Metabolism Is a Very Big Subject
Everyone seems to know about metabolism.
If I let slip at a social occasion that my job is researching into human metabolism, there are not many people who will not express an interest. A typical conversation might be:
Or, more rarely,
But the idea that ‘metabolism’ is just concerned with how fast, or slowly, we might burn off excess energy is a very restricted one. A commentator announced recently on the radio that ‘the economy has slowed by 30%’ (as a result of the coronavirus pandemic). I suppose this refers to the Gross Domestic Product, GDP. But I don’t know what things contribute to this, or how they interact and how each is regulated. My wife and I try to buy produce from local shops, thinking we are doing some good for ‘the local economy’. I guess the overall economy is made up of many ‘local economies’, together with other things like, for instance, manufacturing and garbage disposal. If that is so, then there are many parallels with metabolism. I am guessing that my understanding of economics is very similar to most people’s understanding of metabolism. Yes, there are ways of capturing an ‘overall’ figure for a person’s metabolism, but that in turn is made up a myriad of smaller components. And we can’t understand the ‘overall’ picture, let alone how it might change, without having some knowledge of these components that contribute to it.
Almost every component of our bodies is the result of a metabolic process. The DNA that constitutes our genes is made from smaller components, each of which can be manufactured in our cells. The proteins that make up much of our cells are made from amino acids: there are 20 common amino acids in our proteins, and a few that occur in smaller amounts. Many of these amino acids can be made in our cells from other components, or interconverted, and they can all be broken down in our cells in metabolic processes. These metabolic processes are covered in specialised textbooks of biochemistry, but clearly it would be impossible to cover them in a book of this size. The most active metabolic processes in our bodies, in terms of mass converted, are those involving the nutrients that we eat, and use as building material and to derive energy, and these are therefore the processes on which we shall concentrate our attention. I guess that for many readers it just so happens that these are the very metabolic processes that will be of most interest.
Metabolism Developed Very Early in Evolution
The basic features of human metabolism must have evolved very early in the evolution of life. Our metabolism shares many features with the most distantly related organisms, bacteria and yeasts for example. If you were to give me a yeast cell and a human cell and ask me to study them in the laboratory, I might struggle to tell them apart (without genetic sequencing or looking at them through a microscope) – unless the yeast cell happened to break down sugar to make alcohol, which would be a give-away (yeast cells convert sugar to alcohol in the process called fermentation). One common feature of most life forms is that energy produced from breaking down nutrients is not used immediately (e.g. for movement or growth), but is trapped in a compound called adenosine triphosphate – ATP. ATP is the universal ‘energy currency’ of life. In humans, as we shall see, most ATP is generated in a metabolic process called the citric acid cycle (or Krebs cycle, after its discoverer). Similar processes are found in all life forms, including bacteria. Undoubtedly, many aspects of metabolism were present in whatever early cells predated the split between bacteria and other life forms.
Clearly you are reading this book because you are interested in human metabolism, but should you, in the future, decide to become a plant scientist or to work on exotic marine organisms, or even bacteria, a lot of what we will cover together in this journey will still be very closely applicable.
Human Metabolism Must Depend upon a Flow of Information
We have seen the universality of the underlying pathways of cellular metabolism. (A metabolic pathway is a series of chemical reactions, each brought about by a specific protein [an enzyme], that has the effect of transforming one substance into another. We shall look at metabolic pathways in more detail in Chapter 3.) But the metabolic patterns of humans, indeed of all animals, have some distinct and some common features. Humans mostly have fairly regular patterns of fasting and feeding, not eating overnight and then taking in discrete meals during the day. We are not like smaller mammals, shrews for example, that need to eat pretty well all the time in order to provide enough energy to generate the heat that they need to survive. So it stands to reason that, after we have eaten, we have means to store nutrients beyond our immediate needs, and then to release them from these stores as needed.
Consider, for instance, what happens to the fat that we eat. We eat a juicy burger, maybe with some fries. There will be a lot more fat in that meal than we need for energy for several hours. There is a metabolic pathway that leads the excess fat into specialised cells, fat cells (called adipocytes), where it is stored. But overnight, when we don’t have food available, we will need to draw on those stores. Should I decide to get up in the morning and jog before breakfast, I will still have plenty of energy available to do so: fat stored in my fat cells, and carbohydrates stored in my muscles and my liver. And, should I be out for a very long jog (and fail to take some nourishment with me), my liver can turn on a pathway to make more carbohydrate (glucose, in fact). We see that metabolic pathways – storage of fat, bringing fat out of its stores to be used, and similar pathways for carbohydrates – are not constant in time (as they might be in a bacterial cell, for instance): they are activated at certain times and suppressed at others. This is ‘metabolic regulation’ and is arguably the most interesting aspect of human metabolism.
Immediately we can see that humans, unlike bacteria, must have means of internal signalling, ‘telling’ metabolic processes when to become active and when to shut down. I want to go for a walk. My brain sends signals to my leg muscles telling them to move. My muscles need more energy, from fat stores in my fat cells and from carbohydrate stores in my liver. Signals have to travel around orchestrating these processes. As we shall see, both nerves and hormones play a role in such regulation, and we shall learn that hormones have quite remarkable powers of regulation over metabolic pathways.
Another difference from the pathways of cellular metabolism that a bacteriologist might study is that such pathways involve cooperation between different tissues and organs. Any one of my cells (I have maybe 30 trillion cells) cannot survive on its own for more than perhaps 24 hours if I try to culture it in a laboratory flask. But my body could survive a month or two of complete starvation – indeed, there are records of people surviving more than 3 months’ starvation, that we shall look at later. This involves fuels being transferred around the body, changes in the pathways of metabolism to conserve reserves, and of course all coordinated by nerves and hormones. During exercise, fuels will need to be transferred to the muscles: there must be changes in blood flow to deliver fuels and oxygen to the muscles and remove waste products. These are essentially illustrations of what is called ‘integrative physiology’ – the opposite, in a way, of the reductionist approach of molecular biology that looks in smaller and smaller detail at what goes on within cells.
Integrative physiology has had a rough time in recent decades. Since I started to study biochemistry in the 1960s, many Nobel Prizes have been given for the amazing discoveries that have been made in how DNA stores our genetic information, how protein molecules are made up, processes that transfer materials in and out of cells, and how tiny organisms such as nematode worms are put together. But, as we shall see, this was not always the case: a very large number of Nobel Prizes have also been awarded for studies of metabolism. I should note at this point that Nobel prizes are the tip of an iceberg: most scientists beaver away, making progress that is not recognised in such a public way. But in the case of metabolism, they happen to mark a series of milestones, new developments that have shaped future thinking. Hans Krebs, perhaps the most widely known metabolic scientist, and himself a Nobel Laureate, described the situation thus: ‘Nobel awards are to some measure a matter of good luck, because their number is too small to do justice to all who would merit an award … If I ask myself how it came about that one day I found myself in Stockholm, I have not the slightest doubt that I owe this good fortune to the circumstance that I had an outstanding teacher at the critical stage of my scientific career’ (Krebs in his article ‘The making of a scientist’, 1967).
So, we shall spend some time looking at these pathways of metabolism, especially those concerned with fats, carbohydrates, and proteins, the three substances that provide us with energy to carry on our human lives. (We shall look a little more at this concept of ‘energy’ at the end of this chapter.) Ultimately, these substances release their energy for use in our muscles, our brain, heart, and other tissues by oxidation – that is, chemical reaction with oxygen. This process is related to combustion – burning – but, unlike putting a match to a candle which then burns, the process within cells involves many small chemical changes. This is critical. It enables the energy so liberated to be captured for our purposes efficiently, and also enables regulation of the processes (you can’t do much to regulate a candle burning once you’ve started it off). It also avoids the release of a potentially destructive amount of heat within the cell. A key point that we shall meet several times – but which often seems to be overlooked by people writing about different approaches to diet – is that, ultimately, these three nutrients are broken down in the same way, and enter a ‘final common pathway’ for oxidation and capture of energy, the citric acid cycle, mentioned earlier. They are not independent of one another. If one substance is being oxidised, another will be spared. This is fundamental, and yet often not appreciated.
But before we get into any of these details, it will be useful to take a brief look at the history of studies of human metabolism.
How Did Our Present Views of Metabolism Evolve?
Perhaps the first scientist to begin to understand metabolism, as we now know it, was Antoine Lavoisier, the French chemist, economist, and social reformer. Lavoisier has been called the founder of modern chemistry. Since the time of the Greek philosophers, matter had been supposed to be composed of the four ‘elements’: Earth, Water, Air, and Fire. Lavoisier clarified the meaning of a chemical element – a substance that could not be split further into constituent parts – and he compiled a list of the elements then known. Lavoisier, like a number of scientists, especially in the UK and in France in the latter half of the eighteenth century, was interested in the composition of air, and the process of combustion. Joseph Black, a Scottish physician, and the Rev Joseph Priestley, an English minister and experimenter, had both studied the composition of air and the products of chemical reactions such as burning. These scientists distinguished what were then called different types of ‘air’ (using that term for what we now call a gas), such as ‘fixed air’, which we now call carbon dioxide, ‘inflammable air’ or hydrogen, and ‘nitrous air’ (nitrous oxide). In 1775 Priestley produced what he called ‘pure air’, which we now know as oxygen. He showed that a mouse could survive long periods (a couple of hours, which was longer than in other ‘airs’) in this gas, and tried breathing it in himself, noting that his ‘breast felt particularly light and easy for some time afterwards’.
At that time, theories of burning involved a hypothetical substance ‘phlogiston’, which was emitted from burning matter. A substance could only continue to burn as long as the surrounding air could absorb the phlogiston emitted. Priestley did not contradict this view. He believed that his new gas, which supported combustion better than any other, must therefore initially be devoid of phlogiston, so it had a greater absorptive capacity than other gases, and accordingly he referred to it as ‘dephlogisticated air’. Priestley and Lavoisier were in contact, and indeed met when Priestley visited Paris in 1774. Lavoisier had the inspiration to see that Priestley’s ‘dephlogisticated air’ held the key to understanding combustion. Lavoisier deduced that common air was a mixture of two different components, one the newly discovered ‘pure air’ (oxygen), the other an inert gas that he called ‘mofette’, which we now call nitrogen. He showed that in combustion (the burning of a candle), the ‘pure air’, or as he called it, ‘eminently respirable air’, was consumed, and that when this was used up the combustion ended, rather than the air surrounding the candle becoming saturated with phlogiston. Lavoisier’s overturning of the phlogiston theory was a major breakthrough in chemistry. In 1779, Lavoisier named this ‘eminently respirable air’ oxygen, from the Greek words for ‘acid’ and ‘beget’.
Lavoisier, though, wanted to extend his research beyond the burning of candles and the oxidation of metals. Like Priestley, and Black before him, he showed that when a small animal (usually a mouse) breathed common air, ‘fixed air’ (carbon dioxide) was produced. He also showed that the mofette (nitrogen) passed into the lungs and came out unchanged. He worked with the French mathematician Pierre-Simon Laplace, to study the heat that was produced in combustion. Heat could be measured in an instrument known as a calorimeter (named from the Latin calor for heat, as in the calorie) – an insulated container in which a rise in temperature could be measured. They studied the heat produced by animals, devising a calorimeter in which a guinea pig could be studied for several hours. They measured the heat produced by the animal by surrounding the instrument with ice and seeing how much of the ice melted. They also measured the amount of oxygen used by the animal. In separate experiments, they showed that combustion (burning a candle) using this amount of oxygen produced a very similar amount of heat. ‘La respiration’, declared Lavoisier, ‘est donc une combustion’ – respiration (by which he meant the use of the air breathed in, not just the process of breathing) is a form of combustion. Lavoisier had no way of knowing what was going on inside the guinea pig’s body – the requisite techniques were not available to him. But he must have realised that within the animal’s lungs there was no fire, as in the burning of a candle, although the end result, in terms of heat and carbon dioxide produced, was the same.
Lavoisier extended these observations to the human body. In around 1789, he studied his assistant Armand Séguin, providing him with oxygen to breathe, and using a mask to collect the carbon dioxide produced (Figure 1.1). Lavoisier was able to show that consumption of oxygen and production of carbon dioxide increased when Séguin was digesting a meal, or exercising, or subjected to a cold environment.
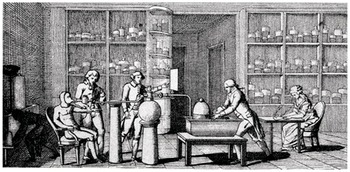
Figure 1.1 Lavoisier’s experiment with his assistant Séguin.
The tragic end to Lavoisier’s experimentation came in 1794 during the Reign of Terror, when the French Revolutionary Tribunal condemned him to death on the guillotine, because he had been a tax gatherer or ‘Farmer-General’. The French mathematician Lagrange remarked ‘Only a moment to cut off that head, and a hundred years may not give us another like it’.
One of Lavoisier’s interests had been the improvement of French agriculture. He felt it lagged behind English agriculture. He worked with the Committee of Agriculture to improve this situation, in part by increasing the number of cows and sheep raised by farmers. During the early part of the nineteenth century the raising of farm animals became an important area of scientific research. European scientists, including Justus von Liebig in Germany and Jean-Baptiste Boussingault in France, studied the role of nitrogen in crops and animals. von Liebig showed the importance of nitrogen for plant growth and speculated that nitrogen-containing substances in plants and animals were similar (we now call them proteins). Boussingault showed that plants do not use nitrogen directly from the air. Gaseous nitrogen must first be converted into other substances (starting with ammonia) from which it can become utilisable. We now know that this is done by bacteria in the soil, associated with the roots of some crops. Nitrogen is needed by animals in the form of protein (we will explore this further in later chapters), and this must arise initially from plants. (In the early twentieth century, an industrial method for converting gaseous nitrogen into ammonia, the Haber-Bosch process, was developed by the German scientists after whom it is named. This is now the major route for ‘fixing’ nitrogen from the air for use as a fertiliser, and ultimately for making the protein on which we depend.)
The nutritional needs of farm animals were studied at this time, essentially by measuring what the animal ate (usually something fed by the experimenters), what was excreted, and how the animal grew – or did not grow. This led to a greater understanding of the need for both protein and ‘energy sources’, carbohydrate and fat, for adequate growth and well-being of an animal. However, the question of what processes were going on inside the animal was still largely unanswered.
It was another French scientist who took this research forward. Claude Bernard was a French physician who studied animals – mainly dogs – in his laboratory and developed the science of the workings of the body that we now call physiology. Bernard emphasised the importance of the internal medium of the body, or ‘milieu intérieur’. He believed that ‘the stability of the internal environment (the milieu intérieur) is the condition for the free and independent life’. He worked on the internal processes that govern this medium, concentrating especially upon the role of the pancreas in digestion, and the liver in maintaining the correct amount of glucose (sugar) in the bloodstream – both processes that we shall explore later.
Metabolic investigations were now going to a deeper level than ‘what goes in and what comes out’. By the end of the nineteenth century the emphasis had shifted further, so that now the metabolism of individual tissues (such as muscle or liver) could be investigated in the laboratory. The twentieth century saw an enormous growth in this research, with key metabolic pathways being discovered.
The German scientist Otto Warburg worked on the biochemical pathway of combustion. We saw earlier that Lavoisier had shown that fuels are used by the human body in a process with similarities to combustion. Warburg investigated just what was going on inside cells. Confusingly to the outsider, as noted before, the term ‘respiration’ is often used by scientists to refer to the process of using fuels by combining them with oxygen (whereas for most people, respiration refers to the act of breathing). Warburg’s studies on the mechanism of respiration led in 1931 to the award of the Nobel Prize in Physiology or Medicine.
Hans Krebs, another ‘giant’ of metabolic research, and perhaps the only one whose name is widely known, studied medicine in Germany and worked with Warburg from 1926 to 1933, when his employment was abruptly terminated by the Nazi Party because of his Jewish ancestry. (As we shall see, this was not uncommon amongst the pioneers of cellular metabolism.) Fortunately for Krebs, and for the world of metabolism, he was offered a position in the UK, initially in Cambridge, where he continued his research into cellular respiration. He soon moved to the University of Sheffield and later, in 1954, to the University of Oxford, where he worked until his death in 1981.
Krebs is remembered especially for the discovery of two important metabolic pathways that take the form of ‘cycles’ – again, a concept we will explore in more detail in later chapters. The better known of these is the cycle now often known as the Krebs Cycle, more officially called the citric acid cycle, or the tricarboxylic acid cycle. We shall examine it in Chapter 5 – and see how it gets its different names. This is the common pathway by which metabolic fuels are oxidised – that is, combined with oxygen in the process analogous to combustion. As we shall explore throughout this book, this process is absolutely crucial to any understanding of what we should, or should not, eat (and whether it matters), and indeed of ‘fast’ or ‘slow’ metabolism.
The other cycle discovered by Krebs is known as the urea cycle, and takes us back to the nineteenth-century work of Boussingault and others. Animals are largely built of proteins. A characteristic of proteins is that they contain nitrogen. As these proteins are eventually broken down, this nitrogen must be disposed of. Some forms of nitrogen are quite toxic to animals, ammonia being one of these. In the urea cycle, which operates in the liver, ‘unwanted’ nitrogen is converted to the substance called urea, which is relatively non-toxic and is excreted in the urine. Like Warburg before him, Hans Krebs was awarded the Nobel Prize in Physiology or Medicine in 1953 for his discovery of the citric acid cycle (Figure 1.2).
Over the course of more than 150 years, then, the study of metabolism had moved from an overall view of nutrients, or fuels, being taken in, and waste products (such as carbon dioxide and urea) coming out, to detailed study of the pathways within cells that bring about these changes. To return to the analogy of economics, the science had moved from measuring GDP to exploring the myriad ‘local economies’ and other processes that make this up. These metabolic processes, or pathways, are what we know as ‘intermediary metabolism’, and we shall explore them further in the rest of this book.
Human Metabolism in a Wider Context
We shall be exploring the means by which we, humans, derive energy from our foodstuffs. It’s useful at the outset just to think about where this energy came from. We eat plant and animal tissues. All the energy ‘stored’ in these comes originally from the sun. Plant cells use the energy in sunlight to make sugars through a process called photosynthesis. These sugars are the starting point for making starches and also fats. As noted above, soil bacteria can use nitrogen from the atmosphere and incorporate it into organic compounds, which are taken up by plants, which we eat in turn. Even the meat-eaters amongst us are dependent upon these plant sources: if you eat beef, for instance, the cow will have derived its bodily materials by eating plants – no animal has the ability to capture and use the energy of sunlight, nor to incorporate nitrogen from the air into its bodily materials. Ultimately, then, when I pedal my bicycle up the hill that connects our residential area to the centre of Oxford, I am using energy derived from sunlight – my bicycle is (indirectly) solar powered!
In general, as molecules get bigger, they store more energy. Life centres on molecules built of carbon (C) atoms. During photosynthesis, plants use carbon dioxide (CO2) from the air and, with energy from sunlight, convert this into sugars. A typical sugar molecule, such as glucose, has six atoms of carbon (C6H12O6, meaning its molecules each contain six atoms of carbon, 12 of hydrogen and six of oxygen). Fats are mostly built of units called fatty acids (more detail in the next chapter). A common fatty acid, palmitic acid, has 16 carbon atoms in each molecule. The process of making more complex molecules from simpler ones is called anabolism.
Breaking down of complex molecules is called catabolism. As these complex molecules are broken down by combination with oxygen, the process of oxidation, the carbon atoms are split apart, combined with oxygen, and energy is liberated. On complete combustion of glucose, just carbon dioxide and water remain, as follows:
One molecule of glucose + six molecules of oxygen → six molecules each of carbon dioxide and water;
or, in short,
When we begin, later, to look at the cellular pathways by which this is brought about, the concept of breaking down larger molecules to make smaller ones, with release of energy, will be central to our thinking. It is illustrated in Figure 1.3.
What Is Metabolism? Conclusion
So we see that metabolism underlies all processes that keep us alive: making the components of our cells, providing energy in a continuous way for living and working, and disposing of bodily components that are no longer needed. This must involve energy stores where excess fuel can be sequestered, to be called upon when needed. We have also seen that, in order to keep metabolism doing what it needs to at any one time, a system of internal communication is needed. We shall explore each of these themes in the next few chapters.






