Non-technical summary
Four new insects are described from the ~220 million year old (Triassic) Cow Branch Formation of Virginia and North Carolina. These insects belong to Hemiptera, the insect order that contains the “true bugs.” The four new hemipteran insects include one pair of isolated wings belonging to the extinct family Hylicellidae, one complete specimen assigned to a distinctive new genus of the extinct family Ipsviciidae, and two heteropterans belonging to unknown families. Only the ipsviciid is preserved with enough morphological detail for systematic study and is described as Solitivicia reducta new genus new species due to the highly reduced venation of its forewings. The peculiar distribution of hemipterans in this deposit, namely the paucity of cicadomorphans and coleorrhynchans which are both otherwise abundant in Triassic deposits, is discussed.
Introduction
Hemiptera Linnaeus, Reference Handlirsch and Schröder1758, the largest order of nonholometabolous insects, contains ~104,000 described Recent and extinct species in > 300 families, the largest number of families in any insect order (Szwedo, Reference Szwedo2018). Although the higher-level phylogeny of Hemiptera remains in question (e.g., Sorensen et al., Reference Sorensen, Campbell, Gill and Steffen-Campbell1995), six suborders are currently recognized: †Paleorrhyncha Carpenter, Reference Carpenter1931 (archescytinoids), Sternorrhyncha Amyot and Audinet-Serville, Reference Amyot and Audinet-Serville1843 (e.g., aphids, scale insects, whiteflies), Coleorrhyncha Myers and China, Reference Myers and China1929 (moss bugs), Fulgoromorpha Evans, Reference Evans1946 (planthoppers), Cicadomorpha Evans, Reference Evans1946 (e.g., cicadas, leafhoppers), and Heteroptera Latreille, Reference Latreille1810 (true bugs) (Szwedo, Reference Szwedo2018). Hemipterans are defined by their unique mouthparts which have been modified into a rostrum for piercing and sucking. The adaptability of this structure for various feeding styles (e.g., phytophagy, predation, hematophagy, parasitism) has enabled hemipterans to occupy diverse habitats and ecological niches throughout the order's long geological history.
The earliest members of Hemiptera, Protoprosbolidae Laurentiaux, Reference Laurentiaux1952 and Aviorrhynchidae Nel et al., Reference Nel, Roques, Nel, Prokin and Bourgoin2013, are known from the Carboniferous Period (~330 Ma; Nel et al., Reference Nel, Roques, Nel, Prokin and Bourgoin2013). Archescytinidae Tillyard, Reference Tillyard1926 (suborder Paleorrhyncha), an extinct, possibly paraphyletic group of small, plant-feeding insects, is also known from the latest Carboniferous; this group likely gave rise to suborder Sternorrhyncha (Szwedo, Reference Szwedo2018). The remaining suborders probably arose during the Permian, with diverse members of Cicadomorpha, as well as the earliest members of Coleorrhyncha (family Progonocimicidae Handlirsch, Reference Handlirsch1906) and Fulgoromorpha known from Permian fossils. The superfamily Scytinopteroidea Handlirsch, Reference Handlirsch1906 (Cicadomorpha) is the taxon from which the true bugs (suborder Heteroptera) are thought to have arisen (Shcherbakov, Reference Shcherbakov and Schaefer1996); scytinopteroids were abundant during the late Permian and were one of the few hemipteran groups to survive the End Permian extinction and proliferate in the Triassic (Shcherbakov, Reference Shcherbakov2000). Paraknightia magnifica Evans, Reference Evans1943 (Paraknightiidae Evans, Reference Evans1950), an unusual insect with expanded paranotal lobes from the upper Permian of Australia, has been suggested as a possible ancestor of Heteroptera (Evans, Reference Evans1943; Shcherbakov, Reference Shcherbakov1984). Besides this single enigmatic taxon, there is currently little fossil evidence of the evolution of basal Heteroptera.
The hemipteran faunas of the Triassic differed greatly from those of the Permian. Cicadomorphans were dominated by now extinct taxa (i.e., Scytinopteroidea Handlirsch, Reference Handlirsch1906, Dysmorphoptiloidea Handlirsch, Reference Handlirsch1906, Pereborioidea Zalessky, Reference Zalessky1930, and Palaeontinoidea Handlirsch, Reference Handlirsch1906), fulgoromorphans from this period are poorly known with only a single family (Surijokocixiidae Shcherbakov, Reference Shcherbakov2000) represented in the Triassic record, and Coleorrhyncha, today a relict group of 37 extant species (Peloridiidae Breddin, Reference Breddin and Michaelsen1897) in Patagonia, New Zealand, New Caledonia, and eastern Australia (Ye et al., Reference Ye, Damgaard, Burckhardt, Gibbs, Yuan, Yang and Bu2019), were abundant and diverse worldwide (Szwedo, Reference Szwedo2018). The first definitive members of Heteroptera are known from this period and were almost exclusively represented by Nepomorpha Popov, Reference Popov1968, the infraorder including giant water bugs (Belostomatidae Leach, Reference Leach and Brewster1815), water scorpions (Nepidae Latreille, Reference Latreille1802), backswimmers (Notonectidae Latreille, Reference Latreille1802), and water boatmen (Corixidae Leach, Reference Leach and Brewster1815), among others. Interestingly, most Triassic heteropterans have been assigned to extant families or superfamilies (Szwedo et al., Reference Szwedo, Bourgoin and Lefebvre2004); this is in stark contrast to the Cicadomorpha and Sternorrhyncha, in which many of the families or superfamilies of their Triassic representatives are extinct.
Hemiptera is the most abundant order of insects in the Late Triassic (Norian) Solite Konservat-Lagerstätte (Cow Branch Formation) of Virginia and North Carolina (USA). This deposit preserves one of the earliest post-Permian extinction assemblages of both terrestrial and freshwater insects, of which Hemiptera comprise ~66% of identifiable specimens. Of these, two groups of Nepomorpha dominate: Triassonepa solensis Criscione and Grimaldi, Reference Criscione and Grimaldi2017, the oldest known member of Belostomatidae (Criscione and Grimaldi, Reference Criscione and Grimaldi2017), and a putative member of Notonectidae or Corixidae (Fraser et al., Reference Fraser, Grimaldi, Axsmith, Heckert, Liutkus-Pierce, Smith, Dooley, Fraser and Sues2017). Non-nepomorphan taxa are far less common, comprising only ~1% of hemipteran specimens, most of which are not identifiable beyond order. In this study, four new hemipteran specimens are presented: one pair of isolated wings belonging to Hylicellidae Evans, Reference Evans1956 (Hylicelloidea: Cicadomorpha), one specimen of a distinctive new genus of Ipsviciidae Tillyard, Reference Tillyard1919 (Scytinopteroidea: Cicadomorpha), and two heteropterans belonging to unknown families. Only the ipsviciid has enough morphological information preserved for a systematic description; the others are described and discussed informally.
Materials and methods
Locality and Material
The specimens described in this study were collected from the Late Triassic Cow Branch Formation, located in the Dan River Basin of southern Virginia and northern North Carolina (USA). The ‘Solite deposit,’ or Solite Konservat-Lagerstätte, comprises the fossiliferous layers of the Cow Branch Formation that outcrop in the former Solite Corporation Quarries at Cascade, Virginia. The majority of the Solite insects come from the aptly named ‘insect layer’ (cycle 2; Olsen, Reference Olsen1979), a 34 mm thick unit preserving abundant insects from at least nine orders, as well as ‘conchostracans,’ rare fish, and the aquatic reptile Tanytrachelos ahynis Olsen, Reference Olsen1979 (Fraser et al., Reference Fraser, Grimaldi, Axsmith, Heckert, Liutkus-Pierce, Smith, Dooley, Fraser and Sues2017). The insects are ~220 Myr old (Norian; Olsen et al., Reference Olsen, Reid, Taylor, Whiteside and Kent2015; Kent et al., Reference Kent, Olsen and Muttoni2017) and are preserved as two-dimensional, silvery carbon films in a matrix of fine-grained, black mudstone. Preservation is often excellent, with many specimens fully articulated and microscopic details such as setae visible. A second formation, the Walnut Cove Formation, is also considered part of the Solite deposit (Criscione-Vastano and Grimaldi, Reference Criscione-Vastano and Grimaldi2024). Slightly older (between 227.5 and 224.5 Ma; Olsen et al., Reference Olsen, Reid, Taylor, Whiteside and Kent2015) than the Cow Branch, it outcrops ~30 km southwest of the Solite Quarries in Madison, North Carolina. Because only a single possible hemipteran specimen is known from Walnut Cove, its material is excluded from this discussion of the Solite Hemiptera.
Methods
Specimens were wetted with 70% ethanol to increase contrast and viewed using a Nikon SMZ1500 stereomicroscope fitted with a fiber optic ring light. The ring light provided a non-directional, diffuse light source necessary for the illumination of the silvery, carbonaceous film in which the Solite insects are preserved. Photographs were taken using a Nikon 16MP camera with Nikon Elements NIS software. For higher resolution study, a Nikon petrographic microscope fitted with 4X, 10X, 20X, and 40X PlanApo lenses having epioptic illumination were used. This technique provided higher resolution imaging of minute details such as tarsal segments and wing veins. Measurements were taken using ImageJ software and all specimens were illustrated using Adobe Illustrator. The venational schemes of Lambkin (Reference Lambkin2020a) and Lambkin (Reference Lambkin2020b) are followed for Hylicellidae and Ipsviciidae, respectively.
Repositories and institutional abbreviations
The specimens described herein are housed at the Virginia Museum of Natural History (VMNH) in Martinsville, Virginia, USA and the American Museum of Natural History (AMNH) in New York, New York, USA. Additional insect specimens from the Solite deposit not included in this study are housed at the Yale Peabody Museum of Natural History in New Haven, Connecticut, USA.
Systematic paleontology
Order Hemiptera Linnaeus, Reference Linnaeus1758
Suborder Cicadomorpha Evans, Reference Evans1946
Superfamily Hylicelloidea Evans, Reference Evans1956
Family Hylicellidae Evans, Reference Evans1956
Subfamily Hylicellinae Evans, Reference Evans1956
Hylicellinae incertae sedis
Figure 1
Material
VMNH 49780, part and counterpart, Fig. 1.
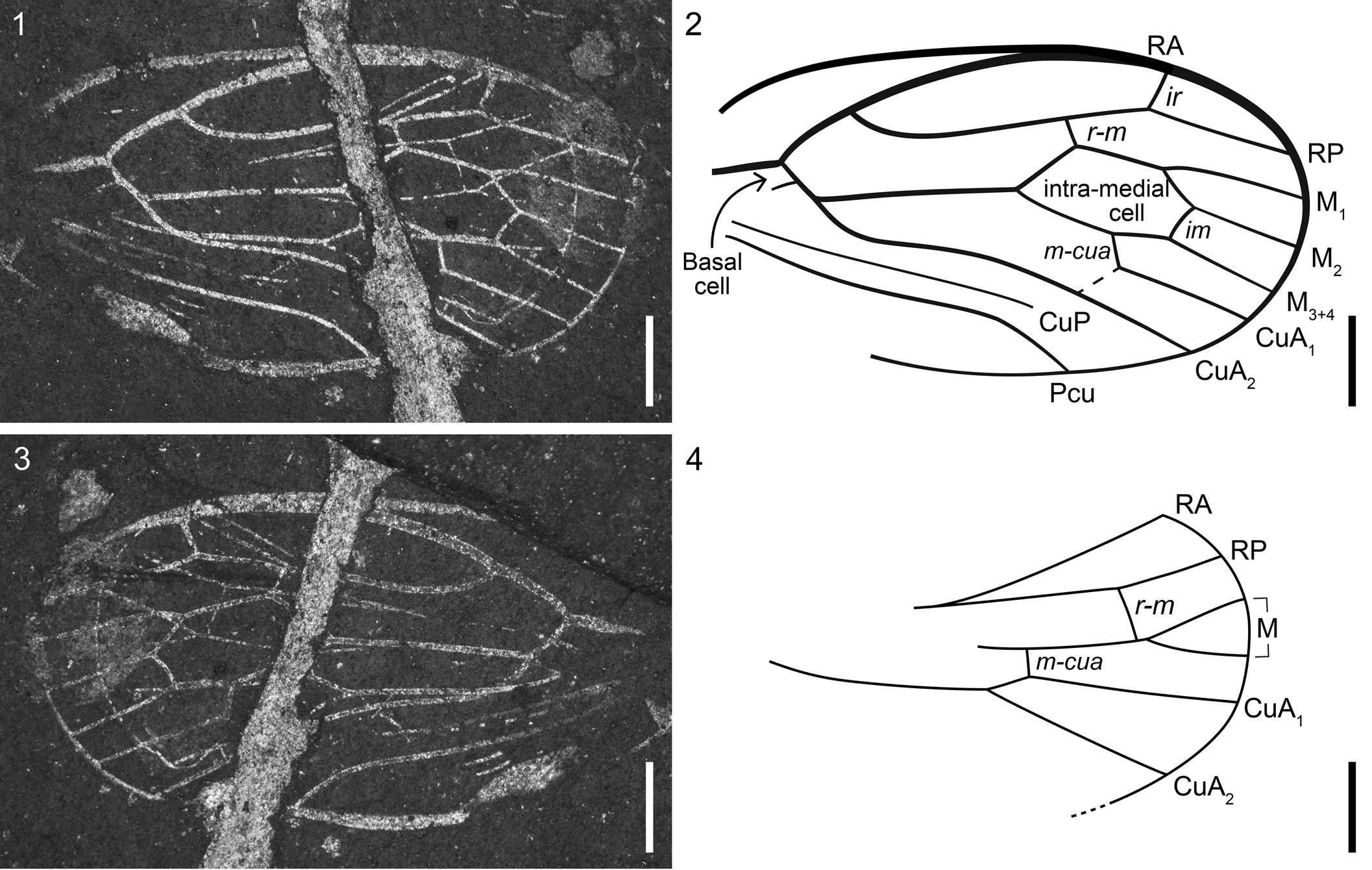
Figure 1. Hylicellinae Evans, Reference Evans1956 incertae sedis, VMNH 49780. Venational scheme following that of Lambkin (Reference Lambkin2020a): (1) part; (2) illustration of forewing showing venation; (3) counterpart; (4) illustration of hind wing showing venation. CuA1, CuA2 = cubitus anterior veins (first and second); CuP = cubitus posterior; ir = inter-radial crossvein; M1, M2, M3+4 = media (first, second, third + fourth); m-cua = crossvein m-cua; Pcu = postcubitus; r-m = crossvein r-m; RA = radius anterior; RP = radius posterior. Scale bars = 1 mm.
Occurrence
Former Solite Quarry, Eden, Rockingham County, North Carolina, USA (36°32'29.7"N, 79°40'12.8"W); Norian, Late Triassic, Cow Branch Formation.
Description
Single specimen consisting of overlapping forewing and hind wing. Forewing: nearly complete with only basal-most portion not preserved. Wing short, broad (5.9 mm long measured from the base of radius (R), 3.5 mm wide), length ~1.7 times the width; fully membranous, no indication of a nodal line; peripheral membrane absent. Costal margin slightly convex, apex broadly rounded. Subcosta (Sc) not visible; basal cell poorly preserved, bounded anteriorly by short stem R + M + CuA (radius + media + cubitus anterior), posterior margin indistinct. R and M + CuA separating near apex of basal cell. R bifurcating in basal third of wing length; radius anterior (RA) convex, meeting costal margin in apical third of wing length, their fusion forming the thickest vein in the wing. Radius posterior (RP) simple, one crossvein ir present, running from slight bend in RP to terminus of RA; one crossvein r-m proximal to ir, connecting to M1+2. M separating from CuA near basal cell, three-branched; intramedial cell large, polygonal in shape, length ~2.3 times the width; one crossvein im between M2 and M3+4. CuA two-branched, apparently bifurcating distal to M bifurcation; crossvein m-cua connecting to CuA1 near bifurcation of CuA. Cubitus posterior (CuP) thinner than other veins, nearly straight, distal portion not preserved. Postcubitus (Pcu) simple, gently bending apically. Hind wing: preservation fragmentary, basal and anal portions not preserved; peripheral membrane absent. Stem R not preserved, apparently long. M two-branched, bifurcating distally, branches short; r-m connecting to stem M just before the point of bifurcation. CuA also two-branched; m-cua connecting stem M and CuA1. CuP and Pcu not preserved.
Remarks
The forewing venation of this specimen most closely resembles that of the extinct family Hylicellidae in the simple RP, large intramedial cell, and the separation of R and M + CuA near the apex of the basal cell. Hylicellidae is one of three families included in the extinct taxon Hylicelloidea (Lambkin, Reference Lambkin2020a). This superfamily is known from the Triassic of Australia (Lambkin, Reference Lambkin2020a) and China (Fu and Huang, Reference Fu and Huang2023) to the Jurassic and Cretaceous of Eurasia (Shcherbakov, Reference Shcherbakov1988). Hylicelloidea has been suggested as ancestral to Clypeata Qadri, Reference Qadri1967 (Shcherbakov, Reference Shcherbakov and Schaefer1996, Reference Shcherbakov2012; Wang et al., Reference Wang, Szwedo, Zhang and Fang2010), the taxon containing the Cicadoidea Latreille, Reference Latreille1802, Cicadelloidea Latreille, Reference Latreille1802, Cercopoidea Westwood, Reference Westwood1838, Membracoidea Rafinesque, Reference Rafinesque1815, and Myerslopioidea Evans, Reference Evans1957 (Szwedo, Reference Szwedo2018). Three subfamilies are currently included in Hylicellidae: Conjucellinae Shcherbakov, Reference Shcherbakov2012, Vietocyclinae Shcherbakov, Reference Shcherbakov1988, and Hylicellinae Evans, Reference Evans1956 (Lambkin, Reference Lambkin2020a).
The Solite specimen differs from Vietocyclinae in the number of r-m crossveins, the number of branches of M, and its more reduced hind wing venation. It also differs from Conjucellinae in the overall shape of the forewing (much more elongated in Conjucellinae) and the shape of the basal cell. The third subfamily, Hylicellinae, is a ‘catch-all’ group without formal definition for the remaining hylicellid genera (Lambkin, Reference Lambkin2020a). Unlike the other two subfamilies which are known from the Jurassic and Cretaceous of Eurasia (England, Germany, Russia, Mongolia, and China), the Hylicellinae are known from the Late Triassic (Norian) of Australia. Within this taxon, the Solite specimen most closely resembles Mesocixiodes Tillyard, Reference Tillyard1922 and Hylicella Evans, Reference Evans1956 in the short, shared stem of M + CuA near the apex of the basal cell and the reduced venation. The Solite specimen differs from Mesocixiodes in its simple RA and RP, larger intramedial cell, and more reduced apical venation. The Solite specimen also differs from Hylicella in its simple RA and the absence of fusion of M3+4 and CuA1. One notable difference between the Solite specimen and most other hylicellids is the lack of sclerotization or punctation in the forewing. Punctures and coriaceous textures are commonly preserved in the Solite insect material, so its absence in this specimen is good evidence for a fully membranous forewing texture. Because of these noted differences, the Solite specimen is placed in Hylicellinae incertae sedis.
Suborder Cicadomorpha Evans, Reference Evans1946
Superfamily Scytinopteroidea Handlirsch, Reference Handlirsch1906
Family Ipsviciidae Tillyard, Reference Tillyard1919
Genus Solitivicia new genus
Type species
Solitivicia reducta n. gen. n. sp. by present designation.
Diagnosis
As for the type species, by monotypy.
Occurrence
Former Solite Quarry, Eden, Rockingham County, North Carolina, USA (36°32'29.7"N, 79°40'12.8"W); Norian, Late Triassic, Cow Branch Formation.
Etymology
The genus name is a combination of the prefix soliti-, for the Solite deposit from which the genus is known, and -vici(a), a stem commonly used in the family Ipsviciidae.
Remarks
Solitivicia n. gen. differs from all other known scytinopteroids in the structure of the forewing, which is elongate, narrow, fully coriaceous, and possesses highly reduced venation.
Solitivicia reducta new genus new species
Figures 2–6
Holotype
VMNH 51992, female, part and counterpart (Figs. 2, 3).
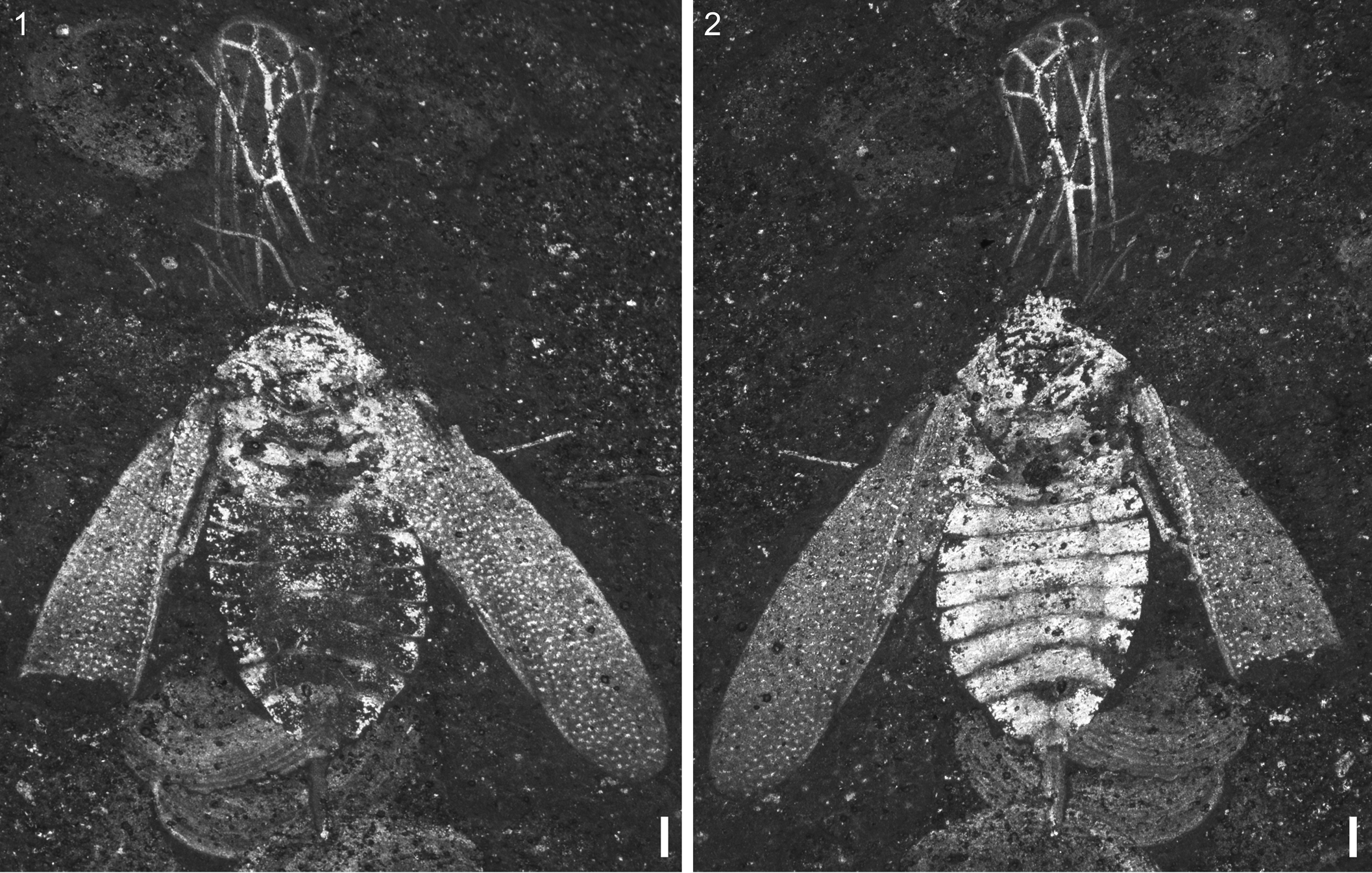
Figure 2. Solitivicia reducta n. gen. n. sp., holotype, VMNH 51992: (1) part; (2) counterpart. Scale bars = 0.25 mm.
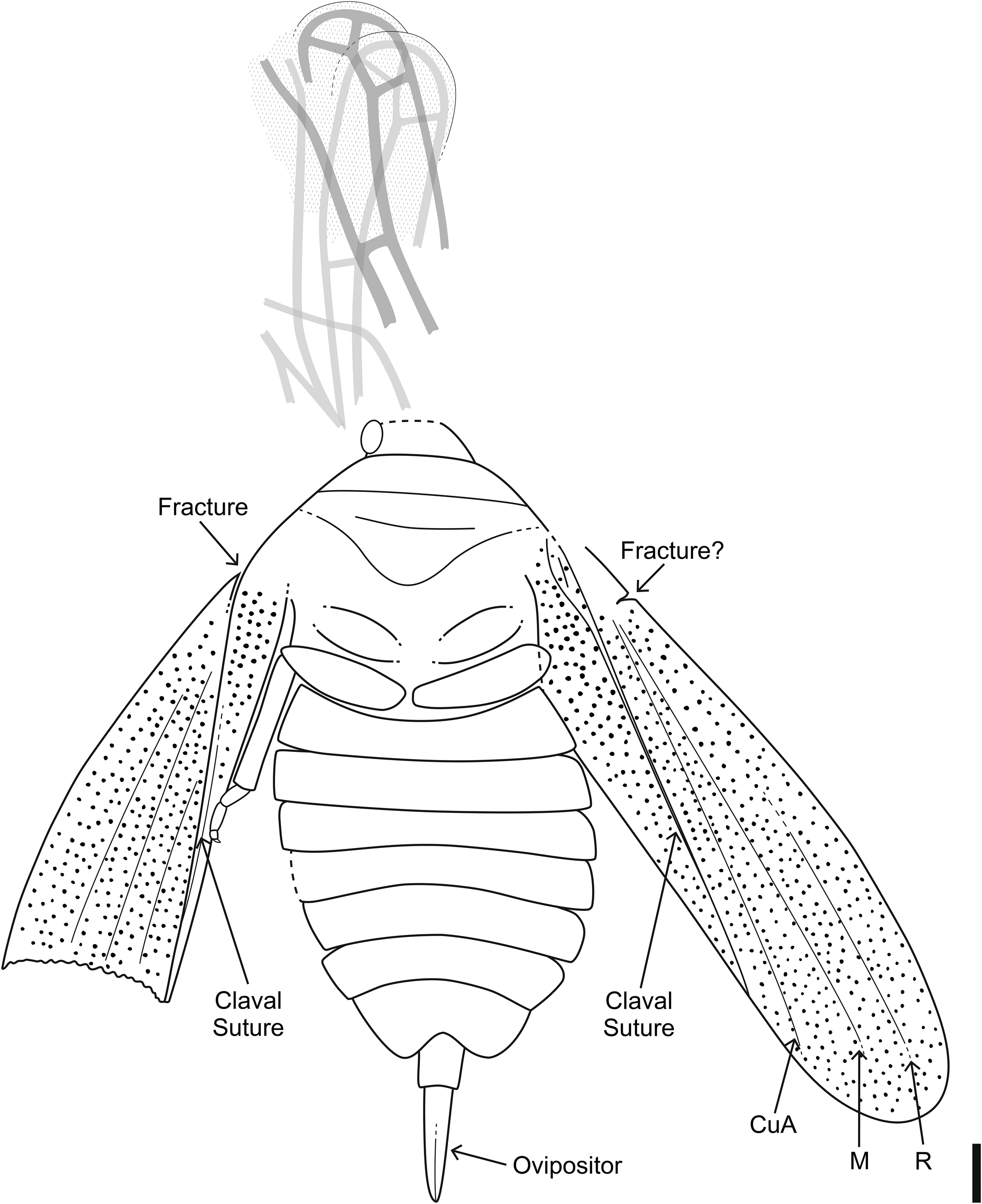
Figure 3. Solitivicia reducta n. gen. n. sp., illustration of holotype, VMNH 51992. CuA = cubitus anterior; M = media; R = radius. Scale bar = 0.25 mm.
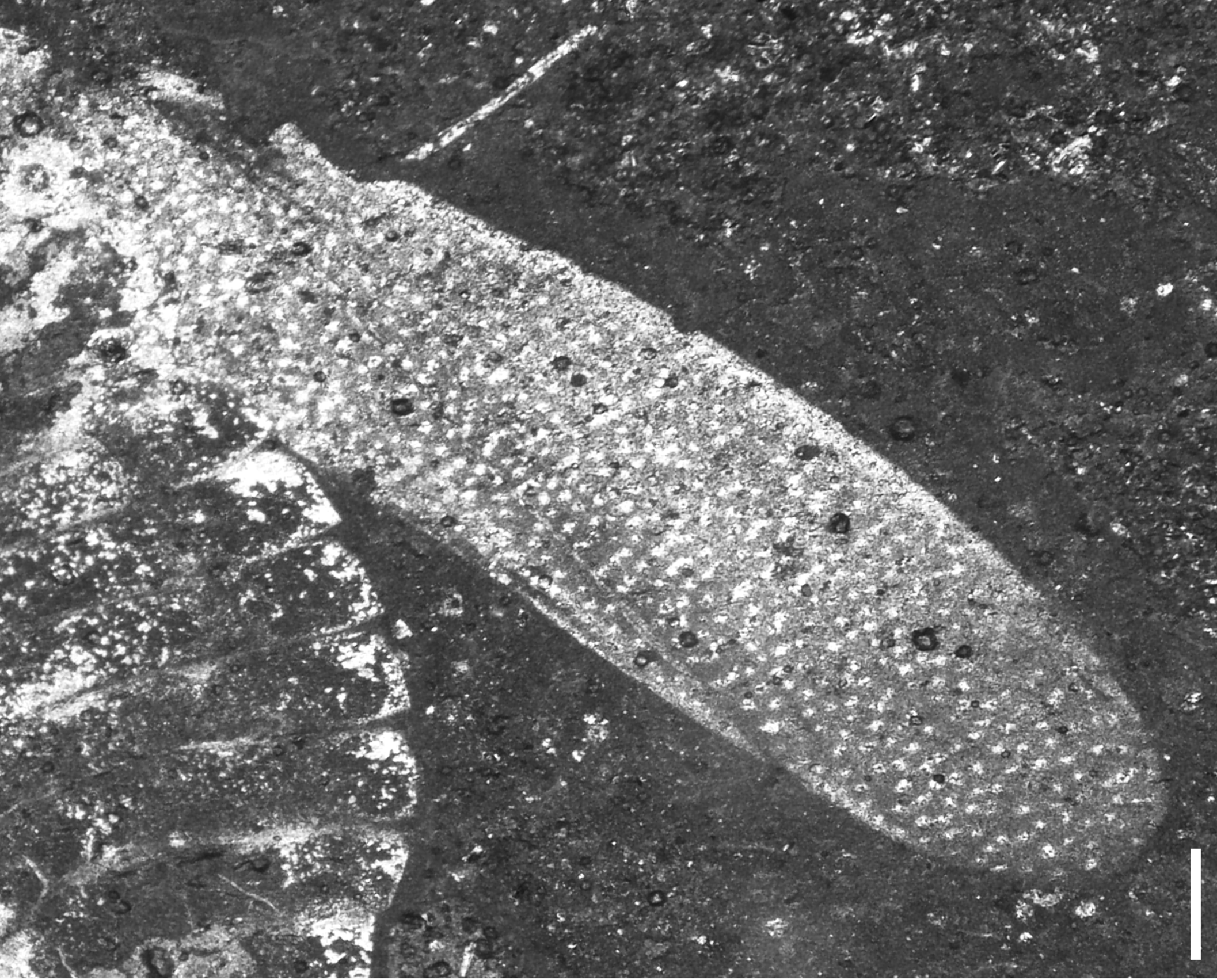
Figure 4. Forewing of Solitivicia reducta n. gen. n. sp., VMNH 51992, showing irregular pigmented spots and linear veins. Scale bar = 0.25 mm.
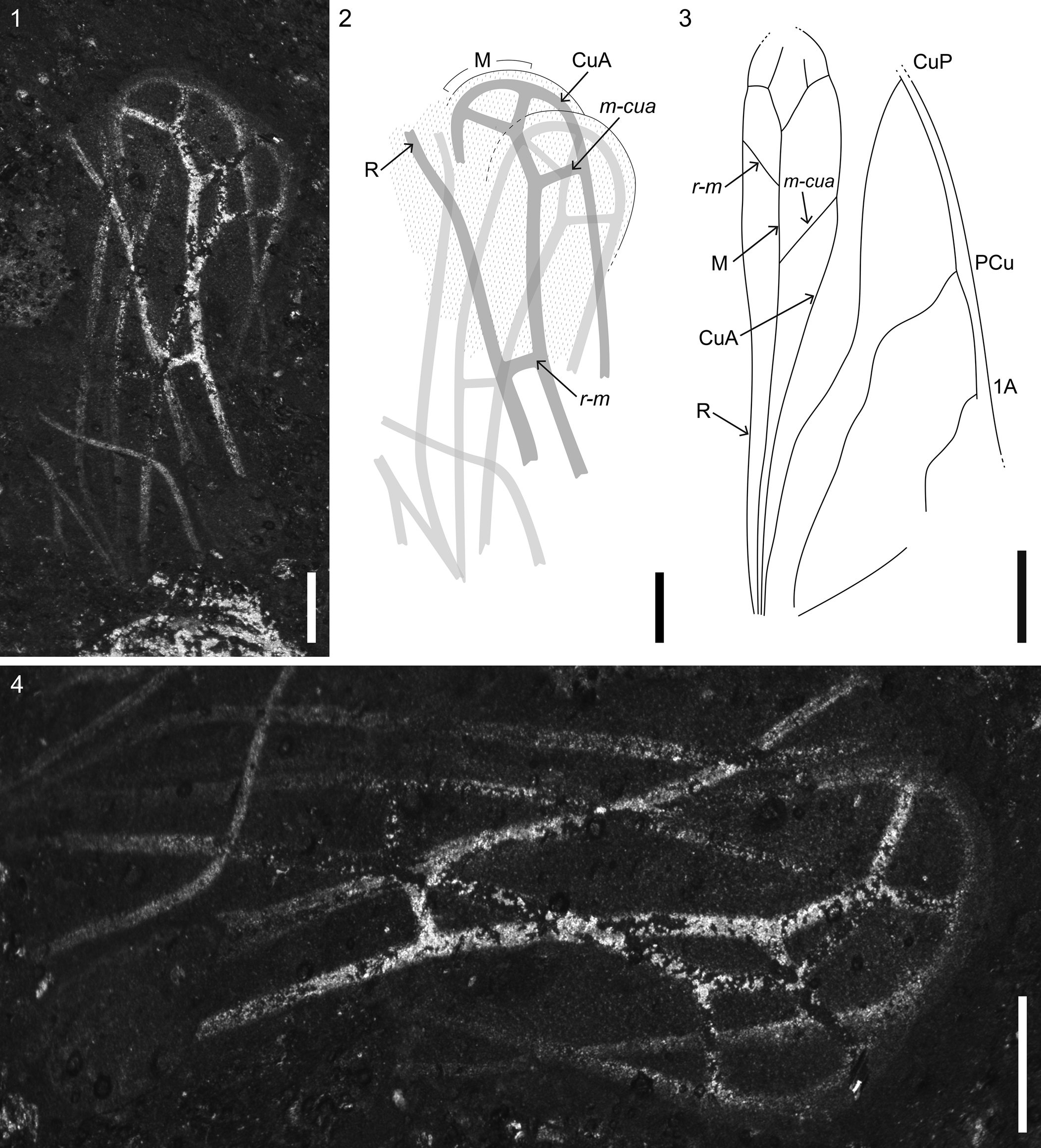
Figure 5. Hind wing venation of ipsviciids: (1) hind wing of Solitivicia reducta n. gen. n. sp., VMNH 51992, part; (2) illustration of Solitivicia reducta n. gen. n. sp. hind wing, showing venation; (3) hind wing of Ipsvicia jonesi Tillyard, Reference Tillyard1919, redrawn from Lambkin (Reference Lambkin2020b); (4) hind wing of Solitivicia reducta n. gen. n. sp. showing dense microtrichia covering surface. 1A = first anal vein; CuA = cubitus anterior; CuP = cubitus posterior; M = media; m-cua = crossvein m-cua; Pcu = postcubitus; R = radius; r-m = crossvein r-m. Scale bars = 0.25 mm (1, 2, 4); 2 mm (3).
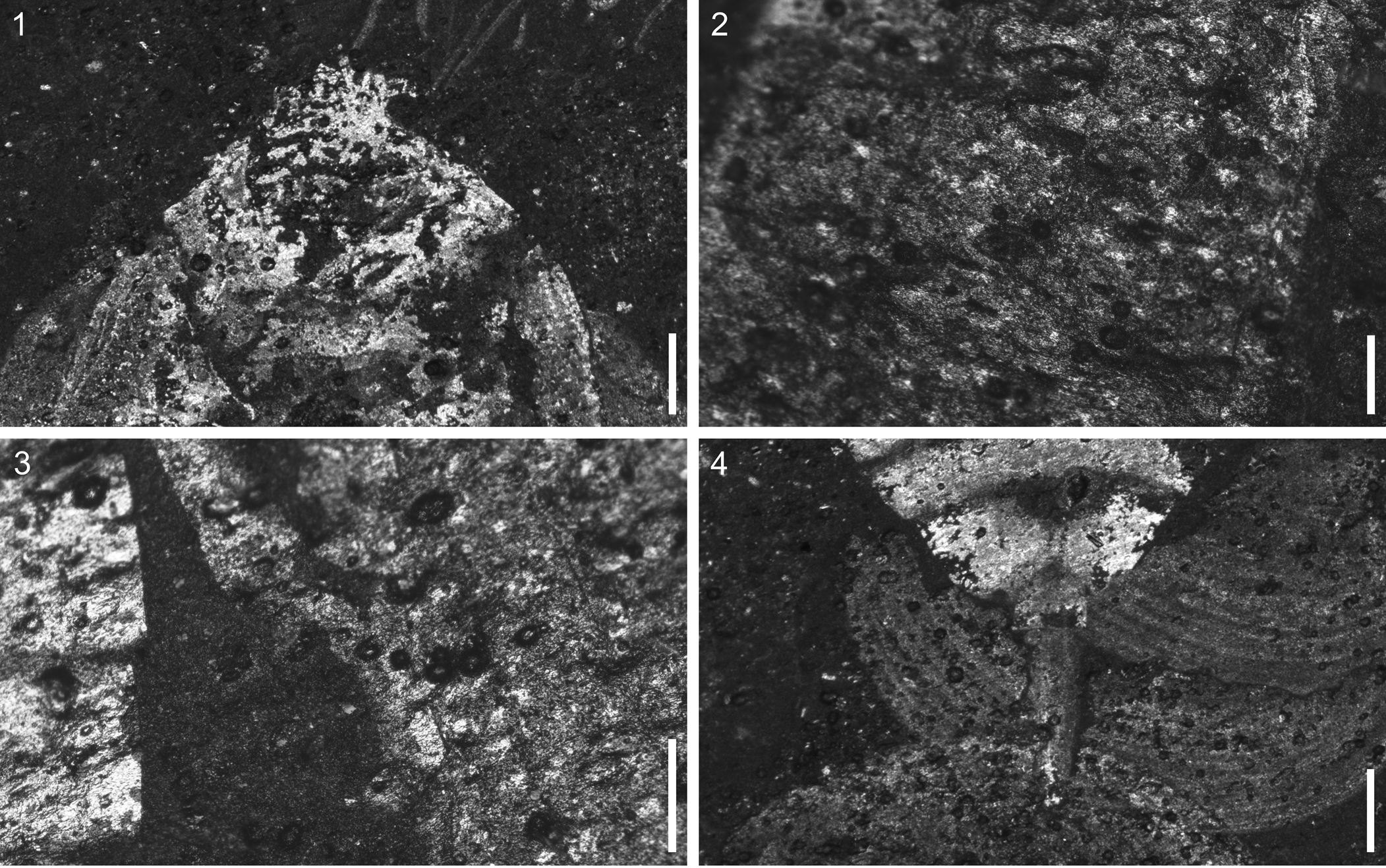
Figure 6. Morphology of Solitivicia reducta n. gen. n. sp., VMNH 51992: (1) head and anterior portion of thorax; (2) detail of forewing showing venation and irregular pigmented spots; (3) hind tarsus showing segmentation; (4) apex of abdomen with ovipositor. Scale bars = 0.25 mm (1, 4); 0.1 mm (2, 3).
Diagnosis
Body small, stout. Forewings slender, length 4 times the width; coriaceous, with irregular pigmented spots covering surface; forewing venation highly reduced, veins linear, crossveins and closed cells absent; corium and membrane not differentiated. Hind wings with simple, linear R and CuA, M bifurcating apically. Hindleg with three tarsomeres. Abdomen with 8 segments, segment VII partially divided by segment VIII; ovipositor robust, well-developed, extending well past abdominal apex.
Occurrence
Former Solite Quarry, Eden, Rockingham County, North Carolina, USA (36°32'29.7"N, 79°40'12.8"W); Norian, Late Triassic, Cow Branch Formation.
Description
Body short, stout. Total length 3.3 mm (2.8 mm excluding ovipositor). Body width 1.3 mm (excluding spread wings).
Head
Small, incompletely preserved. One compound eye visible, located laterally. Antennae and mouthparts not preserved. Ocelli, if present, not visible.
Thorax
Pronotum narrow, trapezoidal in shape; anterior margin straight. Scutellum moderately-sized, somewhat triangular, posterior margin broadly convex. Forewings (tegmina) slender, length ~4 times the width, extending past apex of abdomen; coriaceous with dense pattern of minute, irregular, pigmented spots throughout; small fracture in anteriormost costal margins; clavus and claval suture distinct, clavus occupying ~0.7 times the wing length; membrane not differentiated. Venation incompletely known and highly reduced: Sc and anal veins not preserved; R, M, and CuA linear; no crossveins or closed cells. Hind wings flipped forward, overlapping, stretched out in front of insect; smaller than forewings, anal portion absent; distinct peripheral membrane, hind wing covered in dense microtrichia. Venation highly reduced, R and CuA simple, M with single, apical bifurcation. Prothoracic legs not preserved. Mesothoracic femora partial, slightly smaller than those of metathorax; no other segments visible. Metathoracic leg short; femur small, length ~3.4 times the width; tibia slender, length 6.7 times the width; tarsus three-segmented, third segment quite short; tarsal claw small.
Abdomen
Broad; eight-segmented, segments I–IV subequal, width ~6 times the length; segment VII partially divided by segment VIII (as in Heteroptera). Segment VIII small, parallel-sided, ~0.2 times the width of segments I–IV. No spiracles visible. Ovipositor large (0.5 mm long), robust, extending well past apex of abdomen; fine suture visible medially between valves.
Etymology
The specific epithet highlights the very simple, reduced venation of the species’ forewings.
Remarks
In contrast to Solitivicia reducta n. gen. n. sp., the majority of hemipteran fossils from the late Paleozoic and early Mesozoic are preserved as isolated wings, underscoring the significance of the Solite deposit's fully articulated insects. Because of this limitation, comparisons between early hemipteran taxa may rely solely on forewing characteristics, which is often made possible by their high degree of specialization. Solitivicia reducta n. gen. n. sp. is represented by a single specimen (VMNH 51992) in the Solite deposit, and no other early hemipteran fossil is known to share a similar forewing morphology. However, the shape and patterning of the forewings, the venation of the hind wings, and the robust ovipositor suggest placement in the scytinopteroid family Ipsviciidae.
Scytinopteroidea is the superfamily of Cicadomorpha from which Heteroptera is thought to have evolved (Shcherbakov, Reference Shcherbakov and Schaefer1996). The taxon contains six families: Ipsviciidae Tillyard, Reference Tillyard1919; Paraknightiidae Evans, Reference Evans1950; Saaloscytinidae Brauckmann, Martins-Neto, and Gallego, Reference Brauckmann, Martins-Neto and Gallego2006; Scytinopteridae Handlirsch, Reference Handlirsch1906; Serpentivenidae Shcherbakov, Reference Shcherbakov1984; and Stenoviciidae Evans, Reference Evans1956 (Shcherbakov, Reference Shcherbakov1984, Reference Shcherbakov and Schaefer1996, Reference Shcherbakov2011). Ipsviciidae is recognizable by a series of forewing characteristics, including the unique form of the subcosta, a complex apical forewing venation, and a simple CuA. In most specimens, the forewings are also either tuberculate or punctate. Five genera, Ipsvicia Tillyard, Reference Tillyard1919, Ipsviciella Becker-Migdisova, Reference Becker-Migdisova1962, Ipsviciopsis Tillyard, Reference Tillyard1922, Ipsvicioides Fujiyama, Reference Fujiyama1973, and Strivicia Shcherbakov, Reference Shcherbakov2022, are currently recognized within the family, whereas one additional genus, Triassophyllum Papier et al., Reference Papier, Nel, Grauvogel-Stamm and Gall1997, is tentatively placed within (Lambkin, Reference Lambkin2020b; Shcherbakov, Reference Shcherbakov2022). Ipsviciidae is a predominantly Triassic family, with the exception of Ipsviciella asiatica Becker-Migdisova, Reference Becker-Migdisova1962 from the Early Jurassic. Additionally, most ipsviciids have a Laurasian distribution: Ipsvicia from Germany, Ipsviciella and Strivicia from Kyrgyzstan, Ipsvicioides from Japan, and Triassophyllum from France. Only Ipsvicia and Ipsviciopsis are known from the Gondwanan continents, specifically Australia.
Most ipsviciids are only preserved as isolated wings, but a few less fragmentary specimens are known from the Norian (~226 Ma; Raven et al., Reference Raven, Jell and Knezour2015) Ipswich Coal Measures of Queensland, Australia (Lambkin, Reference Lambkin2020b). The Australian ipsviciids, belonging to Ipsvicia and Ipsviciopsis, were robust insects with tough, coriaceous forewings measuring up to 22 mm long, and a large, shield-like pronotum. Solitivicia reducta n. gen. n. sp. possessed a more generalized body plan than the Australian insects, and was quite a bit smaller, with a wing length of only ~3 mm. Another ipsviciid, Strivicia davidi Shcherbakov, Reference Shcherbakov2022 from the Madygen Formation of Kyrgyzstan (Ladinian–Carnian), is more comparable in size to S. reducta n. gen. n. sp. (forewings < 5 mm in length), and is particularly interesting due to the preservation of a stridulatory area on the underside of the forewing (Shcherbakov, Reference Shcherbakov2022).
The forewings of Solitivicia reducta n. gen. n. sp. (Fig. 4) are unique, most notably in their highly reduced venation and lack of cells. Although other ipsviciids have more complex venation with numerous cells and crossveins, the forewings of S. reducta n. gen. n. sp. share with the family a similar elongate shape (length up to 4 times the width), a simple CuA, and a clavus occupying as much as 0.7 times the wing length (Lambkin, Reference Lambkin2020b). The Early Jurassic species Ipsviciella asiatica also has reduced forewing venation, though the reduction is not as extensive as the near-linear veins of Solitivicia reducta n. gen. n. sp. Additionally, unlike other ipsviciids that have a distinct surface sculpture and are often covered in dense punctures or tubercules, the forewings of Solitivicia reducta n. gen. n. sp. are instead patterned with minute, irregular, pigmented spots. It has been suggested that the strong surface sculpture in this family perhaps allowed for camouflage among the vegetation (Barth et al., Reference Barth, Ansorge and Brauckmann2011); the irregular pigmented spots of Solitivicia reducta n. gen. n. sp. might have served a similar function.
The hind wings of Solitivicia reducta n. gen. n. sp. (Fig. 5), flipped forward, and stretched out in front of the insect, are partially preserved, with only the anterior portion visible (R, M, and CuA; following the venational scheme of Lambkin, Reference Lambkin2020b). The posterior portion (CuP and anal vein(s)) is missing or can be represented by the few indeterminable fragments just above the insect's head. The hind wing venation preserved in the Solite fossil, albeit difficult to unravel because the wings overlap, seems to closely match that of Ipsvicia jonesi Tillyard, Reference Tillyard1919 (Fig. 5.3) from Australia's Ipswich Coal Measures. The only other known scytinopteroid hind wings belong to Scytinoptera Handlirsch, Reference Handlirsch1904 (Becker-Migdisova, Reference Becker-Migdisova1948, figs. 2, 33, 39–43) and a specimen tentatively assigned to Argentinoscytina Lara and Wang, Reference Lara and Wang2016, both belonging to family Scytinopteridae; the venation of these hind wings differs significantly from that of Solitivicia reducta n. gen. n. sp. Due to the infrequent preservation of scytinopteroid hind wings, further comparisons cannot be made between the remaining scytinopteroid taxa, but the striking similarity between the venation of Solitivicia reducta n. gen. n. sp. and Ipsvicia jonesi supports the Solite species’ placement in Ipsviciidae.
Four partial legs of Solitivicia reducta n. gen. n. sp. are preserved, but only a single metathoracic leg has more than one segment visible (Fig. 6.3). This leg, consisting of the femur, tibia, and three-segmented tarsus, is fairly short relative to the size of the body.
The ovipositor of Solitivicia reducta n. gen. n. sp. (Fig. 6.4) is fairly large and robust in comparison to its body size, comprising ~17% of the insect's total body length. Today, true ovipositors are present in most hemipteran groups (Cicadomorpha, Coleorrhyncha, many Heteroptera), though they are usually quite small. Conversely, the world's largest leafhopper, Ledromorpha planirostris Donovan, Reference Donovan1805 (Cicadomorpha: Cicadellidae), is an example of an extant hemipteran with a very large ovipositor, perhaps used to deposit eggs beneath the bark of the eucalyptus trees where it is commonly found (Jones and Deitz, Reference Jones and Deitz2009). Large ovipositors have also been preserved in some fossil cicadomorphan taxa: the Permian Paraknightia magnifica (Scytinopteroidea: Paraknightiidae; Evans, Reference Evans1950), the Triassic ipsviciid, Ipsviciopsis elegans Tillyard, Reference Tillyard1922 (Scytinopteroidea: Ipsviciidae; Lambkin, Reference Lambkin2020b), and the Jurassic cercopoids, Jiania crebra Wang et al., Reference Wang, Szwedo and Zhang2012 and Jiania gracila Wang et al., Reference Wang, Szwedo and Zhang2012 (Cercopoidea: Sinoalidae; Wang et al., Reference Wang, Szwedo and Zhang2012). Although ovipositors are highly adaptive for inserting eggs into or beneath a substrate, they can have disadvantages. Insects with large, robust ovipositors tend to be weak fliers. The large ovipositor of Solitivicia reducta n. gen. n. sp. indicates that it was depositing eggs into a substratum (e.g., in soil or plant material), and the size of the ovipositor in relation to its body length, as well as the long, narrow wings, suggest it was not likely a strong flier.
Suborder Heteroptera Latreille, Reference Latreille1810
Heteroptera incertae sedis
Figure 7
Material
VMNH 49761, part and counterpart (Fig. 7).
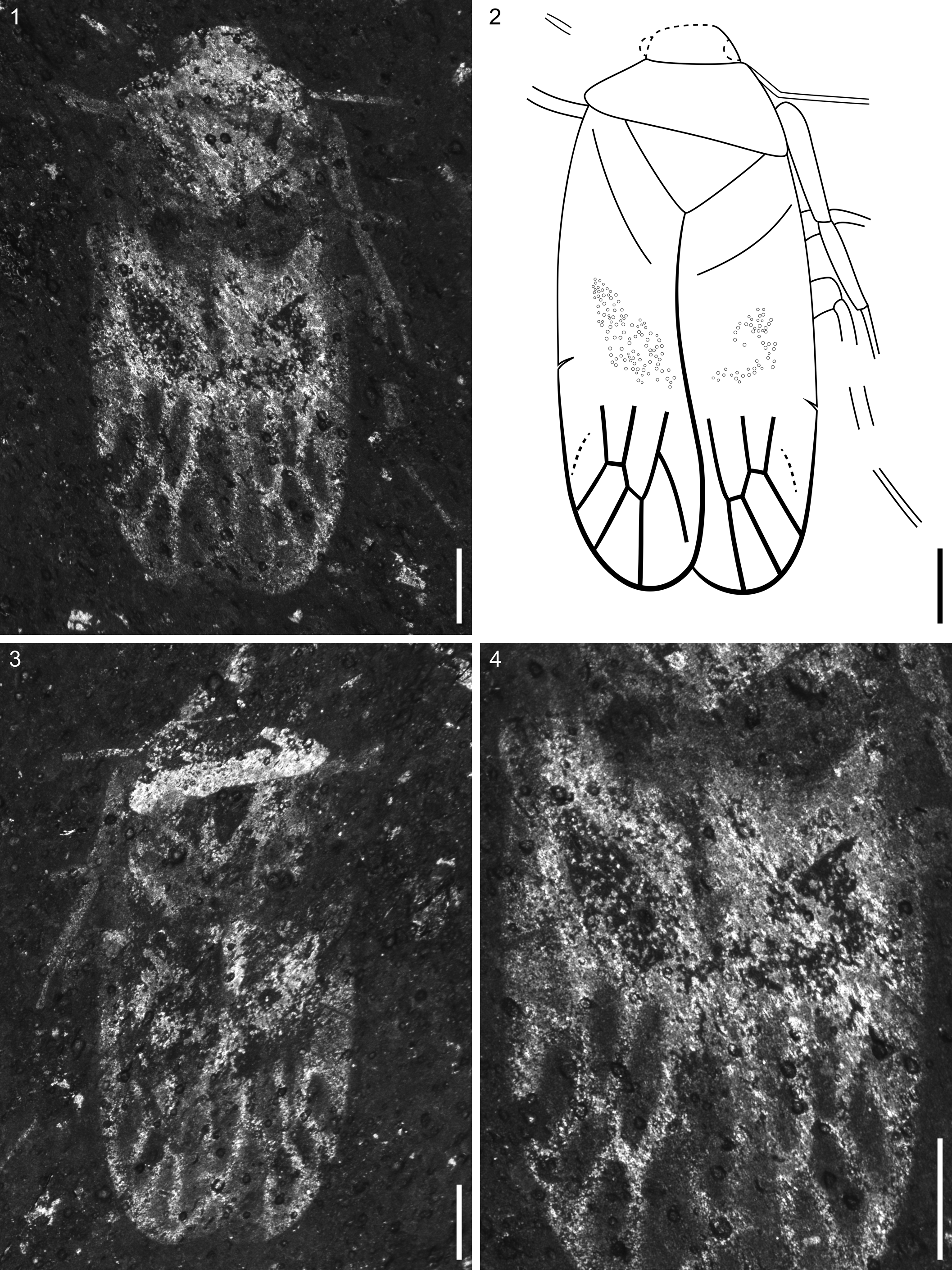
Figure 7. Heteroptera Latreille, Reference Latreille1810 incertae sedis, VMNH 49761: (1) part; (2) illustration of part; (3) counterpart; (4) detail of forewings showing small, textured areas. Scale bars = 0.25 mm.
Occurrence
Former Solite Quarry, Eden, Rockingham County, North Carolina, USA (36°32'29.7"N, 79°40'12.8"W); Norian, Late Triassic, Cow Branch Formation.
Description
Tiny hemipteran (1.9 mm long, 0.9 mm wide), preserved in dorsal view; preservation fair with some deformation. Head small, incompletely preserved, anterior margin convex; small fragments of antennae visible on each side of head, thin, at least as long as combined length of pronotum + scutellum, segmentation not preserved. Pronotum slightly deformed, trapezoidal in shape. Scutellum large, triangular. Forewings with small textured area medially; clavus short, occupying ~0.3 times the wing length; basal venation indistinct, small break in costa near its midlength (possibly costal fracture), apical venation with at least four large cells; veins thick. Legs long, slender: one small fragment on left side; three partial legs on right side; prothoracic leg with elongate femur and tibia, partial tarsus with segmentation indistinct; meso- and metathoracic legs fragmentary with indistinct segmentation. Abdomen not visible.
Remarks
Preservation of this specimen is too poor for definitive taxon placement, but the forewings lacking venation in the anterior two-thirds, along with the presence of dense punctation medially, indicates that this insect possesses the basic features of the heteropteran hemelytra and should therefore be placed in suborder Heteroptera. Although the Solite specimen also retains some characters (e.g., apical forewing venation) of Permian hemipterans such as Kaltanospes kuznetskiensis Becker-Migdisova, Reference Becker-Migdisova1961 (Mitina Formation, Russia), this can be expected given the Solite specimen's presumably basal position within Heteroptera. This insect also resembles Cuneocoris geinitzi Handlirsch, Reference Handlirsch and Schröder1920 (Cuneocoridae Handlirsch, Reference Handlirsch and Schröder1920) from the lower Toarcian (Early Jurassic) of Germany, which has been placed in the heteropteran infraorder Dipsocoromorpha Miyamoto, Reference Miyamoto1961 (Popov and Wootton, Reference Popov and Wootton1977). The Solite specimen, though insufficiently preserved, is generally similar in size, habitus, and apical forewing venation to the Toarcian specimen. However, given the absence of dipsocoromorphan synapomorphies (as in Knyshov et al., Reference Knyshov, Weirauch and Hoey-Chamberlain2021), Cuneocoris geinitzi would be more appropriately placed, along with the Solite specimen, in Heteroptera incertae sedis.
Heteroptera incertae sedis
Figure 8
Material
VMNH 51435, part and counterpart (Fig. 8.1). One additional specimen, AMNH 04-97 (Fig. 8.2).
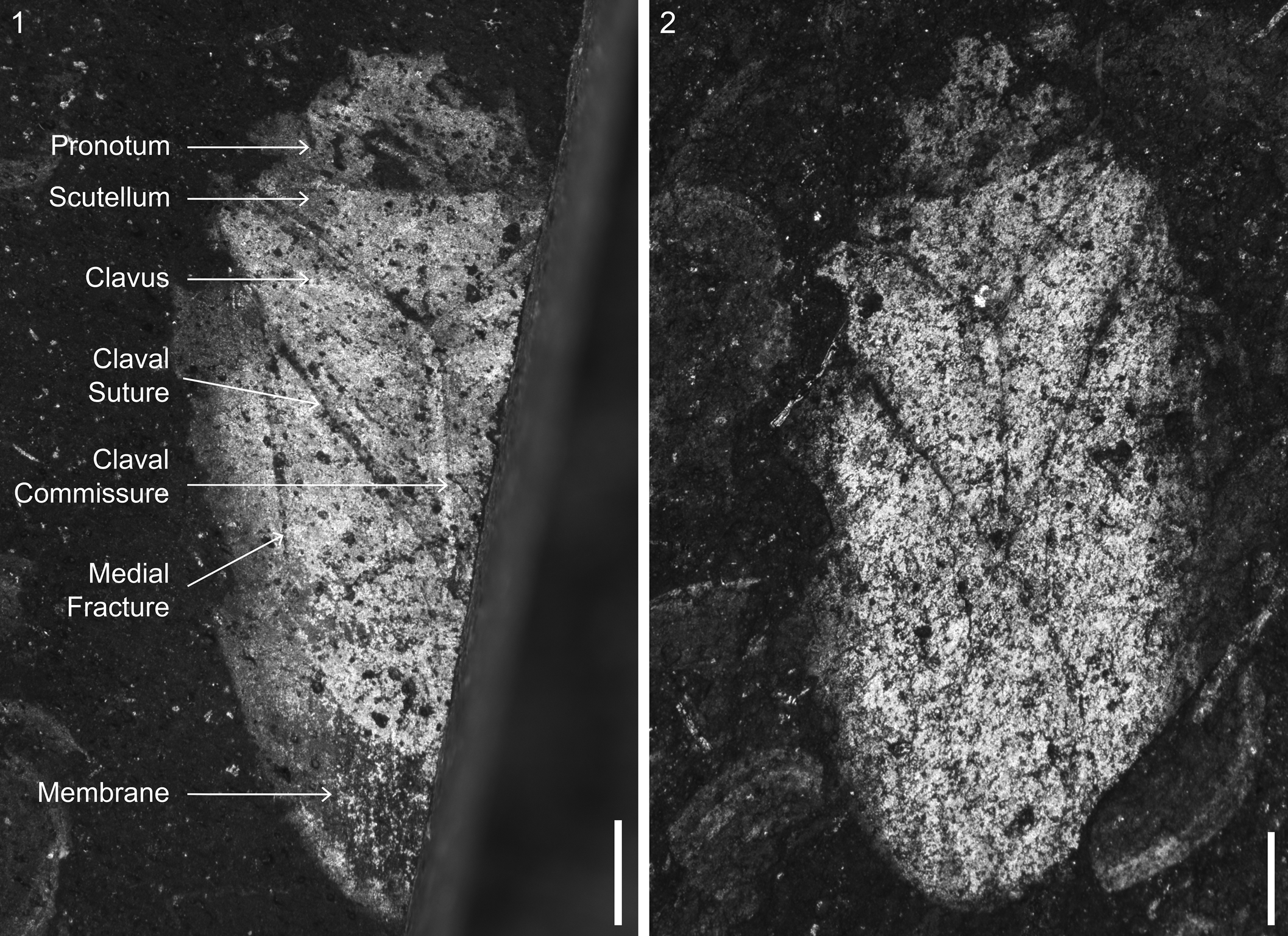
Figure 8. Heteroptera Latreille, Reference Latreille1810 incertae sedis: (1) VMNH 51435, part; (2) AMNH 04-97. Scale bars = 0.5 mm.
Occurrence
Former Solite Quarry, Eden, Rockingham County, North Carolina, USA (36°32'29.7"N, 79°40'12.8"W); Norian, Late Triassic, Cow Branch Formation.
Description
Small hemipteran (4.1 mm long, ~2.3 mm wide), preserved in dorsal view; preservation fair, right side mostly absent due to fracture in the rock. Head small, anterior margin flat; eyes small, laterally located. Pronotum faint, lateral margins indistinct, anterior and posterior margins transverse, parallel. Scutellum wide, triangular, width at least 2.2 times the length. Right hemelytron with only partial clavus preserved. Left hemelytron with clavus, claval suture, claval commissure, and medial fracture; clavus occupying ~0.3 times the wing length; membrane differentiated, venation indistinct. Legs and abdomen not preserved.
Remarks
Preservation of this specimen is too poor to allow for taxon determination beyond Heteroptera. One additional specimen (AMNH 04-97) is similar in size (4.6 mm long, ~2.2 mm wide) and habitus to VMNH 51435 and likely belongs to a related taxon. It differs from VMNH 51435 in a more pointed anterior margin of the head and a less differentiated hemelytral membrane.
The oldest definitive heteropteran fossils almost exclusively belong to Nepomorpha, the earliest of which is a presumed relative of Naucoridae Leach, Reference Leach and Brewster1815, Arlecoris louisi Shcherbakov, Reference Shcherbakov2010 (?Triassocoridae Tillyard, Reference Tillyard1922) from the earliest Middle Triassic Grès à Voltzia Formation of France (Shcherbakov, Reference Shcherbakov2010). Nepomorphans increased in abundance during the Late Triassic, with many representatives from the period's major deposits, including the Solite deposit where nepomorphans comprise ~99% of the hemipteran specimens. During the Triassic, heteropterans of the other infraorders are quite rare, with only a few questionable members reported from the Ladinian of Switzerland (Cimicomorpha: Tingidae: Archetingis ladinica Montagna et al., Reference Montagna, Strada, Dioli and Tintori2018), the Carnian of China (Pentatomomorpha: Coreidae: Kerjiecoris oopsis Lin, Reference Lin1992), and the Rhaetian of the United Kingdom (Leptopodomorpha: Archegocimicidae Handlirsch, Reference Handlirsch1906; Pentatomomorpha: Pachymeridiidae Handlirsch, Reference Handlirsch1906, and Protocoridae Handlirsch, Reference Handlirsch1906). Apart from the two heteropteran taxa described herein, no other non-nepomorphan heteropterans are currently known from the Solite deposit, which can be expected given their apparent rarity during the Triassic.
Discussion
Approximately 8,100 insect specimens have been collected from the Cow Branch Formation, of which 67% are identifiable to order. Hemipterans are the most abundant, comprising 66% of the identifiable specimens, but are overwhelmingly dominated (99%) by two groups of nepomorphans: Belostomatidae (Criscione and Grimaldi, Reference Criscione and Grimaldi2017), and an undescribed species likely belonging to Notonectidae or Corixidae. Specimens previously referred to the extinct family Archescytinidae (Fraser et al., Reference Fraser, Grimaldi, Olsen and Axsmith1996; Reference Fraser, Grimaldi, Axsmith, Heckert, Liutkus-Pierce, Smith, Dooley, Fraser and Sues2017) do not actually belong to Hemiptera; they are more likely related to Psocodea. The remaining Solite hemipterans (~1%) are generally poorly preserved or unidentified, with only two known specimens belonging to Cicadomorpha: Hylicellidae (VMNH 49780; Fig. 1) and Ipsviciidae (VMNH 51992; Figs. 2–6). Both families were previously known only from Eurasia and Australia; the Solite specimens are significant additions to the paleoentomofauna as the first records of these two families from the New World.
The hemipteran community preserved in the Solite deposit is highly unusual when compared to those of other Triassic localities. Coleorrhyncha, unknown from Solite, are diverse and well-represented in other Triassic deposits by members of the extinct family Progonocimicidae (Szwedo, Reference Szwedo2018), and cicadomorphans (especially those belonging to Scytinopteroidea) are abundant. For example, in the Madygen Formation of Kyrgyzstan, ‘Homoptera’ is the third most abundant taxon, comprising approximately one quarter of the 20,000 insect specimens collected; many of these are yet undescribed (Shcherbakov and Popov, Reference Shcherbakov, Popov, Rasnitsyn and Quicke2002). Here, cicadomorphans are the most common, represented by diverse members of Dysmorphoptilidae Handlirsch, Reference Handlirsch1906, Hylicellidae Evans, Reference Evans1956, Chiliocyclidae Evans, Reference Evans1956, and Scytinopteroidea Handlirsch, Reference Handlirsch1906; Sternorrhyncha and Fulgoromorpha are rare (Shcherbakov, Reference Shcherbakov2008).
This same pattern also holds in the major Gondwanan deposits. In South Africa's Molteno Formation, homopterans are the third most abundant group, comprising at least 12% of specimens (~250/2000; Anderson et al., Reference Anderson, Kohring and Schlüter1998). The Los Rastros Formation of Argentina also preserves abundant hemipteran wings (in addition to those of Coleoptera and Blattodea), containing members of Cercopoidea, Dysmorphoptilidae, and Scytinopteroidea (Mancuso et al., Reference Mancuso, Gallego and Martins-Neto2007). In the Denmark Hill Insect Bed (Blackstone Formation) of Australia, Hemiptera is the second most abundant order, comprising 21% of the insects collected (15% ‘Homoptera,’ 6% Heteroptera; Tillyard and Dunstan, Reference Tillyard and Dunstan1924, table C). It is unclear why cicadomorphans are uncommon in the Solite deposit when they are such a large component of other Triassic insect faunas. Given the exquisite, microscopic scale preservation of delicate insects, the rarity of the generally more robust cicadomorphans is almost certainly not an issue with preservational bias, but more likely caused by some paleoecological factor. Given that other phytophagous taxa such as Elcanidae (Orthoptera) are well-represented in the Solite material, perhaps the littoral vegetation surrounding ancient ‘Lake Solite’ consisted of plants less conducive to cicadomorphans. When compared to other Triassic deposits (e.g., Madygen Formation, Molteno Formation), the flora of Solite is significantly less diverse, dominated by conifers and bennettitaleans, with some ginkgophytes and rare ferns (Fraser et al., Reference Fraser, Grimaldi, Axsmith, Heckert, Liutkus-Pierce, Smith, Dooley, Fraser and Sues2017). Perhaps the lack of cicadomorphans at Solite is simply a consequence of this less diverse flora.
Acknowledgments
We thank Lucy Treado, Adam Pritchard, and the staff of the Virginia Museum of Natural History for arranging specimen loans and hosting a visit by J.C.V. We are grateful to S. Alpert, C. Martin, D. Ebel, and the staff of the AMNH Earth and Planetary Sciences Department for enabling access to the petrographic microscope for higher resolution photomicrography of specimens. We also thank D. Shcherbakov for his thoughts on the identity of the Solite ipsviciid and are grateful to V. Hartung and an anonymous reviewer for their thorough and thoughtful comments on this manuscript.
Competing interests
The authors declare no competing interests.










