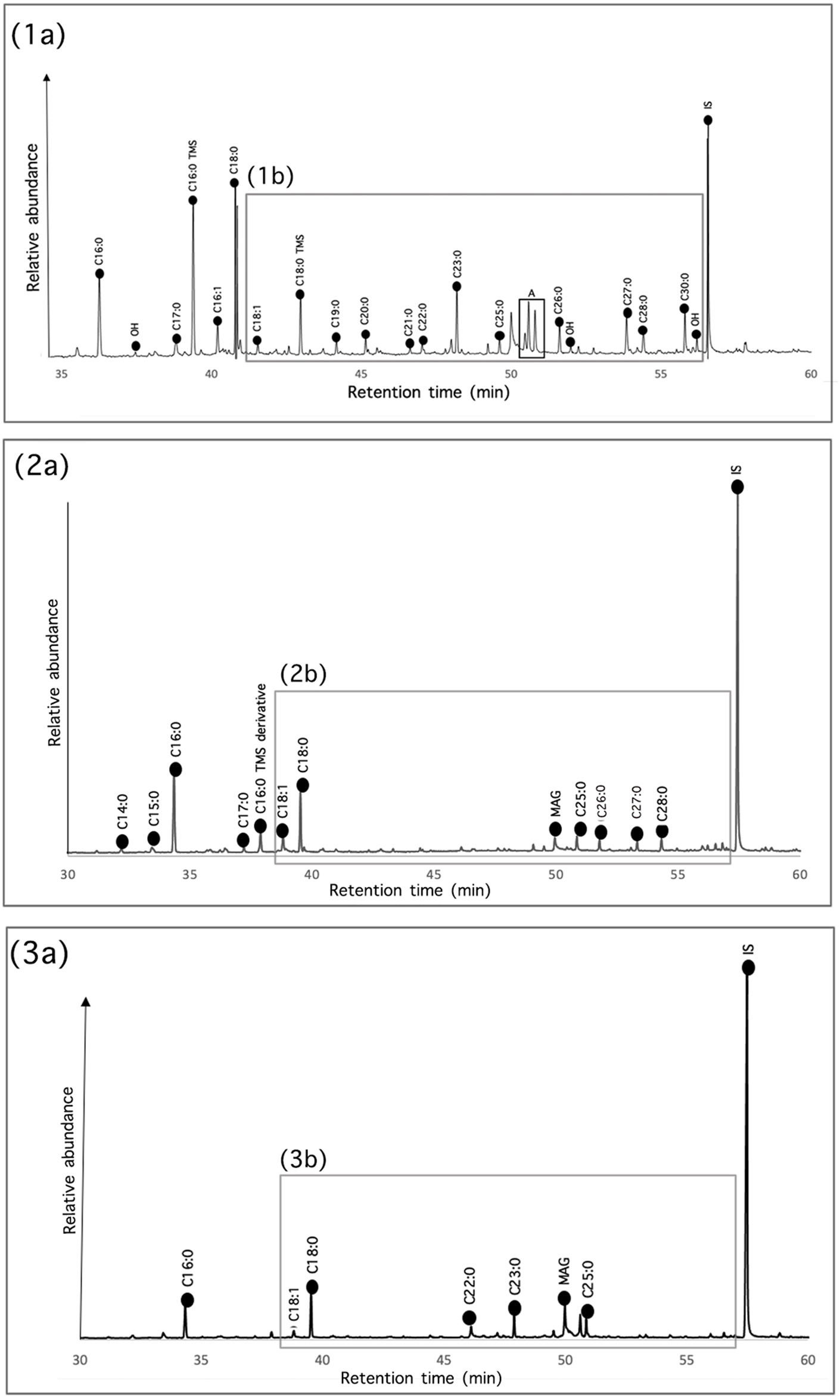
We identified internal standard and derivatization products in all samples, indicating that the extraction and derivatization procedures were effective in both methods of extractions. All samples yielded lipid concentrations higher than 5 µg g−1, making them suitable for interpretation (Whelton et al. Reference Whelton, Hammann, Cramp, Dunne, Roffet-Salque and Evershed2021). Additionally, the presence of unsaturated fatty acids (e.g., C18:1) in some samples suggested a good rate of preservation (Figure 4).

Figure 4. GC-MS chromatograms of samples (1a) LDM_R94_m15, (2a) LDM_R94_m33, and (3a) LDM_R94_M13 obtained by acidic extraction. The trimethylsilyl derivatives (TMS) and methyl ester (ME) derivatives of relevant compounds are marked. A = n-alkane with x carbon atoms, OH = linear alcohol with x carbon atoms, MAG = monoacylglycerol with x carbon atoms, IS = internal standard used for semi-quantification purposes.
We identified the organic compounds associated with archaeological biomarkers preserved in our samples, allowing us to generate hypotheses about the possible foodstuffs processed, stored, or both in the vessels. Overall, we did not observe sharp differences in the lipid yields between the solvent and acidified methanol extractions. However, we observed clear differences in the type of compounds identified by each method (see Supplementary Table 1 for a list of compounds identified with the two extraction methodologies used in the different samples). Cooking pots exhibited higher lipid amounts (up to 132.45 µg g−1), whereas the lowest lipid concentration of 5.56 µg g−1 was found in fine service vessels.
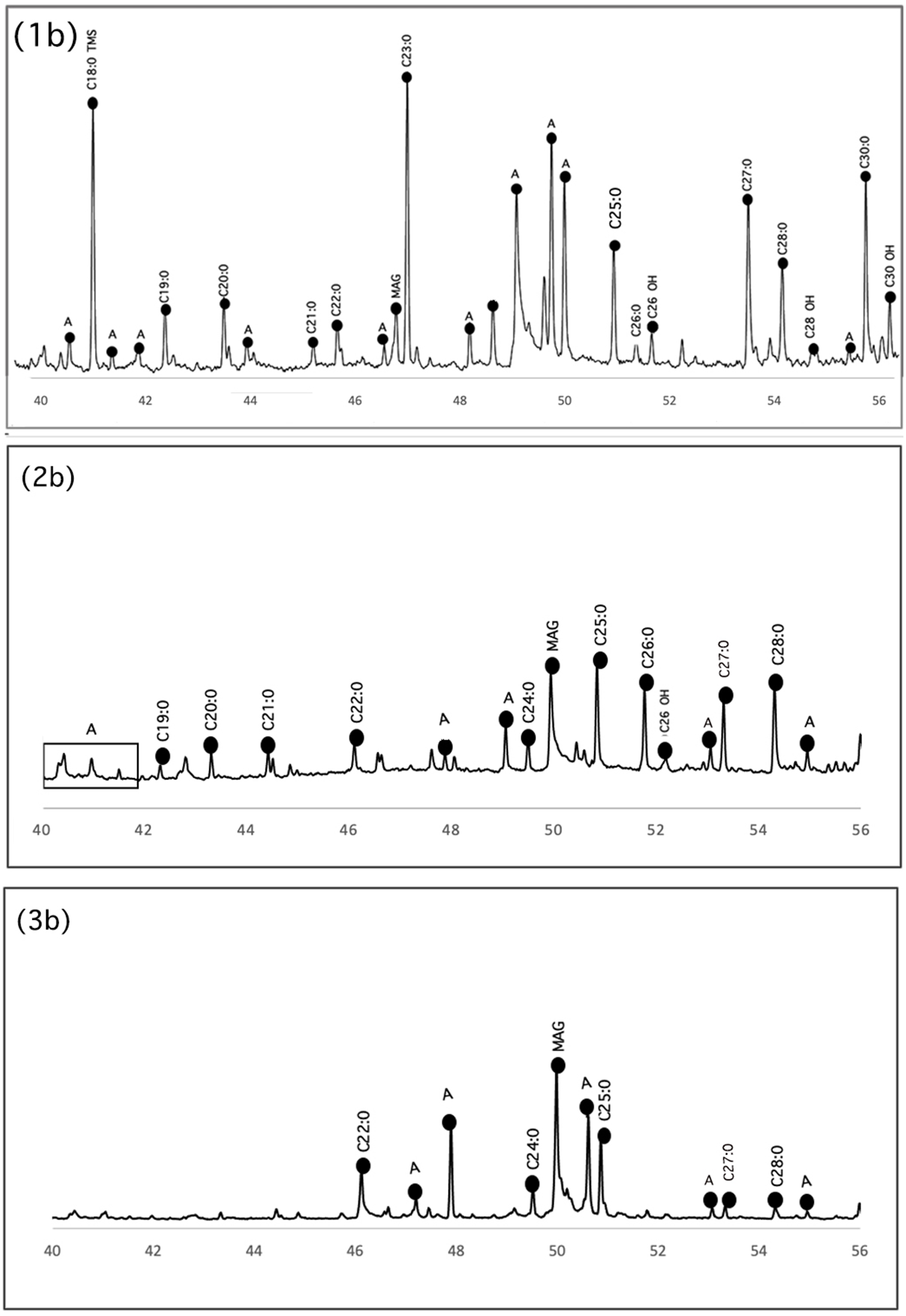
We observed a wide distribution of alkanes within the samples in both extraction procedures, with the number of carbons going as high as 36 (Figure 5). The ubiquitous presence of long-chain alkanes such as n-hexadecane (C16), n-heptadecane (C17), n-octadecane (C18), n-nonadecane (C19), n-eicosane (C20), n-docosane (C22), n-tetracosane (C24), n-hexacosane (C26), n-heptacosane (C27), n-nonacosane (C29), n-triacontane (C30), and n-hentriacontane (C31) could indicate the processing of terrestrial plants in the pots.

Figure 5. Detail of derivatized TMS and MEs long-chain fatty acids, fatty alcohols, and long chain alkanes portion of GC-MS chromatograms from samples: (1b) LDM_R94_m15, (2b) LDM_R94_m33, and (3b) LDM_R94_m13.
We detected fatty alcohols as trimethylsilyl ether derivatives, including the following compounds: 1-dodecanol (C12:OH AL), 1-tridecanol (C13:OH AL), 1-tetradecanol (C14:OH AL), 1-pentadecanol (C15:OH AL), 1-hexadecanol (C16:OH AL), 1-heptadecanol (C17:OH AL), 1-octadecanol (C18:OH AL), 1-nonadecanol (C19:OH AL), 1-eicosanol (C20:OH AL), 1-heneicosanol (C21:OH AL), 1-docosanol (C22:OH AL), 1-tetracosanol (C24:OH AL), 1-pentacosanol (C25:OH AL), 1-hexacosanol (C26:OH AL), 1-heptacosanol (C27:OH AL), 1-octacosanol (C28:OH AL), 1-triacontanol (C30:OH AL) and 1-dotriacontanol (C32:OH AL) (Figure 5). These alcohols are likely the result of the hydrolysis of epicuticular waxes’ esters, because they were found along with straight-chain fatty acids (Beeston et al. Reference Beeston, Palatinus, Beck and Stout2006; Miller et al. Reference Miller, Whelton, Swift, Maline, Hammann, Cramp and McCleary2020). N-dotriacontanol (C32:OH AL) is a biomarker of maize (Reber Reference Reber2021; Reber et al. Reference Reber, Dudd, Van Der Merwe and Evershed2004). Therefore, their presence in one sample is especially useful, although more research is needed to determine their origin.
All samples yielded fatty acids, mostly as fatty acid methyl esters (FAMEs). To a lesser extent, we identified trimethylsilyl ester derivatives in the acidic extraction, which might indicate that, in some cases, the derivatization process before the GC analysis was incomplete. Organic solvent extraction recovered fatty acids with similar results.
Palmitic (C16:0) and stearic acid (C18:0) are the most commonly identified compounds in organic residue analysis; therefore, they have little diagnostic value as biomarkers (Evershed Reference Evershed2008a). In the presence of plant products, C16:0 chromatographic peak areas should be higher than those of C18:0 (Eerkens Reference Eerkens2005). Nevertheless, C16:0 is more water soluble, and assumptions based solely on their ratio might be incorrect (Evershed Reference Evershed2008a; Miller et al. Reference Miller, Whelton, Swift, Maline, Hammann, Cramp and McCleary2020). Samples with an almost certain plant origin can exhibit higher C18:0 peak areas caused by the overrepresentation of this fatty acid compared to the more soluble palmitic acid (Whelton et al. Reference Whelton, Hammann, Cramp, Dunne, Roffet-Salque and Evershed2021). In our samples, it was not possible to determine the fat source based on the C16:0/C18:0 ratios.
In addition to C16:0 and C18:0 fatty acids, we identified saturated long-chain fatty acids with a carbon number range between C20 and C30 in all samples in the acidic extraction (Figure 5) and in samples LDM_R94_m7, LDM_R94_m8, LDM_R94_m10, LDM_R94_m14, LDM_R94_m15, LDM_R94_m19, LDM_R94_m21, LDM_R94_m33, and LDM_R94_m40 when extracted with the chloroform/methanol method. Long-chain fatty acids might be related to degraded plant waxes, indicating the plant origin of absorbed residues (Buonasera Reference Buonasera2007; Bush et al. Reference Calo and Leticia2013; Dunne et al. Reference Dunne2022; Evershed Reference Evershed2008a; Jha et al. Reference Jha, Patalano, Ilgner, Achyuthan, Alsharekh, Armitage and Blinkhorn2024; Miller et al. Reference Miller, Whelton, Swift, Maline, Hammann, Cramp and McCleary2020). In Andean contexts, a combination of C19:0, C20:0, C21:0, C22:0, and C24:0 fatty acids similar to the one we found was identified as evidence of plant waxes in groundstone and ceramics of NWA (Babot and Apella Reference Babot and Apella2003; Bonomo et al. Reference Bonomo, Colobig and Mazzi2012; Lantos et al. Reference Lantos, Palamarczuk, Orgaz, Ratto and Maier2018).
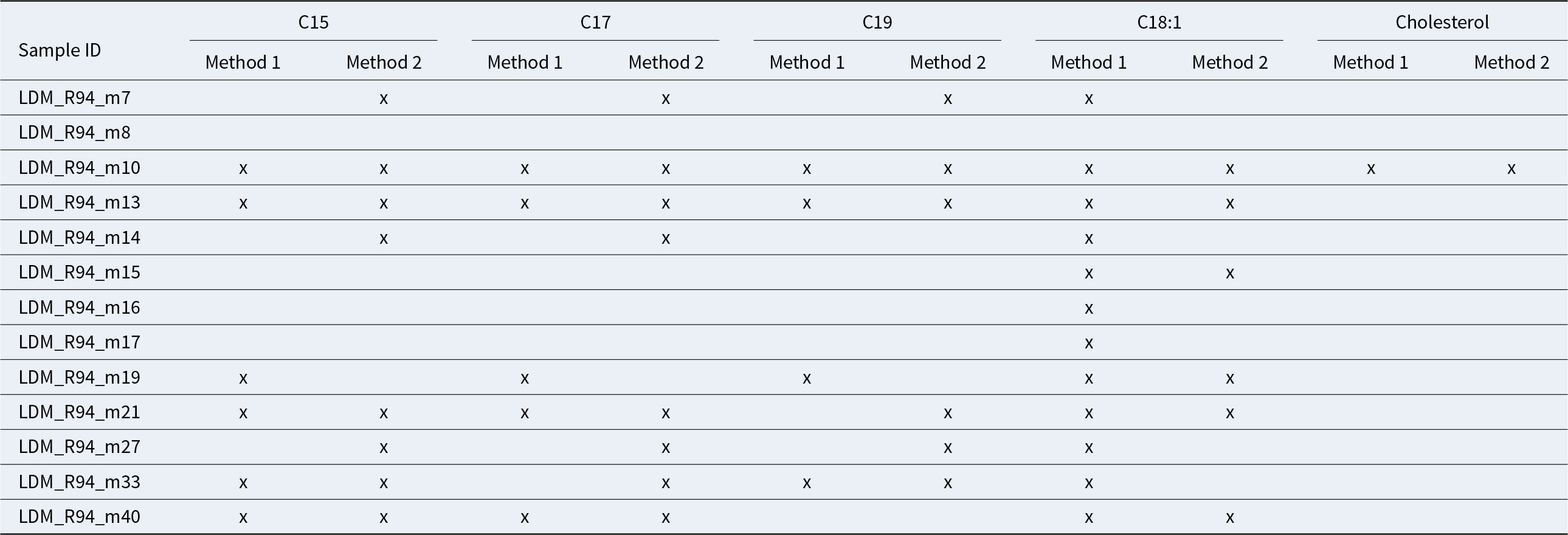
We identified odd-chain fatty acids (C15:0, C17:0, and C19:0) in several samples, but only in specific cases did they appear together (Table 3). The presence of odd-numbered chain fatty acids is, nevertheless, usually associated with the presence of animal ruminant fats (Eerkens Reference Eerkens2005). Fatty acids such as C15:0, C17:0, and C19:0, as well as C18:1 and their isomers, are associated with the bacterial action of the rumen during the digestion process (Fernández Sancha et al. Reference Fernández Sancha, Lantos, Fabiana Bugliani and Maier2021; Lantos et al. Reference Lantos, Careaga, Palamarczuk, Aversente, Bonifazi, Petrucci and Maier2020). These acids might indicate the processing of Andean camelid meat (e.g., Chaile et al. Reference Chaile, Lantos, Maier, Cassiodoro and Tessone2018; Fernández Sancha et al. Reference Fernández Sancha, Lantos, Fabiana Bugliani and Maier2021; Lantos et al. Reference Lantos, Palamarczuk, Orgaz, Ratto and Maier2018, Reference Lantos, Careaga, Palamarczuk, Aversente, Bonifazi, Petrucci and Maier2020). In some cases, these biomarkers can also result from bacterial contamination, but there were no further indicators of that because ergosterol was absent in all samples and only certain ones exhibited this distribution of odd-chain fatty acids (Lantos et al. Reference Lantos, Orgaz, Panarello and Maier2017).
Table 3. List of Samples Selected for Organic Residue Analysis by GC-MS.

Additionally, we identified the presence of unsaturated fatty acids. Several samples yielded C16:1 (palmitoleic acid), C17:1 (margaric acid), and C18:1 (oleic acid; see Figure 4). Palmitoleic acid and oleic acid are widely distributed among the samples, especially in the chloroform/methanol solvent extraction, and their presence may indicate certain plant oils (Evershed Reference Evershed2008b). Linoleic acid (C18:2) is the main constituent of maize and Prosopis, and its presence may suggest the processing or storage of these plant resources (Lantos et al. Reference Lantos, Spangenberg, Giovannetti, Ratto and Maier2015; Naranjo et al. Reference Naranjo, Malec, Pérez, Bárcena and Chiavazza2010; Pérez et al. Reference Pérez, Acosta, Naranjo and Malec2015). The presence of linoleic acid in only one sample can be explained by polyunsaturated fatty acids’ high susceptibility to degradation caused by prolonged cooking events and burial environments (Evershed Reference Evershed2008b; Lundy et al. Reference Lundy, Drieu, Meo, Sacco, Arcifa, Pezzini and Aniceti2021; Whelton et al. Reference Whelton, Hammann, Cramp, Dunne, Roffet-Salque and Evershed2021). Additionally, vessel reuse or mixture with animal products usually masks the presence of C18:2 (Lundy et al. Reference Lundy, Drieu, Meo, Sacco, Arcifa, Pezzini and Aniceti2021).
When present, triacylglycerols (TAGs) can shed light on fat sources, because well-preserved animal and plant fats have different TAGs; however, they are also subject to preferential degradation (Whelton et al. Reference Whelton, Hammann, Cramp, Dunne, Roffet-Salque and Evershed2021). During burial, the ester bonds in the triglycerides become hydrolyzed and subsequent leaching of the fatty acids occurs, limiting the analytical scope of these biomarkers (Evershed Reference Evershed2008a; Whelton et al. Reference Whelton, Hammann, Cramp, Dunne, Roffet-Salque and Evershed2021). Therefore, only the best-preserved archaeological fats will contain TAGs (Manhita et al. Reference Manhita, Martins, Costa, Prazeres, Rocha, Dias, Mirão and Teixeira2014). Indeed, we did not observe TAGs in the study but did find their degradation products, di- and monoacylglycerols (DAGs and MAGs), in some samples (see Figure 4).
Finally, sterols are a very important source of information when evaluating organic residue origins, because they can be easily related to specific sources (Reber Reference Reber2021). Animal products contain high amounts of cholesterol, whereas plant materials produce large amounts of phytosterols and only very minor amounts of cholesterol. Recently, Miller and others (Reference Miller, Whelton, Swift, Maline, Hammann, Cramp and McCleary2020) proposed on an experimental basis that free sitosterol is the most abundant sterol in maize and that C18:1 fatty acid sterol esters should dominate the sterol ester pattern in this American crop. Lantos and others (Reference Lantos, Careaga, Palamarczuk, Aversente, Bonifazi, Petrucci and Maier2020) found that stigmasterol and sitosterol are sterols found in various species, including Prosopis, Phaseolus vulgaris, and Chenopodium quinoa, as well as Zea mays, which could be used as plant food sources in our study area. Unfortunately, we observed degraded plant sterols only in samples LDM_R94_m10 and LDM_m13 (see Figure 4) and cholesterol in sample m10. We also found traces of cholesterol in samples LDM_R94_m40 and LDM_R94_m13. The combination of plant and animal foodstuffs might indicate that La Ciénega’s villagers were using the pots recurrently to prepare different recipes or were combining several food products in the same vessel.
Concluding Remarks
The results of our study indicate that the vessels analyzed were used predominantly to process plant resources, with a limited presence of animal foodstuffs. This preliminary research contributes to debunking previous characterizations of La Ciénega as a specialized herding settlement, instead suggesting the importance of vegetable meals in daily food preparation and consumption. It is important to emphasize that plant-origin products tend to be understated in organic residue analysis because animal products have a higher fat content than plant products. Therefore, it could be possible to infer a greater presence of vegetable-related lipids than in the samples detected here (Evershed Reference Evershed2008a; Whelton et al. Reference Whelton, Hammann, Cramp, Dunne, Roffet-Salque and Evershed2021).
As mentioned earlier, lipid analysis does not allow species-level identification. Nonetheless, it is possible to tentatively identify the plant species processed in these containers based on complementary archaeobotanical information. At the Lomita del Medio site, archaeobotanical (macro- and microremains) analyses revealed the processing of maize, squash, quinoa, and chanar. Moreover, the biomolecular information gathered in this article complements the findings from large grinding stones exposed on the surface of several household complexes across the valley, which show trace evidence of grain grinding, as well as those found in situ during excavations (Figure 6; Table 1). Taxonomic and morphological analyses of the entire botanical and fauna assemblage are in progress.

Figure 6. Grinding stones in La Ciénega Valley (figure by Agustina Vazquez Fiorani). (Color online)
Our organic residue analysis showed that only three samples—LDM_R94_m10, LDM_R94_m13, and LDM_R94_m21—fulfilled all the requirements (low- and mid-odd-chain [<C20] fatty acids and C18:1 isomers) to allow us to infer the presence of ruminant fats; two of these samples correspond to service wares and decorated nonlocal vessels (group C). The third sample with evidence of animal fats corresponds to a plain cooking pot (LDM_R94_m10, group A). This cooking pot could be used to prepare a combination of plant and animal resources based on the combination of fatty acids and cholesterol. However, there is no evidence of ruminant fats in the rest of the cookware (group A) or in group B comprising fine service vessels.
The limited co-occurrence of animal fats within the analyzed samples raises two questions, although we do not rule out that people could have consumed meat without the aid of ceramic containers; for instance, roasting. Could people have used decorated vessels for special occasions or events where nonquotidian, nonstaple foods were prepared and consumed? This scenario may explain why none of the utilitarian wares intended for daily use, such as bowls, pots, and jars, exhibit conclusive evidence of ruminant fats, and only one cooking pot shows traces of animal products. Or could this indicate the application of waterproofing or postfiring surface treatments (using animal fats) in this group of nonlocal ceramics in La Ciénega? Because of our limited sample size and the lack of compound-specific isotopic analysis, we cannot come to a more definitive conclusion. Nonetheless, future research and sampling should take these findings into account.
The data presented here align well with observations in the nearby valleys of Quebrada de los Corrales and Tafí, where the application of stable isotopes for diet reconstruction and archaeobotanical studies on ceramic containers and dental calculus show the exploitation of agricultural products along with different degrees of herding activity, even in high-altitude environments (Franco Salvi, López, and Molar Reference Franco Salvi, Laura López and María Molar2014; Martínez et al. Reference Martínez, Oliszewski, Arreguez, Backwell, Luna, Molar and Eugenia Naharro2020; Molar Reference Molar2022; Oliszewski et al. Reference Oliszewski, Molar, Arreguez, Carrizo and Martínez2019, Reference Oliszewski, Killian Galván, Srur, Olivera and Martínez2020, Reference Oliszewski, Martínez, Arreguez, Coronel, Di Lullo, Gramajo Bühler, Molar, Naharro and Nasif2022). Methodologies that can shed light on discrete aspects of daily life and reconstruct activities at the microscale of everyday use are well suited to help us better understand these complex and dynamic lifeways. We hope this study can serve as a stepping-stone for future multidisciplinary research. By increasing the sample size and further characterizing isotopic ratios by GC-C-IRMS, there is the potential to greatly improve the resolution level and precision of this analysis and bring more attention to research into plant foods in the area.
Acknowledgments
Several people collaborated on this work. JJulian Salazar, Juan Montegú, Lucía Justiniano, Jordi López Lillo, and Gonzalo Moyano Julian Salazar and Ian Kuijt gave crucial advice in the early stages of this manuscript. Hanna Erfenbeck revised the manuscript and considerably improved its quality. We want to thank the Tafí Indigenous community, especially Hilda Mamani and A. Ayala.
Funding Statement
An ARChaeological MATerials science master scholarship, SECyT-UNC Consolidar Res, SECyT 411/18, and FONCyT PICT grants funded this research. The authors have no nonfinancial interests to disclose.
Data Availability Statement
Data published in this article are available at https://www.aacademica.org/eascc.
Competing Interests
The authors declare none.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/laq.2024.41.
Supplementary Table 1. Full List of Compounds Identified by Each Extraction Method.