Introduction
Vitamins and minerals are micronutrients required by the body in small amounts to achieve homeostasis and various physiological processes, such as metabolic regulation, immune function, and modulating gene expression. Reference Anas, Diniz and Menezes1–Reference Palomares3 The availability of these nutrients is often limited by forage quality, requiring supplementation by beef producers. Reference Greene4 However, despite ongoing research into their critical role in fetal development, vitamin and mineral supplementation strategies are largely variable among producers. Reference Arthington and Ranches5–Reference Hurlbert, Baumgaertner and Menezes7 Maternal nutrient requirements vary during gestation due to the increased demand for nutrients to supply the growing fetus. Reference Caton, Crouse and Reynolds8 We have shown that supplementation of vitamins and minerals during the periconceptual period to beef heifers through the first 83 days of gestation increased the concentrations of trace minerals in the fetal liver and muscle. Reference McCarthy, Menezes and Kassetas2,Reference Menezes, McCarthy and Kassetas6 Similarly, vitamin and mineral supplementation to cow-calf pairs grazing native range pastures increased Se, Cu, and Co liver concentrations at pasture removal and weaning for cows and suckling calves compared with the non-supplemented cohort. Reference Hurlbert, Baumgaertner and McCarthy9
Nutritional management during gestation can positively or negatively impact the offspring’s development due to the in utero programming of organ systems. Reference Hyatt, Budge and Symonds10–Reference Wallace, Bourke, Aitken, Milne and Hay12 In ruminants, altered small intestinal mass, villi morphology, hypertrophy, hyperplasia, vascularity, and gene expression were affected due to maternal overnutrition or nutrient restriction. Reference Meyer and Caton13 Vitamin and mineral supplementation combined with a moderate rate of body weight gain to heifers during early gestation increased the fetal small intestinal weight compared with fetuses from supplemented heifers managed at a low rate of gain. Reference Menezes, McCarthy and Kassetas6 Furthermore, we have shown that the hepatic expression of energy- and lipid-related genes in these fetuses was differentially programmed. Reference Diniz, Ward and McCarthy14 These findings suggest that compensatory mechanisms within the developing fetus may regulate the growth rates of metabolic organs, potentially adapting to adjust energy utilization. Reference Menezes, McCarthy and Kassetas6 Such adaptations may have lasting implications on the offspring’s body composition outcomes due to compromised metabolic programming. Reference Caton, Crouse and Reynolds8,Reference Reynolds, Ward, Caton, Scanes and Hill15 Offsprings born to VTM supplemented dams from a similar cohort used in the current study were heavier than CON from weaning through 15 months of age. These adaptations were also observed in greater blood glucose at birth and altered feeding behaviors in VTM offspring compared with CON. Reference Hurlbert, Baumgaertner and Menezes7
We have shown considerable effects on the programming of fetal tissues, metabolism, and placental development in response to maternal nutrition. Reference Diniz, Crouse and Cushman16–Reference Diniz, Reynolds and Borowicz19 Changes in visceral organs during pregnancy may have long-lasting effects on the production efficiency of the offspring. Visceral organs, such as the jejunum, begin development by day 26 of gestation Reference Anas, Diniz and Menezes1 and their development and function may also be programmed in utero. Reference Meyer and Caton13 The jejunum is the main site of absorption of nutrients Reference Collins and Nguyen20 and has a high metabolic activity and rapid turnover. Reference Prezotto, Lemley and Camacho21 Furthermore, the intestinal immune system plays a key role in pathogen recognition and immune response. Reference Cangiano, Villot, Guan, Ipharraguerre and Steele22 Despite its importance, the role of maternal nutrition in the programming of the offspring jejunum remains largely underexplored.Reference Meyer and Caton 13
The observed outcomes of vitamin and mineral supplementation on postnatal growth performance, together with our previous findings under a similar experimental model, Reference Hurlbert, Baumgaertner and Menezes7,Reference Hurlbert, Menezes and Baumgaertner23 led us to investigate the effects of maternal vitamin and mineral supplementation on the gene expression profile of jejunum in neonatal calves. We hypothesized that the maternal vitamin and mineral supplementation throughout gestation would differentially program the jejunal mucosa through changes in the expression of genes involved with immune response, nutrient uptake, and metabolism. Herein, through a transcriptomics approach, we identified differentially expressed genes in the jejunal mucosa of newborn calves, as well as the underlying biological processes affected by maternal vitamin and mineral supplementation. Additionally, we measured gene-gene interactions to uncover regulatory genes in response to maternal diet. Our findings show that the maternal vitamin and mineral supplementation throughout gestation affected genes regulating pathways such as the intestinal immune network for IgA production, cytokine-cytokine receptor interaction, fat digestion and absorption, regulation of immune response, and inflammatory response.
Materials and methods
Care and use of animals
All animal procedures were approved by the North Dakota State University (NDSU, Fargo, ND, USA) Institutional Animal Care and Use Committee (#A21047).
Animals, housing, and diet
The development of the experimental model and study design were previously reported. Reference Hurlbert, Baumgaertner and Menezes7,Reference Hurlbert, Menezes and Baumgaertner23 In brief, 72 crossbred Angus-based heifers (∼ 14 to 15 months of age, initial body weight (BW) = 380.4 ± 50.56 kg; standard deviation) were used to determine the influence of vitamin and mineral supplementation throughout gestation on offspring development. Following a 14 d acclimation to an electronic individual feeding head-gate system, heifers were estrus synchronized using a 7-day Select Synch + CIDR estrus synchronization protocol Reference Lamb, Dahlen, Larson, Marquezini and Stevenson24 and bred via artificial insemination (AI) using female-sexed semen from a single sire. After AI, heifers were blocked by BW and randomly assigned to receive either a basal diet (control; CON; n = 36) or a basal diet plus a vitamin and mineral supplement (VTM; n = 36). Heifers pregnant to the first AI were used in a different experiment. Reference Hurlbert, Baumgaertner and Menezes7 Within their respective treatment groups, heifers not pregnant after the first AI (CON, n = 19; VTM, n = 18) were resynchronized as described above and bred via AI 60 d after the first insemination, and used for the current study. Reference Hurlbert, Menezes and Baumgaertner23
Treatments were applied from 60 d pre-breeding through calving. However, the vitamin and mineral supplement provided from pre-breeding to day 239 of gestation was a loose product (Purina Wind and Rain Storm All Season 7.5 Complete, Land O’Lakes, Inc., Arden Hills, MN, USA; Supplementary Table S1) top-dressed over the basal diet. From day 240 of gestation, the vitamin and mineral supplement was added into the total mixed ration (TMR) for the VTM treatment group. Diet compositions were described elsewhere Reference Hurlbert, Menezes and Baumgaertner23 and are reported in Supplementary Table S1. Individual feed deliveries were monitored through a Calan head-gate system and adjusted during gestation to achieve targeted BW gains of 0.45 kg•heifer-1•d-1 through day 200. After that, feed deliveries were altered to allow for ad libitum daily intake in preparation for calving. Reference Hurlbert, Menezes and Baumgaertner23 Individual intake from day 240 was monitored via the Insentec feeding system (Hokofarm Group B.V., the Netherlands). At parturition, calves were promptly removed from their dams before suckling and were housed in individual pens. Within 2 h of birth, calves were fed 1.4 L of commercial colostrum replacer (LifeLine Rescue High Level Colostrum Replacer, APC, Ankeny, IA, United States) via an esophageal feeder. At 12 and 24 h after the initial colostrum feeding, calves were fed 2 L of milk replacer (Duralife 20/20 Optimal Non-Medicated Milk Replacer, Fort Worth, TX) using an esophageal feeder. At 30 h after the initial colostrum feeding, 14 heifer calves (n = 7 per group) were euthanized via captive bolt and exsanguination. Organs were collected, weighed, and a subsample of mid-jejunum was harvested. The sampling location followed the methods described by Trotta et al. Reference Trotta, Ward and Swanson25 In brief, the small intestine was removed at the pyloric and ileocecal junction, representing portions of the duodenum and ileum, respectively. The remainder of the small intestine was measured and cut in half to represent the proximal and distal jejunum. One-meter segments were cut and sampled from the midpoint of the previously cut sections. Reference Trotta, Ward and Swanson25 The jejunal mucosa was scraped using a glass microscope slide, snap-frozen on dry ice, and stored at − 80°C.
RNA extraction, library preparation, sequencing, and data processing
Total RNA was extracted from the jejunal mucosa (n = 7, VTM; n = 7, CON) using the RNeasy kit (Qiagen Germantown, MA, USA), followed by on-column DNase treatment, according to the manufacturer’s protocol. The integrity and quality (IQ) of the RNA samples were assessed with the Qubit RNA IQ Assay kit (ThermoFisher Scientific), agarose gel electrophoresis, and the Agilent 2100 Bioanalyzer. All samples met the required quantity and quality parameters for library preparation (IQ ≥ 8). Strand-specific RNA libraries were prepared using the NEBNext® Ultra™ II Directional RNA Library Prep Kit for Illumina (New England BioLabs®, Ipswich, MA, USA) with PolyA mRNA enrichment. Paired-end libraries were sequenced on the Illumina® NovaSeq 6000 platform, with 150 bp reads, and a 20 M reads/sample depth. Novogene Co., Ltd (Nanjing, China) performed the library preparation and sequencing.
Raw data quality control was based on filtering out sequencing adaptors and reads with a Phred-Score lower than 30. FastQC v. 0.11.9 Reference Andrews26 and MultiQC v. 1.10.1 Reference Ewels, Magnusson, Lundin and Käller27 were used to perform data quality control and read statistics of the raw reads. The reads were mapped to the ARS-UCD1.2. (release109) Bos taurus reference genome using the STAR aligner v.2.7.5. Reference Dobin, Davis and Schlesinger28 We counted the mapped reads using the quantMode GeneCounts flag from STAR. Reference Dobin, Davis and Schlesinger28 Post-mapping quality control was conducted with MultiQC and edgeR v. 4.0.3. Reference Robinson, McCarthy and Smyth29
Differential expression, regulatory transcription factors, and co-expression network analyses
Genes with zero or low-count expression were filtered out using the filterByExpr function from edgeR. Reference Robinson, McCarthy and Smyth29 A principal component analysis was performed using the factoextra v1.0.7 Reference Kassambara and Mundt30 R-package to identify potential batch effects. To identify differentially expressed genes (DEGs), we used DESeq2 v. 1.42.0, Reference Love, Huber and Anders31 which applies a negative binomial distribution to model the RNA-Seq data. A pairwise comparison was performed between the VTM and CON groups. Genes with a P ≤ 0.05 and absolute log2 fold change > 0.5 were considered significant. The DEGs were classified as up or downregulated based on the sign of the log2 FC in the VTM group. Gene annotation was performed using BiomaRt v. 2.58.2 Reference Durinck, Spellman, Birney and Huber32 based on the bovine ARS-UCD1.2. (release109) reference from the Ensembl database. To visualize the DEGs, a volcano plot was constructed using EnhancedVolcano plot v. 1.20.0 R-package. Reference Blighe and Rana33
To identify transcription factors (TFs) modulating the differential expression between VTM and CON groups, we used the regulatory impact factor (RIF) algorithms, RIF1 and RIF2. Reference Reverter, Hudson, Nagaraj, Pérez-Enciso and Dalrymple34 RIF1 assigns a high score to TFs that are most differentially co-expressed with the highly abundant and differentially expressed genes, while RIF2 assigns a high score to TFs whose expression can predict better the abundance of DEGs. Reference Reverter, Hudson, Nagaraj, Pérez-Enciso and Dalrymple34,Reference Afonso, Fortes and Reverter35 To this end, all genes tested for differential expression were normalized using the VST function from DESeq2. Then, after mining the AnimalTFDB bovine database v.4.0,Reference Shen, Chen and Gan 36 we filtered out those TFs that were not expressed in our dataset (n = 367). The remaining TFs (n = 1,078) were contrasted with the DEG list. The analysis was implemented in FORTRAN 90 based on the source code available from the RIF’s main author,Reference Reverter, Hudson, Nagaraj, Pérez-Enciso and Dalrymple 34 as previously described. Reference Diniz, Crouse and Cushman16,Reference Reverter, Hudson, Nagaraj, Pérez-Enciso and Dalrymple34 Key TFs were selected considering either one of the two RIF scores was greater than |1.96| of the standard deviation (P < 0.05). Reference Diniz, Crouse and Cushman16,Reference Reverter, Hudson, Nagaraj, Pérez-Enciso and Dalrymple34 The co-expression profile of gene pairs for DEGs and TFs was created based on the partial correlation and information theory (PCIT) algorithm. Reference Reverter and Chan37 Significantly co-expressed pairs were selected based on a partial correlation value greater than |0.8| (P ≤ 0.05). Cytoscape v.3.9.0 was used for network visualization. Reference Shannon, Markiel and Ozier38
Functional over-representation and gene set enrichment analyses
To understand the biological roles of the genes in response to the maternal diet, we used two approaches for functional analysis. First, based on the DEGs identified between VTM and CON groups, we performed an over-representation analysis using ClueGO 2.5.10. Reference Bindea, Mlecnik and Hackl39 Redundant terms were grouped based on the kappa score = 0.4. Reference Bindea, Mlecnik and Hackl39 The P-value was calculated and corrected with a Bonferroni step-down (P ≤ 0.05). Over-represented KEGG pathways and Gene Ontology (biological processes) terms were identified and considered significant when P ≤ 0.05. These analyses were carried out based on the B. taurus annotation, and the network visualization was performed on Cytoscape version 3.9.0. Reference Shannon, Markiel and Ozier38
Second, we used a functional class scoring approach based on the Gene Set Enrichment Analysis (GSEA) method considering on all genes tested for differential expression. This approach was used to identify group of genes (gene sets) acting on common pathways rather than DEGs individually. Reference Subramanian, Tamayo and Mootha40 The following equation was used to rank the genes: rank = [sign (log 2 FC)×−log 10(p−value)]. Reference Ziemann41 Considering this equation, the sign of the ranking (positive or negative) will be given by the fold-change, whereas the magnitude will be given by the P-value. Reference Ziemann41 Thus, instead of analyzing individual genes, GSEA evaluates whether predefined gene sets from specific pathways or processes are over-represented among the top or bottom of our ranked gene list. Reference Subramanian, Tamayo and Mootha40,Reference Khatri, Sirota and Butte42
The GSEA analysis was implemented through the WebGestalt (WEB-based Gene SeT AnaLysis Toolkit) Reference Liao, Wang, Jaehnig, Shi and Zhang43 to identify over-represented KEGG pathways at the top or bottom of the ranked list of genes. Reference Subramanian, Tamayo and Mootha40 Based on the GSEA assumptions, the degree of enrichment is represented by the normalized enrichment score (NES). The NES is the enrichment score for the gene set after it has been normalized across analyzed gene sets. A significant positive NES value indicates that members of the gene set tend to appear at the top of the gene list (positive fold change), while a significant negative NES indicates the opposite. Reference Subramanian, Tamayo and Mootha40
Results
VTM supplementation throughout gestation affects maternal and neonatal mineral reserves
The effects of the maternal diet on the dam and neonatal trace mineral concentrations and body weight (BW), blood metabolites, and neonatal organ mass were previously reported. Reference Hurlbert, Menezes and Baumgaertner23 These results are outlined below to give the reader a background and contextualize the current findings. By design, BW for dams was similar (P ≥ 0.25) throughout gestation (CON = 510.1 ± 57.99 kg, VTM = 528.0 ± 65.86 kg). Likewise, VTM supplementation did not affect maternal BW at calving (CON: 558 ± 49.1 kg; VTM: 534 ± 34.4 kg) nor neonatal BW at birth and 30 h later. Reference Hurlbert, Menezes and Baumgaertner23 Similarly, no differences were observed in the neonatal tissue weights. Reference Hurlbert, Menezes and Baumgaertner23 Except for cobalt, no differences were observed in dam serum trace mineral concentrations at calving. Conversely, calves born to VTM-supplemented dams exhibited greater concentrations of Se and Mo in the liver and Co. and Se in the serum 30 h post-calving. Reference Hurlbert, Menezes and Baumgaertner23 Moreover, VTM treatment increased the concentrations of vitamin A in maternal serum and D in the serum of the calf and the dam.
Maternal VTM supplementation affects transcription factor (TF) regulation and gene expression of the jejunal mucosa in newborn calves
The RNA-Seq approach generated, on average, 30.5 M clean reads per sample (ranging from 21.0 to 38.2 M reads per sample). On average, 94.4% of the total reads were uniquely mapped to the reference genome (Supplementary Table S2). Following post-mapping quality control (QC) by removing not expressed or lowly expressed genes, 15,043 out of 27,607 genes remained in the 14 samples for differential expression analysis.
Using DESeq2, we identified 528 differentially expressed genes (DEGs) between the VTM and CON treatment groups (P ≤ 0.05 and |Log2FC| > 0.5; Supplementary Table S3). Among them, 231 genes were upregulated, and 297 were downregulated in the VTM neonatal calves (Figure 1A). Most genes consisted of protein-coding (94.49%); however, long noncoding (3.2%), microRNAs (0.19%), and others (2.12%) were also identified. DEGs with the most significant P-values were KCNMB4, ODF3L2, PTPRNA, BTBD19, TLE2, TMC7, and FLT1. We identified 33 DEGs, which were TFs, including LBX2, ATOH8, and ZBTB7C.
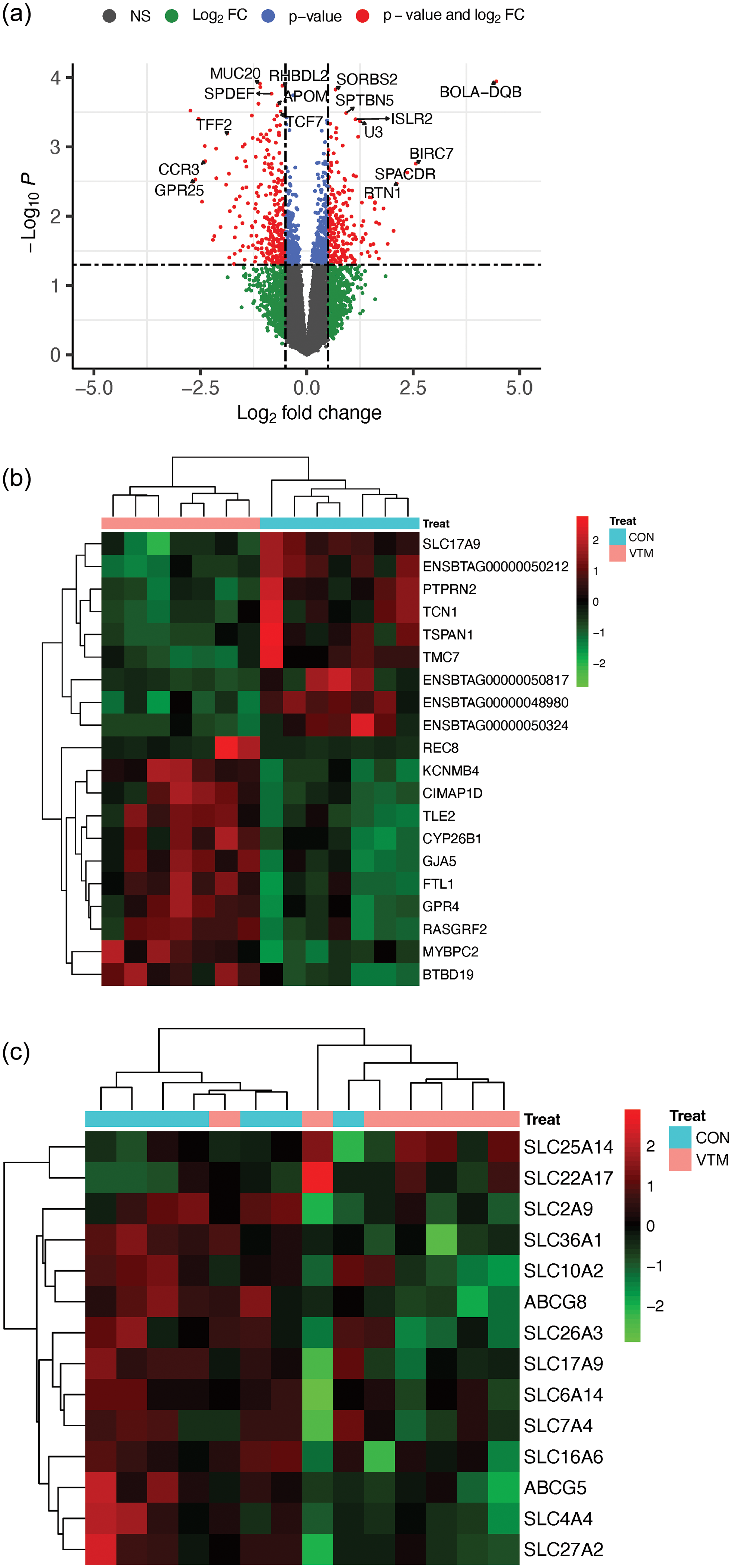
Figure 1. Transcriptomic profile of the jejunal mucosa of neonatal beef heifers born from dams receiving or not receiving vitamin and mineral supplementation (VTM or CON) during gestation. (a) Volcano plot of differentially expressed genes (DEGs) from the jejunal mucosa between VTM vs. CON groups. Each dot represents a gene. The difference in gene expression between the groups is shown as the log2 fold change (x-axis). The -log (base 10) of the P-value is shown on the y-axis. DEGs are color-coded as represented in the legend; (b) Heat map of the top 20 DEGs of the jejunal mucosa of neonatal beef heifers between VTM vs. CON groups; (c) Heat map of genes from the SLC and ABC transporter families identified as DEGs. The heat map colors high and low expression values by red and green, respectively. The expression level of DEGs and groups are color-coded as represented in the legend.
A heat map of the top 20 DEGs is shown in Figure 1B. To understand the potential effects of VTM treatments on programming genes involved with nutrient transport, we screened the DEG list to identify nutrient transporter-coding genes from the SLC and ABC families. We retrieved 12 genes from the SLC and two from the ABC families (Figure 1C). All these genes were downregulated in the calves born to VTM dams, except for the SLC25A14 and SLC22A17.
Based on the RIF metrics, we identified 98 unique significant TFs out of 1,078 tested as potential regulators of differential gene expression (P ≤ 0.05). The TFs identified as regulators for the VTM vs. CON groups are reported in Supplementary Table S4. These TFs were grouped into 25 families. Among them, the zinc finger C2H2 and homeobox were over-represented by 41 and 12 TFs, respectively (Supplementary Table S4). From RIF1, the TFs NFATC1 (z-score = − 2.947) and ONECUT2 (z-score = − 2.40) showed the most extreme negative values, whereas ZSCAN16 (z-score = 5.17) and ZNF274 (z-score = 4.66) showed the greatest positive values. Likewise, for RIF2, we found SMARCAL1 (z-score = − 2.48) and MYC (z-score = − 2.44) as the extreme negative and E2F3 (z-score = 3.42) and NR2C1 (z-score = 3.24) as the extreme positive.
To identify the co-expression profiles between DEGs and RIF genes (TFs), we used partial correlation and information theory. Reference Reverter and Chan37 Our network analysis retrieved 43,606 co-expressed pairs (P ≤ 0.05). To reduce the data dimensionality, we kept 4,783 co-expressed pairs (corresponding to 552 unique genes) with a partial correlation greater than |0.8| (P ≤ 0.05) (Figure 2; Supplementary Table S5). Among the pairs, we had 92 TFs, from which 65 were also RIF genes (Supplementary Table S5). Based on the network analysis, ZNF414, ATOH8, NR1H3, NFATC4, and LBX2 were the most connected TFs (degrees ranging from 23 to 49; Figure 2).
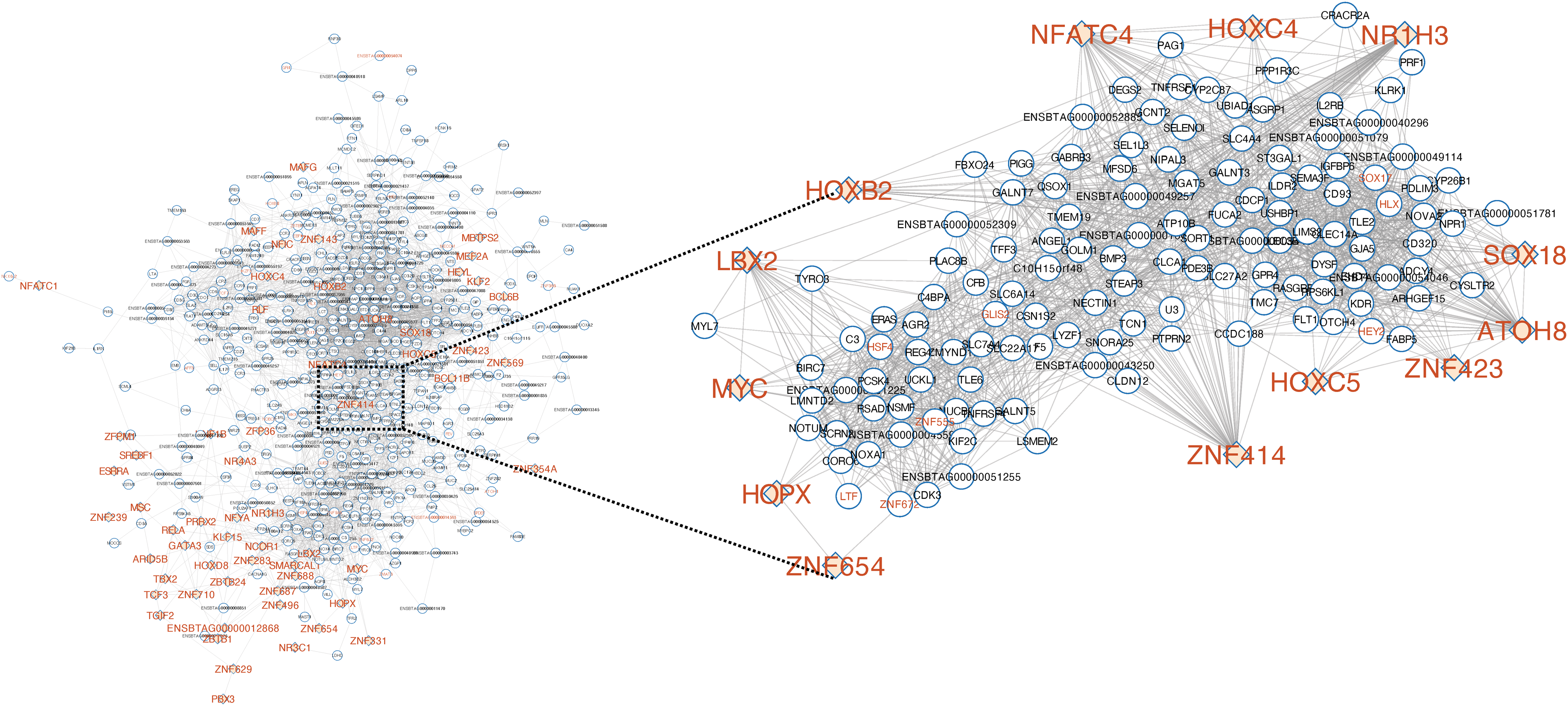
Figure 2. Regulatory network of differentially expressed genes (DEGs) from the jejunal mucosa of neonatal beef heifers born from dams receiving or not receiving vitamin and mineral supplementation (VTM or CON) during gestation. Zoom in on the transcription factors (TFs) with the highest degree and first neighbors. Nodes are DEGs between VTM vs. CON groups and TFs with significant regulatory impact factor (RIF) built through partial correlation information theory (PCIT). Transcription factors are labeled in orange color and those identified as RIFs are represented by a diamond-colored shape. Significantly co-expressed pairs were selected based on a partial correlation value greater than |0.8| (P ≤ 0.05). Nodes with few connections not linked to the main network are not shown. Cytoscape v.3.9.0 was used for network visualization.
Maternal VTM supplementation throughout gestation affects genes underlying immune-related pathways in the jejunal mucosa of newborn calves
To identify over-represented pathways and biological processes affected by the DEGs, we performed an over-representation analysis utilizing ClueGO. Reference Bindea, Mlecnik and Hackl39 Significant KEGG pathways (Figure 3) over-represented by DEGs from the VTM vs. CON groups included glycerolipid, glycerophospholipid, and sphingolipid metabolism, which grouped pathways such as cholesterol metabolism, steroid hormone biosynthesis, and fat digestion and absorption. Pancreatic secretion and cGMP-PKG signaling pathways were grouped with several other pathways, including calcium signaling, insulin secretion, thyroid hormone synthesis, and regulation of lipolysis in adipocytes. KEGG pathways consisting of most downregulated genes in the VTM group were fat digestion and absorption and mucin-type O-glycan biosynthesis.
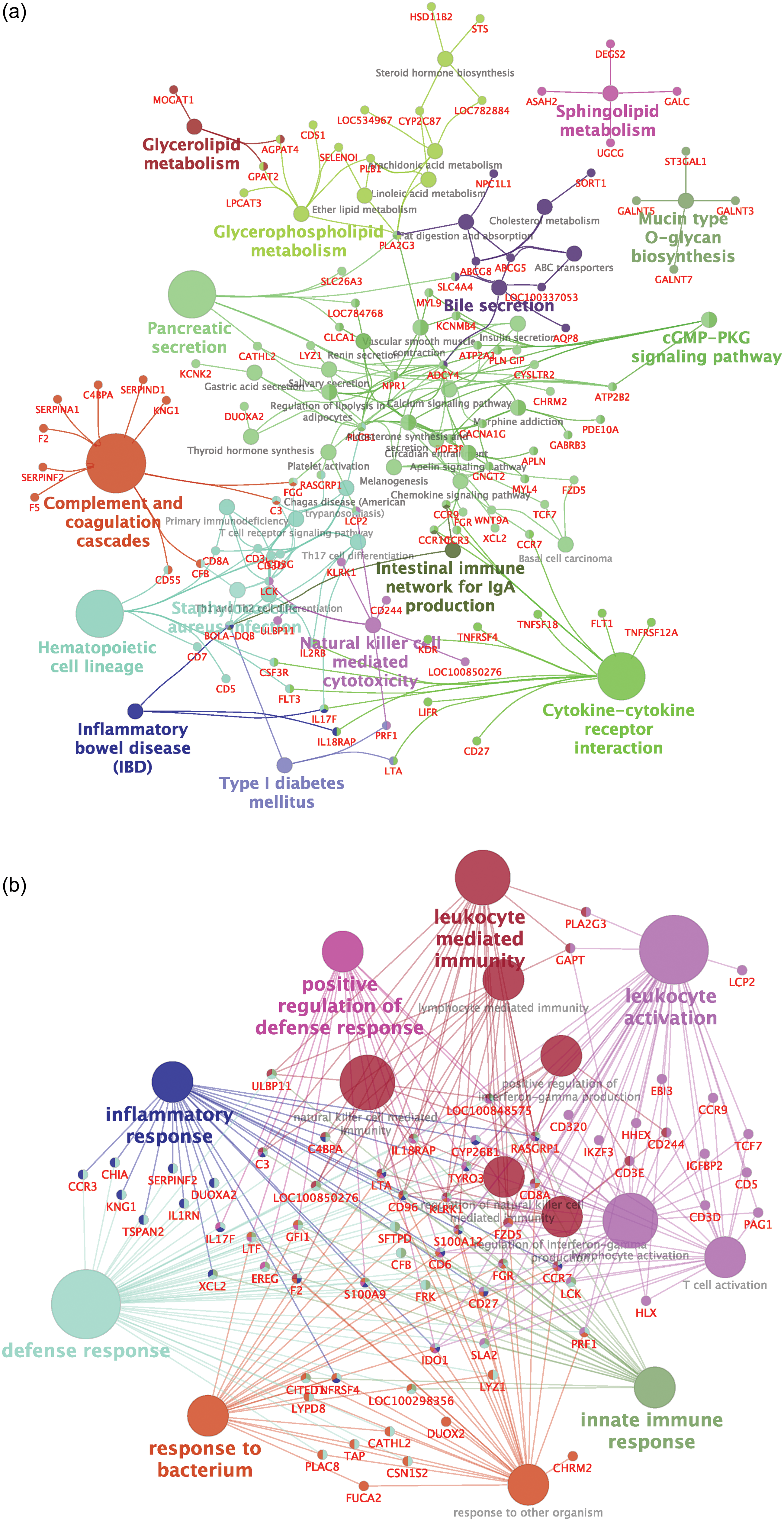
Figure 3. Functional over-representation analysis of differentially expressed genes from the jejunal mucosa of neonatal beef heifers born from dams receiving or not receiving vitamin and mineral supplementation (VTM or CON) during gestation. Network clusters were based on over-represented KEGG pathways (a) and biological processes (b) retrieved from clueGO. Significant terms were taken when P ≤ 0.05. Functionally related groups partially overlap and are arbitrarily colored. The node size represents the significance of pathway enrichment.
KEGG pathways and BPs related to immune related processes were over-represented (Figure 3). Intestinal immune network for IgA production, cytokine-cytokine receptor interaction, and Staphylococcus aureus infection were among the over-represented pathways sharing DEGs. Interestingly, genes underlying the complement and coagulation cascades were mostly downregulated in calves born to VTM dams, including F5, F2, SERPINF2, SERPINA1, C4BPA, CD55, CFB, and C3. On the other hand, the cytokine-cytokine receptor interaction KEGG pathway showed most genes upregulated in the VTM group (LIFR, KDR, TNFRSF4, TNFSF18, FLT1, and TNFRSF12A). Figure 3B shows the over-represented BPs, including leukocyte-mediated immunity, leukocyte activation, innate immune response, and defense response (P ≤ 0.05). Among the underlying genes in most biological processes, we can highlight HEY2, CACNA1G, IGFBP2, IGFBP6, SKAP1, and KLRK1. These results shed light on specific pathways and biological processes likely affected by changes in gene expression influenced by maternal VTM supplementation.
To provide a more complete view of the pathways affected by the maternal diet, we performed a Gene Set Enrichment analysis (GSEA) on 15,043 expressed genes, which were ranked based on a combination of P-value and log fold change (see methods). We retrieved the top 20 KEGG pathways based on the Normalized Enrichment Score (NES; Figure 4). Top pathways involving upregulated genes in the VTM group included focal adhesion, notch, apelin, and hippo signaling pathways. Additionally, beta-alanine metabolism, cGMP-PKG, Rap1, and MAPK signaling pathways (Figure 4) were over-represented. The LOC508879, AOC2, FLT1, RASGRF1, KCNMB4, and TEAD3 genes were commonly identified among the pathways. Pathways over-represented by downregulated genes (negative NES) in the VTM group mainly included those related to immune response, such as complement and coagulation cascades, natural killer cell-mediated cytotoxicity, and primary immunodeficiency. Furthermore, we identified pathways involved with lipid metabolism, fat digestion and absorption, and hormone biosynthesis. All pathways involved significantly downregulated genes in the VTM group, including LOC615045, PLA2G12B, and PLA2G3.
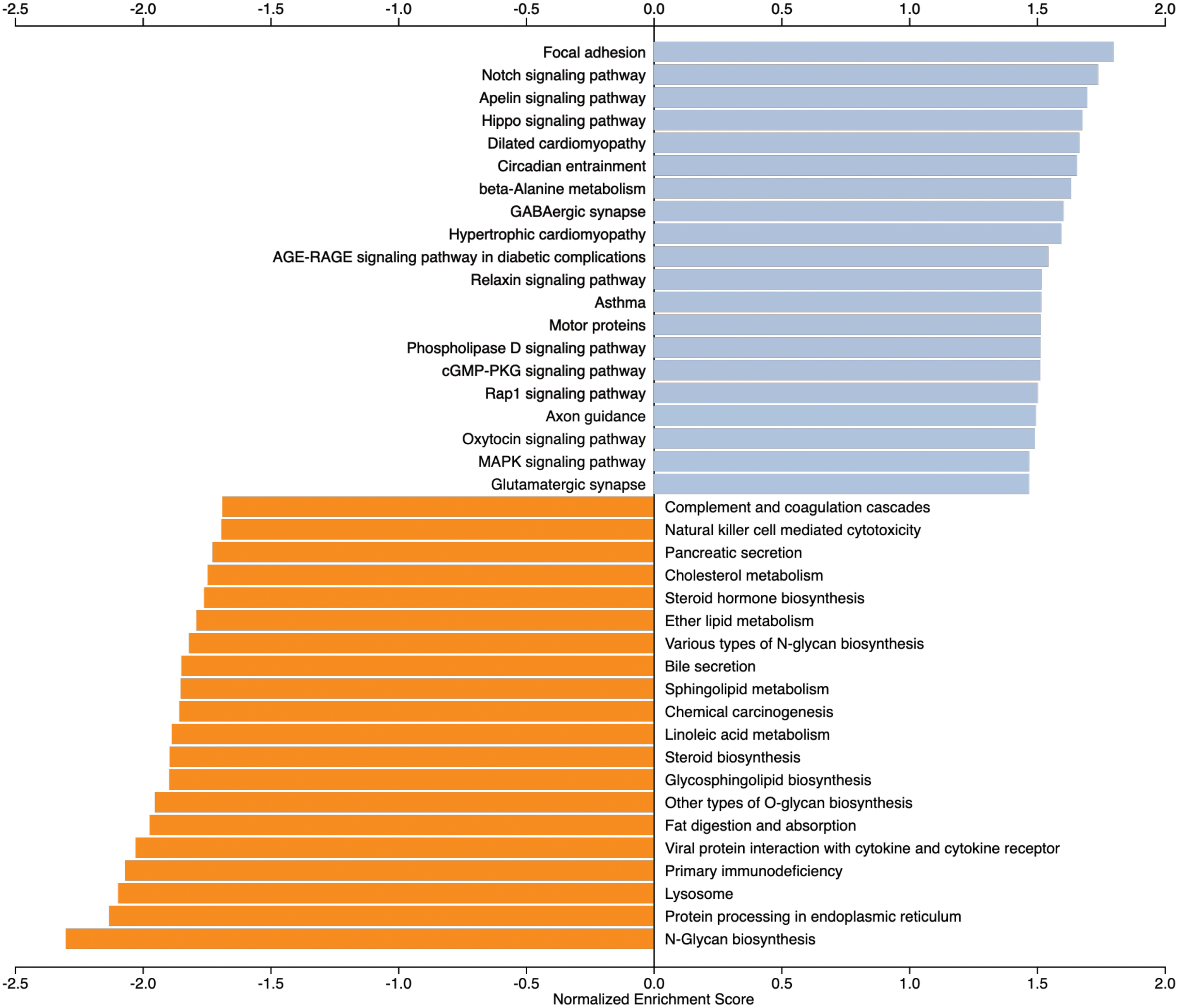
Figure 4. Gene set enrichment analysis-based pathway over-representation of expressed genes from the jejunal mucosa of neonatal beef heifers born from dams receiving or not receiving vitamin and mineral supplementation (VTM or CON) during gestation. The normalized enrichment score (NES) of top enriched (blue bars) and top depleted (orange bars) pathways are based on the comparison of VTM vs. CON calves. Only pathways with a P < 0.05 and NES ≥ |1.5| are shown.
Discussion
We found that maternal vitamin and mineral supplementation throughout gestation affected the expression of 528 genes associated with key metabolic pathways and immune functions in the jejunal mucosa of 30 h old newborn calves. Likewise, the VTM treatment influenced the concentration of Co, Se, and Zn in the serum and Se concentrations in the liver of the offspring calves, despite the fact that the maternal VTM supplementation did not influence the BW or organ mass of the calves. Reference Hurlbert, Menezes and Baumgaertner23 The jejunum is a highly plastic tissue that undergoes morphological and physiological changes throughout gestation. Reference Meyer and Caton13,Reference Meyer, Reed and Vonnahme44 Thus, it is expected that it is responsive to changes in nutrient availability. These changes can differentially program tissue function, affecting energy utilization and maintenance requirements later in life. Reference Reynolds, Ward, Caton, Scanes and Hill15,Reference Prezotto, Lemley and Camacho21 Using the same calves as the ones in the current study, our previous results investigating tissue oxygen consumption indicate an improved mitochondrial function in the small intestine of VTM calves, as evidenced by the greater mitochondrial efficiency of substrate oxidation and ATP production. Reference Menezes, Baumgaertner and Hurlbert45 Collectively, these findings suggest that the observed transcriptomic changes could influence mitochondrial efficiency or vice versa in response to maternal VTM supplementation.
We have previously shown that maternal vitamin and mineral supplementation combined with the rate of body weight gain (GAIN; moderate (MG) vs. low (LG)) increased the liver mass of heifer fetuses at day 83 of gestation. Reference Menezes, McCarthy and Kassetas6 Likewise, there was a VTM × GAIN interaction for fetal small intestinal weight, where fetuses from VTM-MG dams were heavier than those from VTM-LG, with all other treatments being similar. Reference Menezes, McCarthy and Kassetas6 In a follow-up study with a similar experimental design reported here, we showed that vitamin and mineral supplementation throughout gestation improved the performance of F1 female heifers at weaning and post-weaning development, with a significant impact on BW, feeding behavior, and heifer activity. Reference Hurlbert, Baumgaertner and Menezes7 Interestingly, calf birth BW and body measurements were not different. However, calves born to VTM supplemented dams were heavier than that of CON dams from weaning through 15 months of age, Reference Hurlbert, Baumgaertner and Menezes7 suggesting that there might be a lag time from “insult” to observable effect in offspring performance. Additionally, the effects observed may depend on the interaction with other available nutrients and the dam’s nutrient storage, which can buffer the fetus during in utero development. Reference Thayer, Rutherford and Kuzawa46
A growing body of evidence has shown that specific vitamins and minerals set up the functioning of the genome through the modulation of mineral and vitamin-dependent genes or as enzymatic cofactors. Reference Beckett, Yates, Veysey, Duesing and Lucock47 Here, we identified 33 TFs differentially expressed due to maternal diet. Interestingly, 19 were upregulated in the VTM group, which suggests a rewiring of gene networks to modulate the transcriptional output. Reference Pérez-Montarelo, Hudson, Fernández, Ramayo-Caldas, Dalrymple and Reverter48 Our RIF analysis of gene expression retrieved 98 TFs that were differentially co-expressed with DEGs and potential modulators of differential gene expression. ZNF414, HLX, and ATOH8 were the most co-expressed TFs in the network. The protein encoded by the ZNF414 gene has been reported to modulate proliferation, migration, and DNA repair-associated genes. Reference Rodriguez-Martinez, Vuorinen and Shcherban49 Similarly, the HLX TF is required for the development of the enteric nervous system (ENS). Reference Bates, Dunagan, Welch, Kaul and Harvey50 Additionally, it plays a key role in visceral organogenesis, including the liver, gall bladder, and gut. Reference Hentsch, Lyons and Li51 The ENS works cooperatively with other cells to control intestinal homeostasis, Reference Sharkey and Mawe52 which includes nutrient digestion and absorption. Reference Neunlist and Schemann53 The changes in nutrient availability may program tissue development in utero for postnatal function. Reference Meyer and Caton13 Such changes are likely associated with the increased performance post-weaning, as we observed in the contemporary F1 female heifers under a similar experimental approach. Reference Hurlbert, Baumgaertner and Menezes7
Maternal VTM supplementation throughout gestation altered genes involved with nutrient transport, lipid metabolism, and metabolic genes
Vitamins and minerals act as essential cofactors for enzymes that regulate lipid metabolism, highlighting their importance in animal physiology. Reference Diniz W.J., Banerjee and Regitano54 Here, we identified DEGs involved with nutrient transport and fat and lipid metabolism pathways, which included cholesterol metabolism, steroid hormone biosynthesis, and thyroid hormone synthesis. Genes from the SLC and ABC families were mainly downregulated in the VTM group. Among them, the SLC26A3 encodes a key transmembrane chloride ions exchanger. Reference Xiang, Oddy, Archibald, Vercoe and Dalrymple55 The protein encoded by this gene is localized on the columnar epithelial cells Reference Shandro and Casey56 and was reported as important for maintaining the intestinal epithelial barrier function and integrity. Reference Kumar, Priyamvada and Ge57 Decreased expression of SLC26A3 leads to upregulation of antimicrobial peptide expression, altering the immunity protection in young mice. Among the upregulated SLC gene members, SLC22A17 has been associated with the metabolism of drugs and nutrients such as vitamins and flavonoids. Reference Nigam, Bush and Martovetsky58 The ABCG5 and ABCG8 were downregulated in the VTM group. They form an obligate heterodimer that mediates Mg(2+)- and ATP-dependent sterol transport in and out of the enterocytes. Reference Lee, Kinch and Borek59
We identified other genes and pathways related to lipid metabolism. The PLCB1 gene, upregulated in the VTM group, is part of the phospholipase-C family, which requires minerals like Ca and Zn for optimal activity. Reference Singh, Rai and Mathew60 The encoded protein is involved with phospholipid hydrolysis and intracellular transduction of extracellular signals. Reference Liang, Guo and Ma61 This signaling can influence systemic metabolic processes, given that phospholipase-C activity produces inositol triphosphate (IP3) and diacylglycerol (DAG), molecules that regulate calcium signaling and activate protein kinase C pathways in multiple tissues. Reference Bill, Vines and Phospholipase62 Previous studies have associated the PLCB1 gene with feed efficiency, growth, and carcass traits in multiple species. Reference Gao, Zhou and Liu63–Reference Srivastava, Srikanth and Won66 In cattle, PLCB1 was suggested as a candidate to increase fat thickness and weight gain. Reference Srivastava, Srikanth and Won66 The phospholipase D signaling pathway was also over-represented by genes positively ranked in the jejunum tissue based on the GSEA results (genes upregulated in the VTM group). This pathway is involved in fundamental cellular processes that use phosphatidic acid as a secondary messenger Reference Bruntz, Lindsley and Brown67 activating pathways such as mTOR, which controls several processes, including immune response, survival, proliferation, and migration, to maintain cellular homeostasis. Reference Panwar, Singh and Bhatt68 Several pathways underlying fat and lipid metabolism were over-represented by genes negatively ranked within the GSEA analysis (genes downregulated in the VTM group), including fat digestion, absorption, and cholesterol metabolism. From the DEGs acting on these pathways, the PLA2G3 gene was downregulated in the VTM group. The encoded enzyme hydrolyzes phospholipids to release fatty acids and lysophospholipids. Reference Murakami, Yamamoto, Miki, Murase, Sato and Taketomi69 However, the enzyme PLA2G3 also plays a role in immunity-related processes through lipid mediator effects. Reference Murakami, Yamamoto, Miki, Murase, Sato and Taketomi69 Likewise, we identified the LPCAT3 downregulated in the VTM group. This gene is regulated by nuclear receptors, such as the liver X receptor and peroxisome proliferator-activated receptors, and plays an intricate regulatory role in maintaining cellular lipid balance and function. Reference Shao, Qian and Lu70 Modulating lipid metabolism and adipogenesis-related genes could be associated with increased feed efficiency. The observed increased performance of supplemented heifers, particularly the enhanced growth and blood metabolite profiles from weaning through 15 months of age, as reported in Hurlbert et. al., Reference Hurlbert, Baumgaertner and Menezes7 may be attributed to the modulation of lipid metabolism and adipogenesis-related genes, which are associated with improved feed efficiency. Using a similar experimental design, we have reported that calves born to VTM-supplemented dams had a higher weaning weight (17.5 kgs) and a greater ribeye area compared with CON calves at 209 days of age. Reference Hurlbert, Baumgaertner and Menezes7 Therefore, adequate mineral levels throughout gestation are important for the proper functioning of these genes, as well as enhancing overall performance. Further research, however, at different time points is warranted to determine the extent and persistence of any changes observed as a neonate.
Among the DEGs, the cytochrome P450 family was represented by the downregulated genes CYP3A74 and CYP2C87, which are involved with drug metabolism, synthesis of cholesterol, steroids, and other lipids. Reference Lee, Cai, Thomas, Conney and Zhu71,Reference Thelen and Dressman72 Furthermore, the CYP26B1 gene was upregulated in the calves born to VTM dams. The protein encoded by this gene is responsible for retinoic acid (RA) metabolism and homeostasis, intestinal mucosal immune responses, and contributes to vitamin A storage. Reference Chenery, Burrows, Antignano, Underhill, Petkovich and Zaph73–Reference Silveira, Fonseca and Oborn75 We found that vitamin A was increased in the supplemented dams when compared with the CON group at calving, although the calves had similar circulating concentrations of vitamin A at 30 h after birth. Reference Hurlbert, Menezes and Baumgaertner23 While vitamin A is poorly transferred across the placenta, the colostrum would be the primary source for the calf after birth, Reference Quigley and Drewry76 which could elicit different transcriptomic responses. Therefore, further studies should investigate the concentrations of fat-soluble vitamins in the colostrum, their storage in the neonatal liver, and their role in developmental programming.
The insulin-like growth factor (IGF) axis is a complex network involving hormones, receptors, and binding proteins, Reference LeRoith, Holly and Forbes77 which has a key role in growth, metabolism, and intestinal homeostasis. Reference Forbes and Westwood78 The IGF axis also mediates growth hormone (GH) actions. Reference Kuemmerle79 Although no differences were observed in the serum concentrations of GH and IGF-1 in the dams and calves, Reference Hurlbert, Menezes and Baumgaertner23 we identified the IGFBP2 and IGFBP6 genes upregulated in the VTM group. The IGF binding proteins (IGFBP) are physiologic regulators of the interaction of IGFs with their receptors within the gastrointestinal tract and liver. Reference Kuemmerle79 Changes in the expression of IGFBPs can influence IGF activity by altering its bioavailability and interaction with receptors, though this does not directly equate to changes in IGF concentrations. Reference Kuemmerle79 Additionally, minerals such as Se and Zn were reported as determinants of IGF-1 activity. Reference Maggio, De Vita and Lauretani80 Similarly, a relationship between IGF-1 concentrations in serum and vitamin D concentrations has been shown. Reference Gou, Li, Qiao, Maimaititusvn and Liu81 IGFBP2 has been associated with regulating IGF activity in most tissues and organs. Likewise, it can promote transcriptional activation of target genes and contribute to growth and development. Reference Kuemmerle79 Among the regulatory activities, IGF-2 interacts with components of the extracellular matrix, cell surface proteoglycans, and integrin receptors to mediate intracellular signaling. Reference Yau, Azar, Sabin, Werther and Russo82,Reference Faramia, Hao and Mumphrey83 Corkins et al. reported that fetal sheep intestinal fibroblasts were responsive to IGF-2 with greater proliferation. Reference Corkins and Fillenwarth84 The IGF-2 has a role in glucose and lipid metabolism through IGF bioavailability. Reference Faramia, Hao and Mumphrey83 The knockdown of IGFBP6 leads to impaired lipid metabolism and decreases cholesterol biosynthesis. Furthermore, the encoded protein has been reported as an important factor in the immune and inflammatory responses. Reference Laselva, Criscione, Allegretta, Di Gioia, Liso and Conese85,Reference Liso, Venuto, Coda, Giallongo, Palumbo and Tibullo86
Maternal VTM supplementation throughout gestation downregulate genes involved with immune-related pathways and biological processes in neonatal calves
The intestinal epithelium provides support for nutrients, water uptake, and physical barrier protection. Reference Jeon, Klaus, Kaemmerer and Gassler87 Furthermore, the intestine represents the largest compartment of the immune system. Reference Guo, Li and Jia88 Although developmental adaptations during early life coordinate immune function and development, Reference Cangiano, Villot, Guan, Ipharraguerre and Steele22 it has been proposed that the immune system can be programmed in utero. Reference Meyer and Caton13,Reference Meyer, Reed and Vonnahme44 Carroll et al. Reference Carroll, Burdick Sanchez and Broadway89 reported that exposure to multiple low doses of LPS endotoxin in utero had lasting effects on pro-inflammatory and innate immune responses postnatally. The role and importance of minerals in modulating the functioning of the immune system have been discussed in the literature. Reference Cunningham-Rundles, Lin, Ho-Lin, Dnistrian, Cassileth and Perlman90,Reference Weyh, Krüger, Peeling and Castell91 The integrity and functioning of the gut barrier rely on multiple interconnected systems, such as a mucous gel layer, IgA, and junctional proteins. Reference Fakhoury, Kvietys and AlKattan92,Reference Suzuki93 We identified focal adhesion, notch, and hippo pathways over-represented by positively ranked genes. These signaling cascades play multiple roles in the maintenance and function of the epithelial cell types. Reference Jeon, Klaus, Kaemmerer and Gassler87 Differential expression of five genes encoding junction proteins included the claudin (CLDN2 and CLDN12) and connexin (GJA5, GJB3, and GJC1) gene families.
Among the DEGs, the downregulation of SGPP2 has the potential to positively influence the gut barrier defense. Reference Huang, Liang and Nagahashi94 Studies in knockout mice have shown that SGPP2 deficiency enhances mucosal barrier integrity and barrier function. Reference Huang, Liang and Nagahashi94 We also identified several upregulated genes encoding structural proteins, cytoskeleton organization, and tissue development (GAJ5, MYL7, MYL9, MYO5C, DNAH9, TUBB6, TNNT3, and MAST1). Genes positively ranked within the GSEA analysis (genes upregulated in the VTM group) were over-represented in the focal adhesion pathway. These results may suggest the continuous cell turnover, renewal, and differentiation in the intestinal epithelium due to significant changes in enteral nutrition. The CATHL2 gene was reported to exhibit antimicrobial activity against various pathogens, Reference Chandel, Singh and Bhardwaj95 and was identified as downregulated in the VTM group. While primarily associated with immune function, peptides like CATHL2 may indirectly influence gut barrier integrity by modulating the microbiota and defending against pathogenic invasion, possibly impacting aspects of metabolism and feed efficiency indirectly through effects on gut microbiota. Reference Kościuczuk, Lisowski and Jarczak96 From the same calves used in the current study, we have reported that the offspring microbial community in the rumen (ruminal fluid and tissue-associated microbiota) was affected by the maternal vitamin and mineral supplementation. Reference Luecke, Holman and Schmidt97 Such changes were related to the community structure (ruminal fluid), diversity (ruminal fluid and tissue), and composition, suggesting the role of maternal diet during fetal development on the initial microbial colonization of the neonatal calf gut. Reference Luecke, Holman and Schmidt97 Research using the intestinal epithelial cells from mice reared in the presence or absence of microbiota has shown changes in the host gene transcription. In cattle, host-microbial interactions in the rumen were associated with genes underlying immune system-related biological processes. However, the regulatory mechanism between this interaction and the role of nutrients remains unclear. Thus, understanding the interplay between maternal nutrition and the offspring’s intestinal function provides opportunities to positively program the offspring outcomes.
Neonates are immunocompetent at birth and rely on the passive transfer of immunoglobulins (Ig) from maternal colostrum to acquire protection. Reference Cangiano, Villot, Guan, Ipharraguerre and Steele22 We identified multiple biological processes associated with the immune system and inflammatory responses, including the intestinal immune network for the IgA production pathway. The IgA antibodies mediate the humoral adaptive immune response at mucosal surfaces, serving as the first line of defense against microorganisms. Reference Holmgren and Czerkinsky98,Reference Penny, Domingues and Krauss99 Overall, the underlying genes acting on immune-related biological processes and pathways were downregulated in the VTM calves, except for those on the cytokine-cytokine receptor interaction pathway, in which six out of eight genes were upregulated. The GSEA analysis showed the same downregulation pattern with the pathways over-represented by negatively ranked genes. We have reported that among 15 tested serum cytokines, the concentration of IP-10 was increased while IL-4 and IL-17A tended to be decreased in VTM calves compared with CON. Reference Luecke, Holman and Schmidt97 Among the interleukin-related genes, we found IL2RB, IL17F, IL1RN, IL18RAP downregulated and ILDR2 upregulated in the VTM group. It is possible that the pro-inflammatory and anti-inflammatory cytokines produced by the intestine would affect their levels in the blood. However, the panel used was limited to only 15 cytokines. Further research is warranted to fully understand the effects of minerals and vitamins on immune response.
The list of upregulated DEGs with regulatory impact effects on differential expression included the ATOH8 gene. This TF acts in fundamental processes and functions as both a transcriptional activator and a repressor. Reference Divvela, Saberi and Brand-Saberi100 Additionally, ATOH8 acts as a regulator of intestinal microfold cells (M cells), which are responsible for immune sensing in response to intestinal pathogens/antigens. Reference Ohno101 The M cells are found in the epithelium, covering mucosa-associated lymphoid tissues. Reference Corr, Gahan and Hill102 In addition to the M cells, the mucosal surface is protected by physical shields composed of mucin and glycocalyx layers and chemical barriers such as antigen-specific IgA. Reference Kobayashi, Takahashi, Takano, Kimura and Hase103 We identified several chemokines downregulated in the VTM calves, except for the CCR10, which was upregulated. The protein encoded by the CCR10 gene regulates the intestinal IgA response and memory maintenance. Reference Hu, Yang, Yang, Li and Xiong104 Chemokines and chemokine receptors are key players in attracting lymphocytes and leukocytes to lymphoid tissues. Reference Mabbott, Donaldson, Ohno, Williams and Mahajan105 We found leukocyte-mediated immunity, leukocyte activation, and lymphocyte-mediated immunity among the over-represented biological processes.
The immune system is a complex, tightly regulated, cooperative network that requires a balance between positive and negative immune modulators. Reference Chase, Hurley and Reber106,Reference Kaushik, Kandavel, Nalpathamkalam and Pasman107 Nutrients, such as vitamins and minerals, play unique regulatory roles in the programming of the immune system during in utero fetal development, with potential effects on the postnatal immune response. Reference Cunningham-Rundles, Lin, Ho-Lin, Dnistrian, Cassileth and Perlman90 Therefore, the implications of maternal nutrition on turning on or off these genes warrant further investigation.
Our results highlight that maternal VTM supplementation throughout gestation can have a significant impact on many important genes associated with tissue structure, nutrient transport, and immune system pathways. Although individual changes in gene expression may not have major phenotypic impacts, our analysis showed changes in several TFs, which can have downstream effects on regulatory pathways. Additionally, our in-silico analysis pointed out key transcriptions factors that are likely modulating the differences in gene expression. While this study provides novel insights into the potential programming of jejunum development at birth, it has some limitations. Our study relied on a single-time-point analysis, which limits the ability to capture the dynamic changes in gene expression. Thus, to provide a comprehensive understanding of gene expression dynamics and interactions, longitudinal studies at multiple pre and postnatal time points are necessary to determine whether the observed gene expression changes persist and lead to lasting physiological effects. Additionally, our study was limited to the jejunal mucosa, and further research is needed to investigate the impact of maternal supplementation on other tissues and organs integral to growth and metabolism. The long-term implications in animal health due to the downregulation of immune-related genes in the supplemented group warrant further research. Likewise, research is needed to determine the mechanisms involved with fetal programming and whether the extensive alterations in immune-associated transcript abundance at the neonatal timepoint are sustained into further postnatal life and culminate into alterations in long-term outcomes on cattle herd health.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2040174425000157.
Data availability statement
All relevant data are within the paper and its Supplementary Information files. All additional datasets generated and analyzed during this study are available from the corresponding author upon reasonable request.
Acknowledgements
The authors would like to thank the North Dakota State Board of Agricultural Research and Education and the North Dakota Agricultural Experiment Station for their support of this effort and additional product support from Zoetis Animal Health (Parsippany, NJ, USA) and ST Genetics (Navasota, TX, USA). Appreciation is expressed to personnel at the Central Grasslands Research Extension Center, the NDSU Beef Cattle Research Complex, and the Animal Nutrition and Physiology Center for assistance with animal handling and feeding. This work used resources from the Center for Computationally Assisted Science and Technology (CCAST) at North Dakota State University and the Auburn University Easley Cluster.
Author contributions
Conceptualization, CRD, ACBM, KCS; Formal analysis, AJC, WJSD, PB; Funding acquisition CRD, ACBM, KCS; Investigation, CRD, ACBM, JLH, PB, FB, KAB, SA, KKS, KCS; Methodology, CRD, ACBM, JLH, PB, FB, KAB, SA, KKS, KCS, WJSD; Project administration, C.R.D.; Software, AJC, PB, WJSD; Supervision, CRD, WJSD; Writing — original draft, AJC; Writing — review and editing: all authors. All authors have read and agreed to the published version of the manuscript.
Financial support
This research was funded by the United States Department of Agriculture National Institute of Food and Agriculture’s Agriculture and Food Research Initiative Award #2022-67016-36479 and the North Dakota State Board of Agricultural Research and Education. A. J. Craner was funded through the National Needs Graduate and Postgraduate Fellowship Program (USDA-NIFA-NNF: #2021-38420-34060). Financial support for W.J.S.D. was provided by the Agricultural Research Service, U.S. Department of Agriculture, under Agreement No. 58-6010-1-005, by the Alabama Agricultural Experiment Station - Hatch program of the National Institute of Food and Agriculture, U.S. Department of Agriculture.
Competing interests
The authors declared no conflict of interest. The funders had no role in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results that were done entirely independently by North Dakota State University and Auburn University personnel.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of animals and have been approved by the North Dakota State University Institutional Animal Care and Use Committee (#A21047).






