Introduction
Penicillin allergies are reported by approximately 10% of patients. However, clinically significant IgE-mediated penicillin hypersensitivity reactions are found in <5% of patients. Reference Khan, Banerji and Blumenthal1 True IgE-mediated allergic reactions to penicillin often present with rapid onset of urticaria, respiratory distress, angioedema, and/or hypotension after exposure, and may lead to anaphylaxis. Many patients are inappropriately labeled as penicillin allergic for various reasons, including experiencing an adverse drug effect such as nausea, vomiting, or diarrhea, rather than an allergic reaction. Additionally, approximately half of patients with a true IgE-mediated penicillin allergy lose sensitivity five years after reacting, and 80% lose sensitivity in 10 years. Reference Khan, Banerji and Blumenthal1,Reference Bernstein, Gruchalla, Lee, Nicklas and Dykewicz2 There are many barriers and limitations to addressing penicillin allergy labels, including accessibility of formal drug allergy evaluations, provider time constraints, lack of education, insufficient training, and patient hesitation to challenging their drug allergy label. Reference Stone, Trubiano, Coleman, Rukasin and Phillips3 Thus, providers generally avoid prescribing beta-lactam antibiotics if a penicillin allergy label is present. Reference Khan, Banerji and Blumenthal1,Reference Castells, Khan and Phillips4
The American College of Obstetricians and Gynecologists (ACOG) recommends the use of intravenous penicillin G for intrapartum group B Streptococcus (GBS), a common gram-positive organism that colonizes the genitourinary tract. 5 Women are screened for this between 36- and 37-weeks gestational age. It is estimated that 10% to 30% of pregnant women are colonized at any given time, which increases the risk of intraamniotic infection, endometritis, preterm labor, and stillbirth. Additionally, GBS can be transmitted to the fetus at the time of labor and delivery resulting in infection of the newborn. This can lead to complications such as apnea, need for blood pressure support and need for neonatal intensive care, as well as neonatal death. Reference Stoll, Hansen and Sánchez6 Antibiotic prophylaxis with intravenous penicillin G during labor reduces the likelihood of transmission of GBS to the fetus. Penicillin G is preferred due to its narrow spectrum of activity against gram-positive bacteria, which reduces the likelihood of resistance by other vaginal organisms. 5 For women with a listed penicillin allergy, cefazolin is the preferred alternative. Although cefazolin is also a beta-lactam antibiotic, its structure creates a low risk for cross-sensitivity and updated guidelines support the use of cefazolin for patients with a penicillin allergy label, regardless of their reaction history. Reference Khan, Banerji and Blumenthal1 However, this is a fairly new concept and some obstetricians may feel more comfortable using clindamycin or vancomycin for patients with a more severe penicillin allergy. 5,Reference Shenoy, Macy, Rowe and Blumenthal7 As these agents provide broad spectrum coverage, antibiotic resistance and the potential for adverse drug reactions are a concern. Reference Khan, Banerji and Blumenthal1,Reference Castells, Khan and Phillips4
Given the large percentage of patients that may be able to tolerate a penicillin despite having a penicillin allergy label, allergy evaluation and testing is now recommended for pregnant women. Reference Wolfson, Mancini and Banerji8 As this practice has grown over the last fifteen years, literature is available regarding rates of allergy de-labeling in pregnancy. Reference Wolfson, Mancini and Banerji8–Reference Trubiano, Vogrin and Chua13 At our healthcare system, the allergy/immunology division has worked with obstetricians, the antimicrobial stewardship team, and pharmacy to address evaluating penicillin drug allergies in obstetrics patients. In June 2021, through a joint effort that also included representatives from the hospital information technology team, an electronic medical record (EMR) best practice advisory (BPA) was implemented to recommend and assist with referral of obstetric patients who report a penicillin allergy to an outpatient drug allergy clinic (DAC) (Figure 1). The purpose of this study was to evaluate the safety of penicillin allergy evaluation and testing in obstetric patients utilizing oral drug challenges with or without prior penicillin skin testing (PST) depending on characteristics of the patient’s medication reaction, and to determine whether removal of the drug allergy label led to an increased use of first-line guideline-recommended antibiotic prophylaxis for group B Streptococcus (GBS).
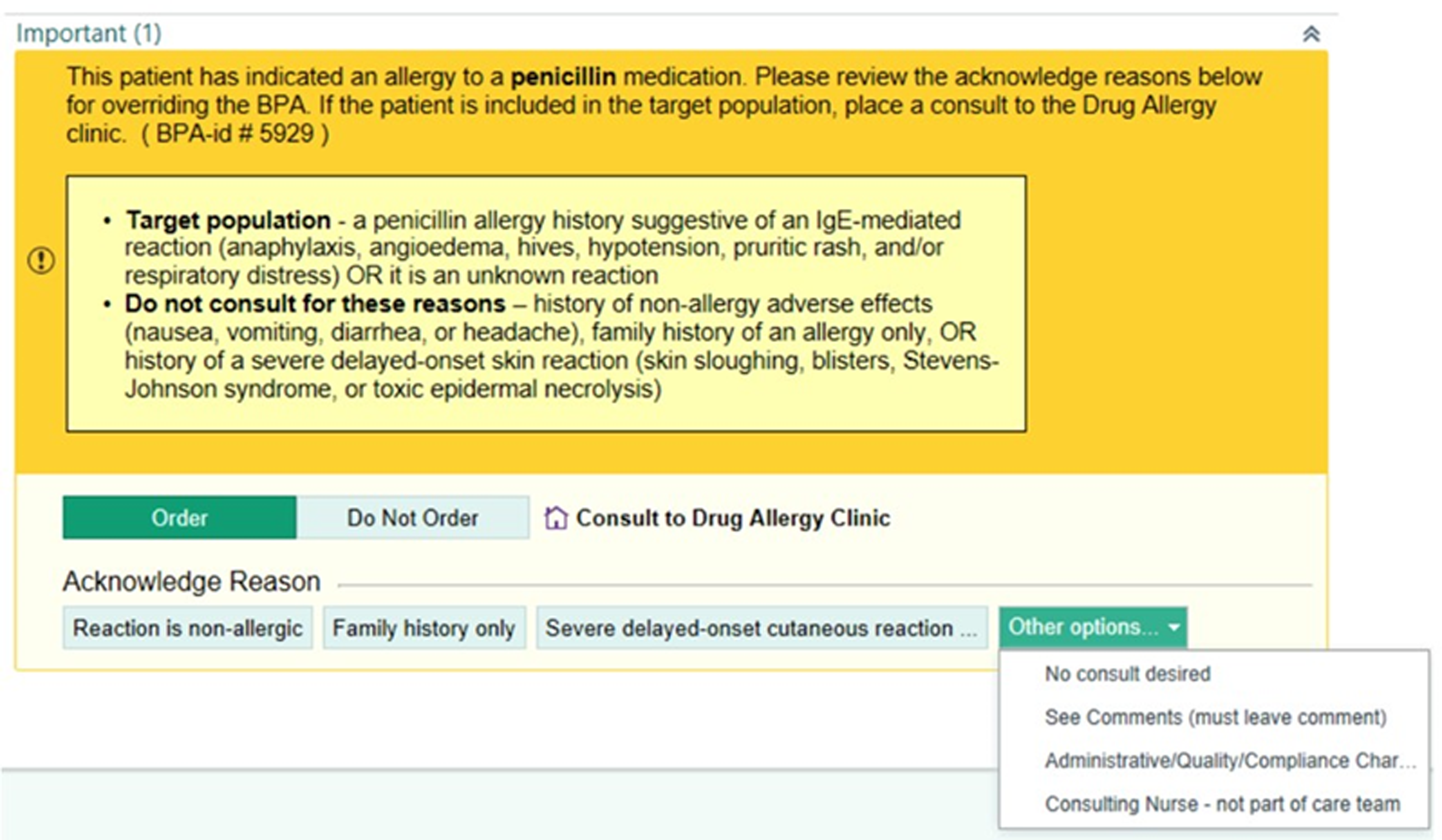
Figure 1. Best Practice Advisory. The best practice advisory (BPA) created at our institution to alert obstetric staff of a pregnant patient’s penicillin allergy.
Methods
We conducted a single-center, retrospective cohort study of obstetric patients identified by an EMR BPA to have a penicillin allergy label between June 30, 2021 and July 1, 2023. Approval to conduct the study was obtained from the University of Iowa Institutional Review Board.
In the DAC, patients were asked to provide a detailed history of their reaction to the penicillin derivative. If the characteristics of the drug reaction suggested a recent or severe IgE-mediated allergy, it was recommended that the patient undergo PST with penicillin G and Pre-Pen® (benzylpenicilloyl polylysine). If testing was negative, the patient underwent a subsequent single-dose oral drug challenge to amoxicillin or a penicillin derivative, with observation for 30 minutes. Patients with a lower risk allergy, defined as a benign cutaneous reaction, such as a morbilliform drug eruption or isolated urticaria, that occurred more than 5 to 10 years earlier, were offered the option to undergo a direct drug challenge to a penicillin with observation for 60 minutes, without prior skin testing. This practice is supported by literature and the 2022 updated Drug Allergy Practice Parameter. Reference Khan, Banerji and Blumenthal1 Although this parameter was released during the period of study, clinicians in the DAC used this as a practice standard for several years beforehand. The type of testing employed was determined by the type and severity of reaction, time since the reaction occurred, and patient preference. If the patient had a negative PST and subsequent direct drug challenge with no reaction, tolerated a supervised direct drug challenge alone, or if based on history alone the label was deemed not to be an allergy, the allergy label was removed from the patient’s medical record. Patients who reported a cephalosporin allergy in addition to a penicillin allergy underwent evaluation of the cephalosporin allergy, generally during a second appointment. The focus of this study, however, was the results of the penicillin allergy evaluation. Patients who were not seen in the DAC were categorized based on the penicillin drug allergy label that was recorded in the EMR.
GBS status prior to delivery and information about labor and delivery was collected from the EMR for all patients for whom the BPA alert was triggered by July 1, 2023 and who delivered by November 1, 2023 if these details were known. Labor and delivery information included the date and type of delivery, the choice of prophylactic antibiotic(s) and reason(s) for use, any adverse drug reactions for chosen antibiotics, and newborn culture results within 7 days of delivery. For women who had more than one delivery in the two-year time frame, only the first delivery that occurred after being evaluated in the DAC was included. We compared the antibiotic prophylaxis used during labor and delivery by patients who were seen in the DAC and de-labeled, those who were seen but not de-labeled, and those for whom the BPA fired but were not seen in the DAC. We also calculated a validated PEN-FAST Score to help stratify the risk of a patient’s drug allergy. Reference Trubiano, Vogrin and Chua13
The primary outcome was the percentage of obstetric patients seen in the DAC with a reported penicillin allergy who safely underwent oral drug challenge with or without prior PST. Secondary outcomes included the percentage of patients seen in the DAC who were able to have their penicillin allergy removed and the percentage of patients able to use first-line guideline-recommended therapy for GBS prophylaxis because of allergy label removal by the DAC. Additionally, we assessed the rate of neonatal GBS colonization or infection in the first 7 days of life.
Data were collected utilizing Microsoft Excel and analyzed with IBM SPSS Statistics (Version 29). We used Student’s t-test, Chi-Squared as well as Fischer’s exact tests for data analysis. A P value of ≤0.05 was considered statistically significant.
Results
During the study period, the BPA fired for 728 patients who were seen in one of the seven obstetric clinics within our health care system. The demographics and allergy history of the patients seen in the DAC and those who were not seen were similar (Table 1). A referral was placed for 549 (75.4%) patients for whom the BPA fired, and 299 (41.1%) were seen in the DAC, while 429 (57.4%) were not seen in the DAC for various reasons (Appendix 1). Most of the patients seen in the DAC were in the third trimester of pregnancy, had a reaction during childhood, and were at low risk for a positive penicillin allergy test based on their PEN-FAST score (Table 2).
Table 1. Demographics
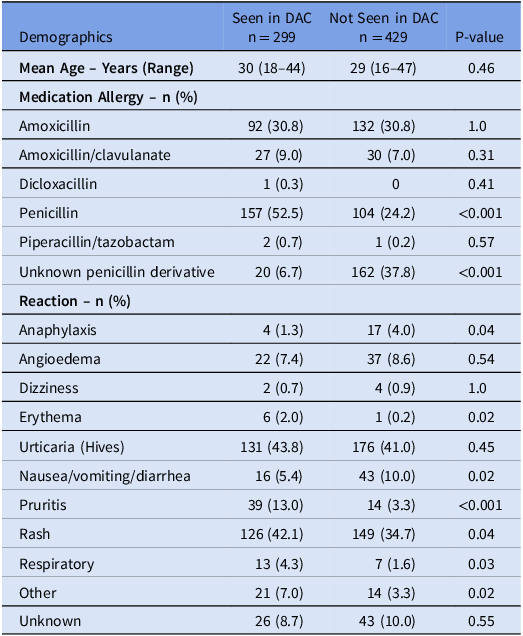
Table 2. Allergy information for those seen in drug allergy clinic

* PEN-FAST Score: 0 (very low risk of positive penicillin allergy test <1%), 1-2 (low risk of positive penicillin allergy test 5%), 3 (moderate risk of positive penicillin allergy test 20%), 4-5 (high risk of positive penicillin allergy test 50%)Reference Trubiano, Vogrin and Chua13
Of the 299 patients evaluated in the DAC, the majority of patients were able to undergo direct oral challenge (Figure 2). Thirty-two patients (10.7%) did not undergo further evaluation for various reasons, the most common being the ability to rule out an IgE-mediated reaction based on history. A total of 108 PSTs were performed and 105 (97.2%) were negative. One hundred of the patients with a negative skin test went on to do an oral drug challenge and 5 patients declined an oral drug challenge with plans to return for testing after pregnancy due to personal preference or time constraints. Two hundred fifty-nine patients underwent an oral challenge with amoxicillin (95.4%), amoxicillin/clavulanate (4.2%), or penicillin VK (0.4%). Two hundred fifty-six (98.8%) patients had no reaction to the challenge and 3 (1.2%) patients had an observed reaction after the challenge. None of the patients that had a reaction to the oral challenge had anaphylaxis and thus, no patient required epinephrine. Two hundred seventy (90.3%) patients had their drug allergy label removed after being seen in the DAC. Twenty-nine patients were not de-labeled for various reasons (Table 3), including 3 with a positive skin test, and 3 that had an immediate reaction to the oral challenge (Table 4).
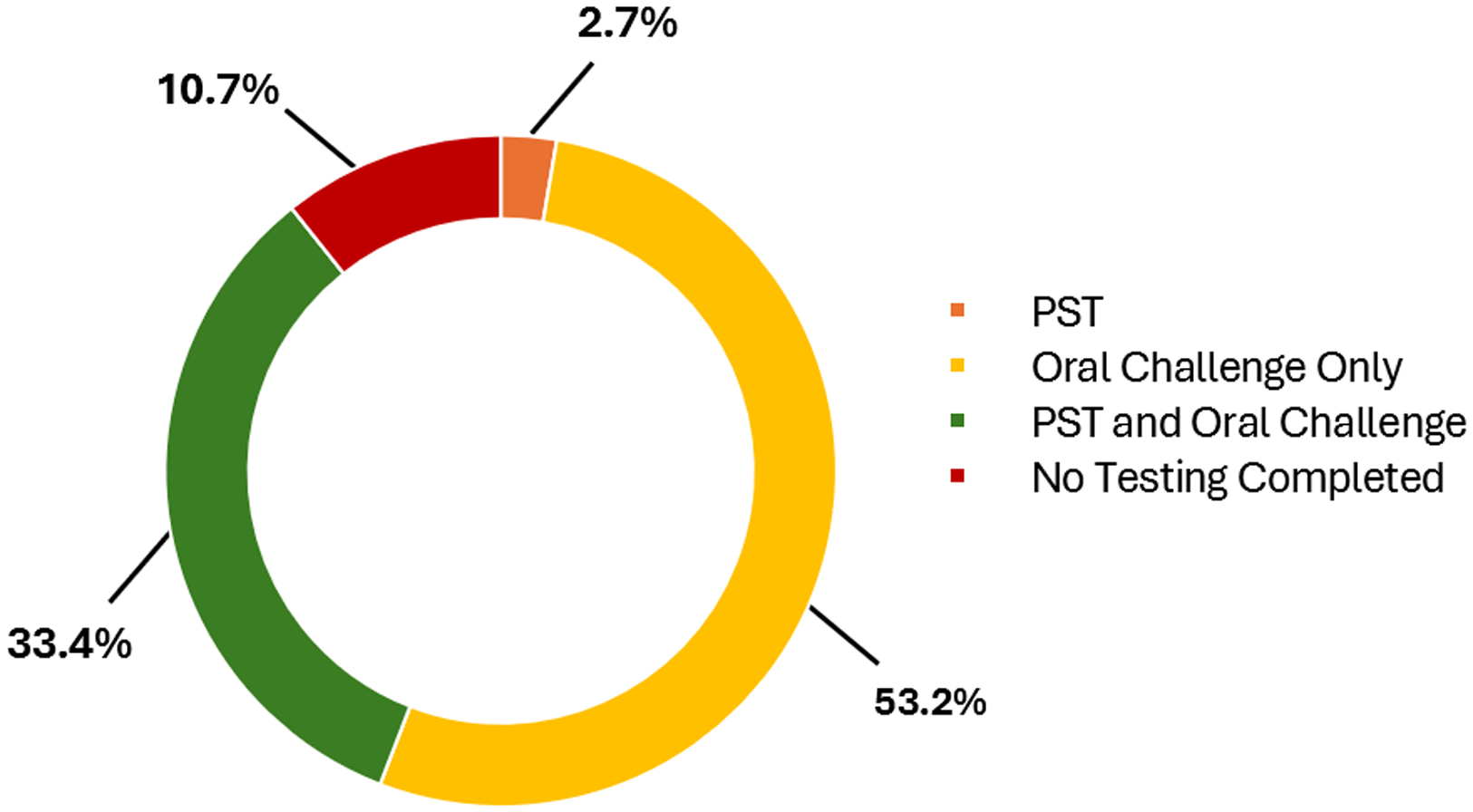
Figure 2. Testing done in the drug allergy clinic. Testing completed during drug allergy evaluation. PST: Penicillin Skin Testing.
Table 3. Reason patient was seen but not de-labeled (n = 29)
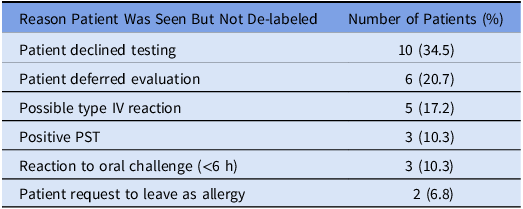
Table 4. Patients with positive oral challenge or penicillin skin testing results
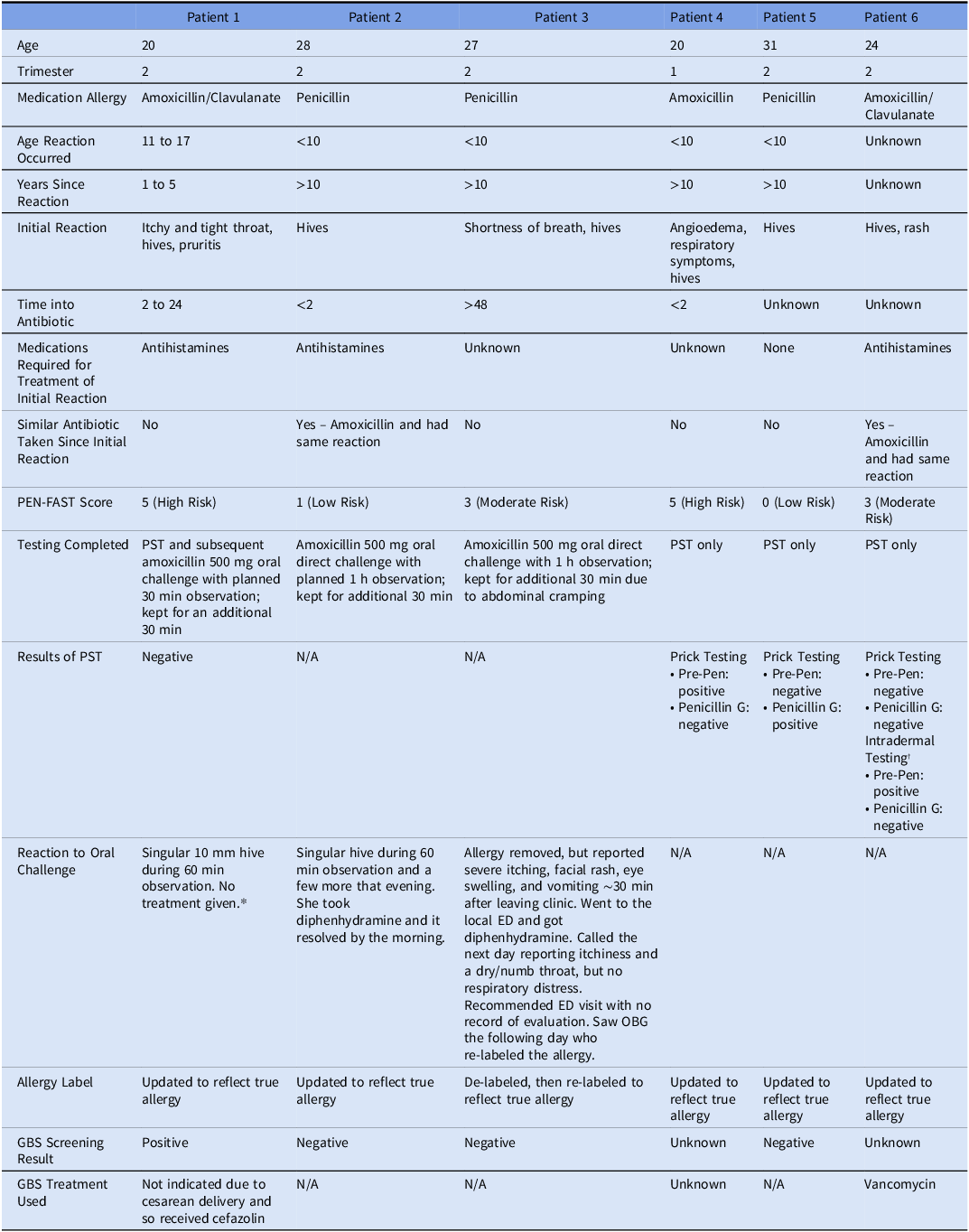
* Patient had a negative skin test and still developed 1 round lesion on her palm in the 30 min observation.
† She experienced a wheal to skin prick testing to both penicillin G and Pre-Pen, however, she also had a wheal with her negative control (though not as impressive as the antibiotic component). Given this, we opted to proceed with intradermal testing (non-duplicative). This returned positive to Pre-Pen. This is indicative of continued sensitization to penicillins.
Screening results for GBS were obtained for 615 of the 728 BPA-identified patients. There were 113 patients with unknown GBS status at the time of delivery due to various reasons, primarily early delivery prior to GBS testing or late pregnancy care at an outside hospital. One hundred and one (16.4%) required GBS prophylaxis due to GBS PCR positivity or unknown GBS status at delivery. This excludes patients who underwent planned cesarean delivery as GBS prophylaxis was not indicated. Of the 48 patients requiring GBS prophylaxis who were previously seen in DAC, 42 (87.5%) received penicillin G. Significantly more patients seen in the DAC compared to those not seen in the DAC received penicillin G (87.5% vs 24.5%, P < 0.001). The majority of patients not seen in the DAC received cefazolin (54.7%) for GBS prophylaxis and 10 received second-line antibiotics including vancomycin (17%) or clindamycin (1.9%). It should be noted that 14 patients not seen in the DAC received a penicillin (Figure 3). The reasons for ordering a penicillin derivative despite the allergy label are unknown. No patients had a drug allergy reaction after administration of an antibiotic during labor. Additionally, none of the infants of mothers who were GBS positive had cultures that were positive for GBS in the first 7 days of life.
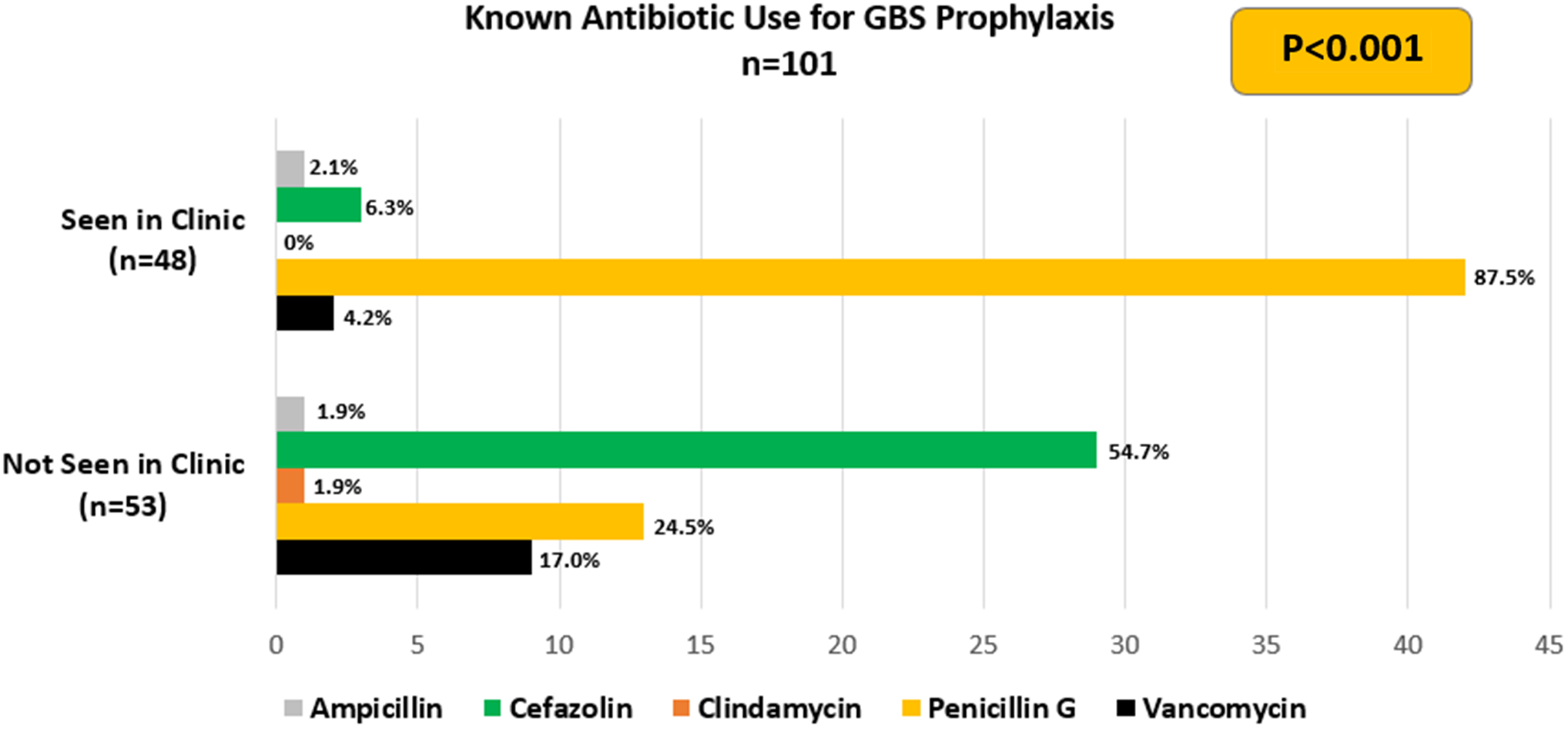
Figure 3. Antibiotic use. The antibiotic used for GBS prophylaxis during delivery.
Discussion
The purpose of this study was to evaluate the safety of penicillin allergy evaluation and testing in obstetric patients utilizing oral drug challenges with or without prior penicillin skin testing (PST) depending on characteristics of the patient’s medication reaction, and to determine whether removal of their drug allergy label led to an increased use of guideline-recommended antibiotic prophylaxis for GBS if indicated. Our study supports the concept of penicillin allergy testing and adds to the literature on the safety of drug allergy testing during pregnancy specifically. The rate of allergy de-labeling was consistent with other studies. Reference Wolfson, Mancini and Banerji8,Reference Patel, Gleeson, Delaney, Ralston, Feldman and Fadugba10–Reference Trubiano, Vogrin and Chua13 In September 2022, the updated Drug Allergy Practice Parameter was released and de-emphasized skin testing prior to oral challenge. Reference Khan, Banerji and Blumenthal1 While this practice was undertaken in the DAC prior to the Parameter release, an increase was seen after the release; in this study, 68% of patients underwent a direct oral challenge after the release, compared to 33.3% of patients seen prior to the release. This was a unique feature of our study, with over half (53.2%) of patients undergoing a direct oral challenge without prior PST over the entire study period. We did not see a difference in the type of allergy testing completed based on trimester of pregnancy. Only 6 of our patients had a true penicillin allergy after evaluation, 3 with positive skin testing and 3 with a reaction after the oral challenge. Only 2 received antihistamines in clinic, and none developed anaphylaxis or required hospitalization. Additionally, none of the patients who required GBS prophylaxis had an allergic reaction to the antibiotic during labor and delivery.
Our results demonstrate that the use of first-line antibiotic prophylaxis for GBS was increased in patients who were seen in the DAC, as most patients who reported penicillin allergies were not truly penicillin allergic or had lost sensitization through time decay. In our study, 270 (90.3%) patients were de-labeled after consultation in the DAC. As a result, 42 of the 48 patients whose penicillin allergies were de-labeled and for whom GBS prophylaxis was indicated received penicillin G at delivery and 1 received another type of penicillin. Notably, the most frequently administered medication for patients not seen in clinic was cefazolin, which is a cephalosporin and beta-lactam antibiotic. Based on literature demonstrating an extremely low cross-reactivity rate, and changes to guidelines, our institution approved an update to our institutional beta-lactam allergy guideline in September 2021. Reference Khan, Banerji and Blumenthal1,Reference Macy9 The guideline suggested that cefazolin can be utilized in the setting of a penicillin allergy, regardless of the severity of the reaction. This was communicated hospital-wide, with particular targeting of surgical staff. This likely contributed to an increase in cefazolin utilization in our patient population and a decrease in the use of clindamycin and vancomycin. These results indicate that de-labeling patients prior to delivery does increase the use of first-line prophylaxis. The reduction in the use of second-line antibiotics is important because, although these agents can be effective for prophylaxis, they have toxicities such as ototoxicity and nephrotoxicity. These toxicities can lead to longer hospital stays and readmissions. Additionally, the use of broader spectrum antibiotics can lead to antibiotic resistance which can lead to difficulty treating infections later in life. However, these antimicrobial stewardship efforts to support the use of cefazolin for penicillin allergic patients was a potential confounder during the study period. In addition to decreasing the use of broader spectrum antibiotics, it may have decreased the obstetrician’s desire to place a DAC referral, given that they could give cefazolin to penicillin allergic patients for GBS prophylaxis or for cesarean delivery prophylaxis. Regardless of the antibiotics used, we saw no cases of early-onset neonatal GBS.
One limitation of our study is that it was likely underpowered to see an impact on the incidence of neonatal GBS colonization. A second limitation was that we only could rely on the description of the allergy label in the EMR for patients who were not seen in the DAC. A third limitation is the majority of patients referred to the DAC were not seen for various reasons, most of which were unknown. Some reasons include no-shows, cancelling appointments, and inability to schedule patients (either due to limited clinic availability prior to delivery or personal conflicts with the DAC schedule). Additionally, we are a referral institution from other remote obstetric offices for more complex patients, which is a barrier for a DAC visit. Lastly, the current workflow in the DAC does not include any steps to remove a patient’s drug allergy from outside EMRs and pharmacy dispensing systems. If our patients were to deliver at another hospital, they may not receive penicillin G or cefazolin due to the drug allergy remaining on their local chart. Patients are given a drug allergy wallet card to show their providers and pharmacies that they no longer have a penicillin allergy, but this may not always be done. It could be beneficial to call or fax a patient’s primary care provider and pharmacy after their appointment to discuss removing the drug allergy in their system.
Our study reinforces the safety of drug allergy testing in obstetric patients. Most patients are appropriate candidates for a direct oral challenge only or PST followed by an oral challenge. Most of these patients (90.3% in our study) will have their drug allergy label removed and only a small number will have evidence of a true IgE-mediated reaction (2.0% of the patients seen for drug allergy testing in our study). Additionally, the likelihood of anaphylaxis in response to drug allergy testing or with subsequent penicillin use during labor and delivery after de-labeling is rare (affecting no patients in our study). Overall, evaluation in the DAC led to increased use of first-line GBS prophylaxis. This study highlights the work of an interdisciplinary team to create and implement a BPA that assisted in effecting a change in practice. This required collaboration between obstetricians, allergists, infectious diseases specialists, pharmacists, nurses, medical assistants, and healthcare information technologists to succeed.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ash.2025.10033.
Acknowledgements
None.
Financial support
None reported.
Competing interests
All authors report no conflicts of interest relevant to this article.








