Key results
-
• O25 was the most prevalent O-serotype among E. coli isolates, consistent with previous studies
-
• 422/637 E. coli isolates from patients aged ≥60 years were EXPEC9V O-serotypes, corresponding to a vaccine serotype coverage of 66.2% in this high-risk population, although this vaccine is no longer in active clinical development
-
• EXPEC9V O-serotype prevalence did not substantially differ when stratified according to sex, presence of a positive blood culture, sepsis, fatality, or multidrug resistance; this suggests EXPEC9V serotype coverage would not substantially differ among these subpopulations
Introduction
Extraintestinal pathogenic E. coli (ExPEC) is an important E. coli pathogroup capable of causing infections outside the gastrointestinal tract [Reference Geurtsen1]. Besides being the most common cause of urinary tract infections (UTIs), ExPEC is the leading – yet under-recognized – cause of bacteraemia in adults worldwide, as well as a major cause of community-onset sepsis and subsequent hospitalization, and is associated with high morbidity and mortality [Reference de Kraker2–Reference Rhee6]. Invasive E. coli disease (IED), which includes E. coli bacteraemia and sepsis, can be clinically defined as an E. coli infection with acute systemic consequences, which is microbiologically confirmed either by the isolation and identification of E. coli from the blood or in any other normally sterile body site; or by the isolation and identification of E. coli from the urine of patients with urosepsis and no other identifiable source of infection apart from the urinary tract [Reference Geurtsen1, Reference Poolman and Wacker5, Reference Rhee6].
In the US, data from 2003 suggested that up to 40,000 patient deaths are caused by E. coli sepsis each year. Assuming mortality increased in line with septicaemia-related hospitalizations, this figure is estimated to have increased to >85,000 deaths in 2014 [Reference Poolman and Wacker5, Reference Russo and Johnson7]. In a cohort study of 17,430 US patients with culture-positive community-onset sepsis, E. coli was identified as the most common causative pathogen, responsible for 33.7% of community-onset sepsis cases [Reference Rhee6]. A more recent systematic study on the global burden of bacterial antimicrobial resistance demonstrated that sepsis-related mortality rates sharply increased in 2020/2021 and, by 2019, the likelihood that these deaths were the result of drug-resistant infections was 33% higher than in 1990 (8.5% vs. 6.4%) [8].
ExPEC strains possess a broad range of virulence traits and exhibit considerable genomic diversity [Reference Geurtsen1]. O-antigens are important surface-exposed virulence factors necessary for pathogen survival, which makes them attractive targets for vaccine development. However, to date, over 180 different E. coli O-serotypes have been described representing a broad structural diversity [Reference DebRoy9, Reference Iguchi10]. Yet, recent studies suggest that for IED, like for invasive pneumococcal disease, the majority of disease is associated with a specific subset of serotypes, with O25, O6, O1, and O2 among the most frequently associated, which considerably narrows down the number of O-serotypes that would be relevant for vaccine development [Reference Chorro11–Reference Weerdenburg17].
Given the high incidence of IED, especially in adults aged 60 years and older, with an associated case-fatality rate of >10%, prevention of IED, including E. coli bacteraemia and sepsis, has been identified as an important unmet medical need [Reference Russo and Johnson7, Reference Frenck18]. Despite available treatment options, the clinical and economic impact of IED remains substantial and novel preventive strategies, including vaccines, are needed [Reference Russo and Johnson7, Reference Frenck18, Reference Huttner19].
Based on the overall prevalence of O-serotypes among E. coli isolated from patients with IED, EXPEC9V – a 9-valent bioconjugate vaccine targeting nine E. coli serotypes (O1A, O2, O4, O6A, O15, O16, O18A, O25B, and O75) – was developed and was being evaluated in a pivotal phase 3 vaccine efficacy trial (E.mbrace; NCT04899336) for the prevention of IED in adults ≥60 years with a recent history of UTI at the time of study conduct, although it is no longer in active clinical development [Reference Qiu20].
To inform the development of EXPEC9V, this study describes the prevalence of O-serotypes of E. coli isolated from hospitalized patients with IED in 17 tertiary care hospitals across eight different countries. We report the serotype coverage of the EXPEC9V vaccine among patients with IED, with a focus on those aged 60 years and older. To estimate vaccine coverage in subgroups of interest, results were stratified by sex, the absence or presence of a positive blood culture, sepsis, a fatal outcome, and multidrug resistance.
Methods
Study design and participants
This retrospective, multicentre, non-interventional study analysed data collected across 17 tertiary care hospitals in Europe, North America, and Asia. IED isolates collected from eligible patients across sites in Canada (2), France (2), Germany (2), Italy (2), Japan (2), Spain (3), the United Kingdom (2), and the United States (2) were included in the analysis [Reference Doua21]. Sites were selected based on their ability to access demographic and clinical data of eligible patients retrospectively. The detailed study methods and primary results – which describe the clinical profile of IED in this cohort of patients and the utility of a proposed IED case definition – have been published previously [Reference Doua21].
The study was initiated on 28 September 2018 and included data collected between 09 January 2018–08 November 2019. The primary data sources for this study were the medical records of each participating patient from the study site (i.e., any clinical records – including microbiology laboratory records – and hospital records) [Reference Doua21].
Patients with IED of any age were identified from these medical record databases using a list of International Classification of Diseases (ICD) codes, which included culture-confirmed bacteraemia and sepsis. Patient records found to include the relevant ICD codes during the 12 months prior to the start of the study were assessed for eligibility as described previously [Reference Doua21]. Patients were included in the study if they had been hospitalized for IED or had nosocomial IED, with either E. coli as a single pathogen or a mix of pathogens where E. coli was present. For this, the E. coli infection had to be laboratory confirmed by culture, with the patient showing signs and symptoms of invasive disease based on the presence of systemic inflammatory response syndrome (SIRS), sepsis, or septic shock.
Patient consent
This study was approved by the Independent Ethics Committee and/or Institutional Review Boards. Physicians sought waivers and/or consent from eligible patients for inclusion of their data into the study according to local regulations. A waiver for informed consent was obtained for Canada, the United Kingdom, and the United States. In Germany, all patients signed a participation agreement/informed consent form (ICF)/informed assent form (IAF); and for deceased patients, a participation agreement/ICF/IAF was signed by the patient’s next of kin. In Spain and Italy, attempts to contact the patients were made, but waivers were obtained if it was too difficult to contact the patient. In France, letters of nonobjection were sent to the patients, which explained that the patients were included without consent if no objection was made. In Spain, France, and Italy, no consent was required for deceased patients. In Japan, no consent was required, but patients were given an opportunity to refuse study participation [Reference Doua21].
Species identification and antimicrobial susceptibility testing
Suspected E. coli strains isolated from patients with IED were shipped to a certified central laboratory using Stuart Transport Medium at ambient temperature. The shipped strains were checked for purity by culture, and E. coli species identity was confirmed by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry. Automated susceptibility testing was performed using the BD Phoenix™ system with NMIC-417 or NMIC-408 panels (depending on the strain), according to the broth microdilution assay as per Clinical and Laboratory Standards Institute (CLSI) guidelines (M07 supplement) [Reference Weinstein and Lewis22], with interpretations regarding susceptibility or resistance reported according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) established breakpoints, as appropriate [Reference Weinstein and Lewis22, Reference Brown23]. Resistance to antibiotics of the following classes was tested at a minimum: aminoglycoside, fluoroquinolone, folate pathway inhibitor, nitrofurantoin, and β-lactam. For a full list of antibiotics included in susceptibility testing, see Supplementary Table S1. Multidrug resistance was defined as resistance to at least three antimicrobial drug classes.
Determining O-serotypes
All E. coli isolates were analysed by agglutination O-serotyping [Reference Orskov24]. Whole-genome sequencing (WGS) was performed using Illumina® technologies, and the identification of O-serotypes was achieved using unique O serotype-specific sequences of wzy, wzx, wzt, and wzm genes following guidelines and using reference sequences described elsewhere [Reference DebRoy9, Reference Iguchi10, Reference Weerdenburg17]. To estimate the serotype coverage of EXPEC9V, the cumulative prevalence of the following nine O-serotypes (based on agglutination or WGS) was reported: O1, O2, O4, O6, O15, O16, O18, O25, and O75. E. coli isolates for which the O-serotype could not be determined were excluded from the analyses. Further subgroup analyses were performed to assess O-serotype prevalence among isolates from patients stratified according to age (all patients with IED; patients aged ≥60 years), sex, whether they had a positive E. coli blood culture, sepsis, a fatal outcome, or a multidrug-resistant (MDR) infection or not.
Statistical analysis
Comparisons were summarized using descriptive statistics (mean, standard deviation (SD), maximum, upper quartile, median, lower quartile, interquartile range, minimum, and number of observations, as appropriate) for continuous parameters, and frequency and proportions for categorical parameters. No formal statistical hypothesis testing was conducted for this study. All 95% confidence intervals (CIs) were two-sided.
Results
IED was diagnosed across different ethnic groups, equally affected both males and females, and occurred most frequently in older adults
A total of 924 patients were screened, of whom 902 patients were included in the study. Reasons for screening failure included: absence of culture confirmation (10 patients); no IED diagnosis within the last 12 months (five patients); no hospitalization for IED or not being in the hospital at the time of IED occurrence (four patients); multiple inclusion criteria not met (two patients); and inability to acquire informed consent (one patient). A total of 898 patients (99.6%) completed the study; reasons for discontinuation included: E. coli isolate was lost (two patients); E. coli isolate was not sent (one patient); and E. coli isolate was not frozen (one patient).
Baseline characteristics and the clinical profile of patients enrolled in this study have previously been published [Reference Doua21], and are summarized in Table 1. Briefly, the median age of the study population was 71 (range, 61–82) years; 465 patients (51.6%) were male, and 690 (76.5%) patients were aged ≥60 years. Most patients had IED with a positive E. coli blood culture (n = 844 [93.6%]), while 326 (36.1%) patients showed signs or symptoms of a UTI. 698 (77.4%), 588 (65.3%), and 127 (14.1%) patients presented with SIRS, sepsis, and septic shock respectively. Overall, 180 (20.0%) patients died, of whom 171 had a cause of death recorded (52 [30.4%] related to IED; 84 [49.1%] related to underlying conditions; and 35 [20.5%] related to other causes) [Reference Doua21].
Table 1. Patient demographics and characteristics

a Interquartile range. BMI, body mass index; IED, invasive E. coli disease; SIRS, systemic inflammatory response syndrome; UTI, urinary tract infection.
O25 the most common O-serotype, consistent with previous epidemiological reports, and EXPEC9V had a vaccine coverage of around 66%
Out of 896 E. coli isolates that underwent WGS, O-serotype prevalence could be determined in 837 (93.4%) isolates, and 76 unique O-serotypes were identified. Among these, the most observed serotypes were O25 (17.3%), O2 (11.7%), O6 (9.3%), O1 (6.3%), O15 (5.3%), O75 (5.0%), O8 (4.2%), O16 (4.1%), O17/O44/O73/O77/O106 (4.1%), O4 (3.9%), O18 (3.0%), and O9 (2.7%) (Supplementary Table S2). Based on this WGS analysis, a total of 552/837 E. coli IED isolates were identified as EXPEC9V O-serotypes, corresponding to a vaccine serotype coverage of 66.0%. In patients aged ≥60 years, 422/637 IED isolates were identified as EXPEC9V O-serotypes, corresponding to a vaccine serotype coverage of 66.2%. The EXPEC9V vaccine had a cumulative serotype coverage of just over 60% among all isolates O-serotyped by WGS and of approximately 55% assessed by agglutination, with similar prevalence in all patients with IED and in patients aged ≥60 years (Figures 1 and 2).

Figure 1. Prevalence (%) of EXPEC9V O-serotypes based on agglutination and whole-genome sequencing with cumulative prevalence (%) for all IED isolates; full analysis set. 95% confidence interval based on the exact Clopper–Pearson method. AGG, agglutination; IED, invasive E. coli disease; WGS, whole-genome sequencing.

Figure 2. Prevalence (%) of EXPEC9V O-serotypes based on agglutination and whole-genome sequencing with cumulative prevalence (%) for IED isolates from patients aged ≥60 years old; full analysis set. 95% confidence interval based on the exact Clopper–Pearson method. AGG, agglutination; IED, invasive E. coli disease; WGS, whole-genome sequencing.
After stratifying the prevalence of IED isolates among patients aged ≥60 years by those who presented with a positive E. coli blood culture (n = 595) and those who did not (n = 42), serotypes O25, O2, and O6 were among the most prevalent of the EXPEC9V serotypes in both subgroups: O25 (IED with a positive E. coli blood culture, 18.2% vs. IED without a positive E. coli blood culture, 11.9%), O2 (11.8% vs. 9.5% respectively), and O6 (10.1% vs. 7.1% respectively) (Table 2).
Table 2. Prevalence of EXPEC9V O-serotypes based on whole-genotype sequencing, stratified by the recording of a positive E. coli blood culture, in patients aged ≥60 years
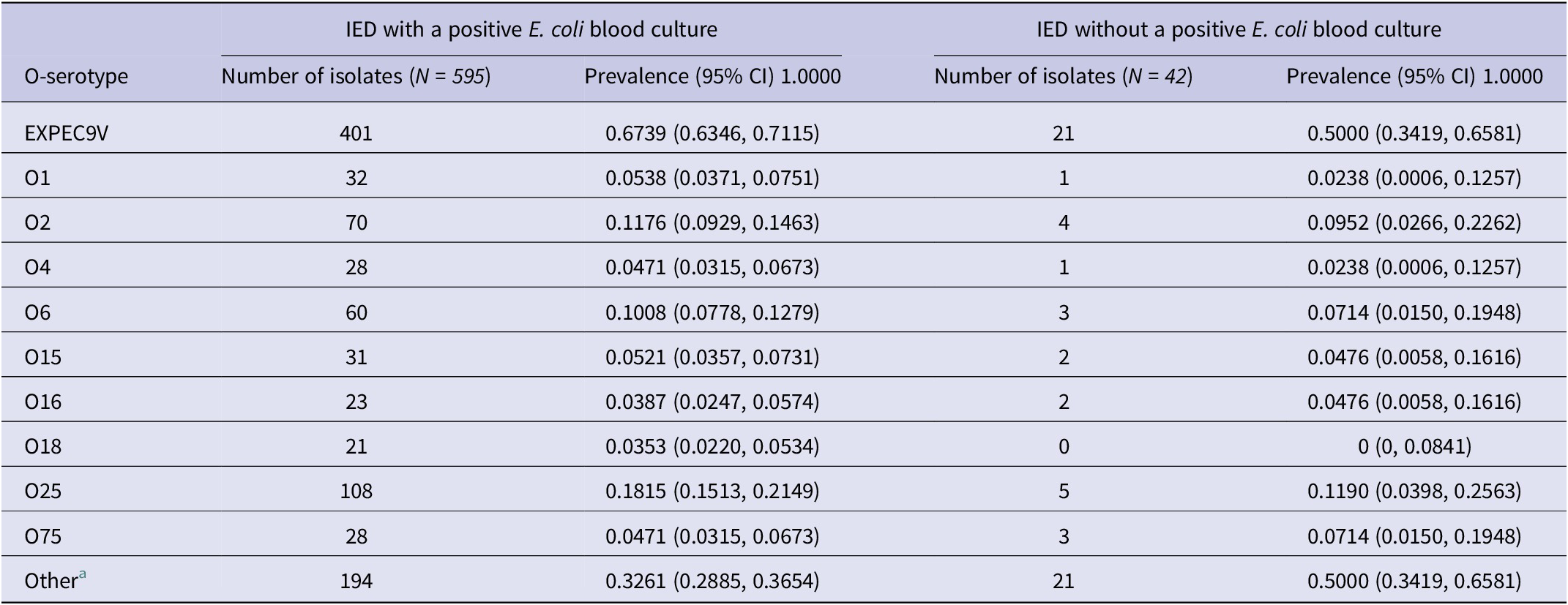
a Non-EXPEC9V O-serotypes.
Note: IED, invasive E. coli disease.
Subgroup analysis of EXPEC9V O-serotype prevalence among patients aged ≥60 years stratified by sex is presented in Table 3. O25 was the most prevalent O-serotype in both females (17.7%) and males (17.8%). Notably, serotype O1 was more common in IED isolates from females compared to males (8.3% vs. 2.6% respectively), as was serotype O16 (6.3% vs. 2.0% respectively). Overall, the prevalence of EXPEC9V IED isolates among female and male patients with IED aged ≥60 years did not substantially differ (70.8% and 62.5% respectively).
Table 3. Prevalence of EXPEC9V O-serotypes based on whole-genotype sequencing, stratified by sex, in patients aged ≥60 years

a Non-EXPEC9V O-serotypes.
Resistance to ≥1 drug and ≥1 drug class was common, at around 66% of isolates
In the total study population, 587 out of 895 E. coli isolates (65.6%) tested for susceptibility were found to be resistant to ≥1 antibiotic in ≥1 drug class, with >25% of the isolates being resistant to amoxicillin, piperacillin, amoxicillin/clavulanate, trimethoprim/sulfamethoxazole, ciprofloxacin, and levofloxacin (Supplementary Table S1). Among patients aged ≥60 years with IED, 450 (65.9%) E. coli isolates were found to be resistant to ≥1 drug class, with 134 (19.6%) considered as MDR (Table 4). The prevalence of EXPEC9V O-serotypes among MDR E. coli isolates from patients with IED aged ≥60 years is presented in Supplementary Figure S1, indicating a cumulative prevalence of approximately 65% among MDR isolates O-serotyped by WGS, which is similar to the vaccine serotype coverage of EXPEC9V in the total study population and patients aged ≥60 years (Figures 1 and 2).
Table 4. Antibiotic resistance in patients aged ≥60 years

Note: Susceptibility results are based on EUCAST 2022 breakpoints. EUCAST, European Committee on Antimicrobial Susceptibility Testing; IED, invasive E. coli disease; MDR, multidrug resistant.
a 683/690 E. coli isolates from patients aged ≥60 years in the full analysis set underwent antibacterial susceptibility testing.
b Susceptible was based on susceptibility to representative antibiotics in the following five classes of antimicrobial drugs: aminoglycoside, fluoroquinolone, folate pathway inhibitor, nitrofurantoin, and β-lactam. Susceptibility was defined as the absence of resistance to 5/5 antimicrobial drug classes.
c MDR was based on resistance to representative antibiotics in the following five classes of antimicrobial drugs: aminoglycoside, fluoroquinolone, folate pathway inhibitor, nitrofurantoin, and β-lactam. MDR was defined as resistance to at least 3/5 antimicrobial drug classes.
EXPEC9V O-serotype prevalence was similar irrespective of survival and sepsis status
From the total amount of IED isolates for which the O-serotype could be determined (n = 837), 165 (19.7%) were from patients who died, while 672 (80.3%) were from patients who survived for >28 days. The prevalence of EXPEC9V O-serotypes by survival status for patients aged ≥60 years with IED is shown in Table 5. Overall, the prevalence of EXPEC9V O-serotypes was similar among patients regardless of 28-day survival status (61.1% among patients with IED who died and 65.8% among patients with IED who survived); however, the prevalence of individual EXPEC9V O-serotypes varied between the two groups. Serotypes O1, O2, and O6 were more prevalent in IED isolates from patients alive after the 28-day follow-up period, while O25 was more prevalent among patients who had died. Similar variations in prevalence rates were observed among all patients with IED (Supplementary Table S3).
Table 5. Prevalence of EXPEC9V O-serotypes based on whole-genotype sequencing, stratified by mortality status, in patients aged ≥60 years

a Non-EXPEC9V O-serotypes.
Four hundred and sixty-five (67.4%) patients with IED aged ≥60 years were diagnosed with sepsis by their physician, and 105 (15.2%) experienced septic shock (Table 1). Among the patients for whom the O-serotypes could be determined, the prevalence of EXPEC9V O-serotypes stratified by sepsis status is shown in Table 6. Overall, the prevalence of EXPEC9V O-serotypes was comparable among patients with and without sepsis (65.7% and 67.8% respectively), as was the overall prevalence of individual EXPEC9V O-serotypes. The prevalence of EXPEC9V O-serotypes by sepsis status for all patients with IED was also similar and is shown in Supplementary Table S4.
Table 6. Prevalence of EXPEC9V O-serotypes based on whole-genotype sequencing, stratified by sepsis status, in patients aged ≥60 years

a Non-EXPEC9V O-serotypes.
Discussion
Despite the key role that E. coli has in causing bacterial invasive disease – being the number one pathogen globally – there is a lack of national, regional, and global surveillance programmes to provide insight into disease incidence as well as associated O-serotype prevalence, in particular. This retrospective study describes O-serotype prevalence in general and of the now-discontinued EXPEC9V vaccine serotype coverage across a multinational cohort of patients hospitalized with IED. Serotypes O25, O1, O2, O6, O15, and O75 were identified as the most common across the IED isolates, with serotype O25 alone being reported for 17.3% of isolates from all patients with IED and 17.7% of isolates from patients ≥60 years old (based on WGS).
The observed overall O-serotype prevalence is consistent with previous multinational epidemiological reports. For example, the most common E. coli O-serotypes causing bloodstream infections in patients aged ≥60 years were identified as O25 (22.9%), O2, O6, O1, O75, O15, O8, O16, O4, O18, O77 group, O153, O9, O101/O162, O86, O13, O46/O134, and O83, all at a prevalence of ≥1% of isolates [Reference Weerdenburg17]. In another recent 10-year study by Lipworth et al. (2021), O-serotypes O1, O2, O4, O6, O8, O15, O16, O18, O25, and O75 made up 72% of 3,278 bloodstream E. coli isolates analysed in the United Kingdom [Reference Lipworth14]. Separately, studies in France and the Netherlands reported that the most common O-serotypes associated with bloodstream infections were O25, O8, O2, O6, and O15 [Reference Royer15, Reference Verboom16]. Furthermore, in a recent multicentric, prospective observational study among hospitalized patients with IED (N = 238), the analysis of blood and urine isolates demonstrated that O25, O6, and O1 were the most prevalent serotypes across three continents [Reference Arconada Nuin25].
Previous studies have described how IED incidence increases and clinical prognosis, including risk of death, worsens with age [Reference Poolman and Wacker5, Reference Bonten26]. When comparing all patients with IED with patients aged ≥60 years in this study, a similar EXPEC9V vaccine serotype coverage was observed between the two groups (~66%). Overall, the prevalence of EXPEC9V O-serotypes among patients aged ≥60 years was found not to substantially differ when stratified by survival at 28-day follow-up or sepsis status. However, serotypes O1, O2, and O6 were more prevalent among patients who survived during the 28-day follow-up period, while O25 was more prevalent among those who had died. While this may be explained by O25 being associated with ST131 – a virulent E. coli clone – further investigations with increased numbers of isolates are needed to establish the link between specific O-serotypes and disease severity. When results were stratified by the absence or presence of a positive E. coli blood culture, O25, O2, and O6 were the most prevalent O-serotypes in both subgroups, although absolute prevalence rates varied. Yet, due to the low number of patients without a positive blood culture (n = 42), confidence intervals for these data are wide, and data should therefore be interpreted with care. The observed difference may be driven by potential selection bias as well as differential geographical representation.
E. coli serotype O25 was particularly prevalent among MDR isolates, reaching a proportion of almost 50%, which is consistent with the high prevalence of this O-serotype among MDR E. coli isolates in previous studies [Reference Lipworth14, Reference Arconada Nuin25]. The prevalence of MDR E. coli strains, such as those belonging to sequence type (ST) O25B:ST131, is increasing [Reference Arconada Nuin25, Reference Manges27], and the global emergence of MDR E. coli represents a major challenge for the prevention and management of E. coli infections that may often be lethal [Reference Weerdenburg17, Reference Mathers, Peirano and Pitout28].
Strengths and limitations
A key strength of this study is that it was a multinational study across eight countries and three regions with relatively large sample sizes of clinical isolates linked to detailed clinical information. The results presented here align with other studies demonstrating the high prevalence of O25 and other IED-associated O-serotypes. This study also provides important real-world data on a pathogen that has historically been underappreciated in national healthcare systems.
As this was a retrospective, observational study, the quality of data was largely dependent on the availability and completeness of individual patients’ medical records. The retrospective nature of the study may have contributed to a low inclusion of patients where E. coli was only confirmed from a positive urine culture, as such isolates may not be routinely stored at hospital sites. The inability to randomly select study sites may have resulted in undetectable measurement errors from several sources, and systematic variation in how patients from different cultural backgrounds perceived illness and sought care.
While agglutination is considered the gold standard for O-serotyping, WGS is more versatile and less labour-intensive. Differences in O-serotype prevalence observed between the agglutination and WGS results may be related to the difference between phenotype and genotype, impacted by false-negative results, or O-antigen expression being absent in some E. coli isolates under in vitro testing conditions.
An important limitation is the relatively low number of sites and E. coli isolates analysed from each individual country, which hinders the ability to compare O-serotype prevalence and vaccine serotype coverage at a country level. Therefore, similar country-specific studies analysing larger numbers of IED isolates from a range of local sites are needed. Additionally, given the geography of participating sites and hospitals, which were predominantly located in high-income countries, further studies are needed to understand serotype coverage across low-to-middle-income countries. Finally, there was a lack of data on prior hospitalizations in other hospitals, which may have led to the misclassification of infections as community-acquired when they were healthcare-associated.
Conclusion
In conclusion, there appears to be overall limited information on disease incidence and O-serotyping data for E. coli due to a lack of robust surveillance and disease awareness. Epidemiological data such as those presented here can help inform the development and adoption of novel preventative strategies, including vaccines. This study described the O-serotype prevalence and coverage of the EXPEC9V vaccine, which is no longer in active clinical development, and identified O25 as the most prevalent O-serotype among IED isolates. Overall, EXPEC9V vaccine serotype coverage was found to be approximately 66% independent whether this was analysed for all patients with IED, or patients aged ≥60 years. EXPEC9V O-serotype prevalence did not substantially differ when results were stratified according to whether patients aged ≥60 years had a positive E. coli blood culture, sepsis, survival at 28-day follow-up, as well as sex.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0950268825100344.
Data availability statement
Janssen’s official data sharing statement (‘The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access [YODA] Project site at http://yoda.yale.edu’) will be provided to a journal/congress upon request.
Acknowledgements
We want to thank Amy Maxson, Kathryn Shute, Todd A. Davies, Ron de Winter, James Peterson, Delores Etheredge, Sonal Munshi, Amy Lwin, Atsushi Momose, Trish Childers, Thijs van den Hoven, Brian Morrow, Alvaro Agustin Rodriguez, Frank Struyf, Moussa Aitabi, Yohei Doi, Phillippe Lanotte, Allison Mcgeer, Keith Morris, Miquel Pujol, Luigia Scudeller, Katsunori Yanagihara, Thomas Verstraeten, and Stefan Zimmermann. We also thank Amy Bray of Eloquent Scientific Solutions for medical writing assistance.
Author contribution
All authors contributed to the manuscript writing. All authors provided a full review of the article and are fully responsible for all content and editorial decisions, were involved in all stages of manuscript development, and have approved the final version.
Funding statement
This study and medical writing assistance were funded by Janssen Research and Development, LLC.
Competing interests
FW has participated in advisory boards of Janssen. JTT has served as an advisor to Resonantia Diagnostics, Inc. and Sanofi. LM-M has participated in educational programmes organized by MSD, Pfizer, and Shionogi and had conducted research studies financed by Janssen, MSD, Pfizer, and Shionogi. MaS has served as principal investigator or sub-investigator for clinical trials run by Adaptive Phage Therapeutics; Affinity Biosensors; Applied BioCode; AstraZeneca; Biotest AG; ContraFect Corp; Crestone; Dompé Farmaceutici; Epigenomics AG; Finch Therapeutics Group; Janssen Research and Development; Leonard-Meron Biosciences; Locus Biosciences; Merck and Co; OpGen; Pfizer; PHAST; Prenosis; QIAGEN; Roche; Seres Therapeutics; Summit Therapeutics; and Vedanta Biosciences. He has been an advisor/consultant to Applied BioCode; OpGen; Prenosis; and Venatorx Pharmaceuticals. JG, OG, and BS are employees of Janssen, Pharmaceutical Companies of Johnson & Johnson. JD, MiS, DA, PIP, and JP were employees of Janssen, Pharmaceutical Companies of Johnson & Johnson at the time of analysis. JF, OB, PH, PL, and TM have nothing to declare.










