Introduction
The human desire to explore extreme and uncharted places has endured for centuries. Today, this quest has shifted toward the exploration of nearby planets or moons in our solar system, driven by the captivating possibility of discovering existing life on one of them. Since we are currently not capable of traveling to another planet or moon, we have to turn or focus to extreme environments on Earth which can be considered analog environments such as the deep sea (McClain et al., Reference McClain, Bryant, Hanks and Bowels2022) or hypersaline brines (DasSarma et al., Reference DasSarma, DasSarma, Laye and Schwieterman2020; Hallsworth et al., Reference Hallsworth, Mancinelli, Conley, Dallas, Rinaldi, Davila, Benison, Rapoport, Cavalazzi, Selbmann, Changela, Westall, Yakimov, Amils and Madigan2021). Here we want to draw the focus to hypersaline brines as they are of high interest for astrobiological studies as several studies have shown the possible presence of hypersaline waters on other planets and moons (Spencer and Nimmo, Reference Spencer and Nimmo2013; Chevrier et al., Reference Chevrier, Rivera-Valentin, Soto and Altheide2020; Chivers et al., Reference Chivers, Buffo and Schmidt2023). It is well established that halophilic Archaea are able to cope well with detrimental environmental conditions such as desiccation (Oren, Reference Oren2014), different types of ionizing and non-ionizing radiation (Jones and Baxter, Reference Jones and Baxter2017; Leuko and Rettberg, Reference Leuko and Rettberg2017), microgravity (Dornmayer-Pfaffenhuemer et al., Reference Dornmayr-Pfaffenhuemer, Legat, Schwimbersky, Fendrihan and Stan-Lotter2011; Thombre et al., Reference Thombre, Shinde, Dixit, Jagtap and Vidyasagar2017), stratospheric conditions (DasSarma et al., Reference DasSarma, Laye, Harvey, Reid, Shultz, Yarborough, Lamb, Koske-Phillips, Herbst, Molina, Grah, Phillips and DasSarma2017) and even exposure to outer space which has been shown during the BIPOAN mission (Mancinelli et al., Reference Mancinelli, White and Rothschild1998), the EXPOSE-R mission (Mancinelli, Reference Mancinelli2015) and the EXPOSE-R2 Mission (Leuko et al., Reference Leuko, Stan-Lotter, Lamers, Sjöström, Rabbow, Parpart, Rettberg, Seckbach and Stan-Lotter2020). However, as a recent review has shown, there are still knowledge gaps in the Halobacteria class, particularly regarding astrobiological stress tests for the genera Haloarcula and Haloferax, while more research has focused on other genera such as Halobacterium and Halococcus (Wu et al., Reference Wu, McGenity, Rettberg, Simoes, Li and Antunes2022).
On Earth, halophilic Archaea are typically found in hypersaline environments such as the Dead Sea (Bodaker et al., Reference Bodaker, Sharon, Suzuki, Feingersch, Shmoish, Andreishcheva, Sogin, Rosenberg, Maguire, Belkin, Oren and Béjà2010; Jacob et al., Reference Jacob, Hussein, Shakhatreh and Cornelison2017), the Atacama Desert (Flores et al., Reference Flores, Hoyos, Venegas, Galetović, Zuniga, Fabrega, Paredes, Salarzar-Ardiles, Vilo, Ascaso, Wierzchos, Souza-Egipsy, Araya, Batista-Garcia and Gomez-Silva2020; Runzheimer et al., Reference Runzheimer, Lozano, Boy, Boy, Godoy, Matus, Engel, Pavletic, Leuko, Armengaud and Moeller2024), Antarctica (Franzmann et al., Reference Franzmann, Stackebrandt, Sanderson, Volkman, Cameron, Stevenson, McMeekin and Burton1988; Williams et al., Reference Williams, Allen, DeMaere, Kyrpides, Tringe, Woyke and Cavicchioli2014), deep-sea anoxic basins (Antunes et al., Reference Antunes, Karen Olsson-Francis and McGenity2020), modern day stromatolites (Goh et al., Reference Goh, Leuko, Allen, Bowman, Kamekura, Neilan and Burns2006), the salt glands of seabirds (Britto-Echeverría et al., Reference Brito-Echeverría, López-López, Yarza, Antón and Rosselló-Móra2009) and indications for halophilic Archaea can even be found associated with deteriorating ancient wall paintings (Piñar et al., Reference Piñar, Saiz-Jimenez, Schabereiter-Gurtner, Blanco-Varela, Lubitz and Rölleke2001). Of high astrobiological interest is the fact that halophilic organisms have been isolated from halite formations around 250 million years old (Gruber et al., Reference Gruber, Legat, Pfaffenhuemer, Radax, Weidler, Busse and Stan-Lotter2004; Schubert et al., Reference Schubert, Lowenstein, Timofeeff and Parker2010; Jaakkola et al., Reference Jaakkola, Ravantti, Oksanen and Bamford2016). If the isolated organisms were entrapped within the halite at the time of formation (250 Mio. years ago) or got entrapped at a later time is still under discussion. Still, isolating living organisms out of such ancient formations give an excellent indication of the robustness and astrobiological significance of these organisms. One highly intriguing and particularly tricky to sample ancient halite reservoir is the remnants of the Zechstein sea, an epicontinental ocean that extended over an area of approximately 600,000 km2 and covered the European Permian Basin during the late Permian (García-Veigas et al., Reference García-Veigas, Cendón, Pueyo and Peryt2011). Chemical analysis of fluid inclusions in marine halite suggests that the Upper Permian Zechstein sequences were deposited over approximately 7 million years, spanning from 258 to 251 Ma. (Zhang et al., Reference Zhang, Krause and Mutti2013). One mayor factor leading to the evaporation of the ocean is believed to be a series of volcanic eruptions in Siberia, leading to the mass extinction of living organisms in just over a million years (252-251 Ma ago) (Benton and Twitchett, Reference Benton and Twitchett2003). The salt layers that have been formed during the different evaporation cycles have been moved around as a result of regional tectonics happening from the Triassic to early Cenozoic, which changes the original distribution of salts, resulting in the formation of different salt structures such as pillows or diapirs (Zhang et al., Reference Zhang, Krause and Mutti2013). Salt meadows and saline springs were also formed over the course of time, which attributed to the salinization of the groundwater. Using brine for the production of salt has been a worldwide industry for thousands of years, with first reports of its utilization as early as the fourth millennium B.C. in Europe (Akridge, 2006).
Beginning in the 12th century (or earlier), the brine originating from the Zechstein sea was used for commercial salt production all over Europe and one of the most successful salt-producing cities at the time was Lunenburg, Germany (Stephan, Reference Stephan, Jakubowski-Tiessen, Masius and Sprenger2014). The city of Lunenburg with its approx. 77.000 inhabitants is located at the lower stretch of the river Ilmenau about 50 km south-east of Hamburg. Lunenburg was one of Europe’s most productive cities for salt production from brine in the middle ages by collecting the brine in large pans and heating it to remove water (panning) (Trüper, Reference Trüper and Ventosa2004). The legend on how the salt deposits in Lunenburg were discovered is quite amusing: “More than a thousand years ago, hunters came across a sleeping wild boar. Its fur glistened white in the sun. The hunters shot the poor sow. They realized that its black bristles were covered in fine salt crystals. Astonished, they followed the animal’s tracks to a pond with salty water. The wild boar had been wallowing there, as pigs like to do. Its fur was drying in the warm sun. The water evaporated and the salt crystals got stuck in the bristles. This is how the people of Lüneburg discovered the salty spring that brought the town its wealth” (translated from the German Salt museum Deutsches Salzmuseum | Salz Sage). There are several more examples of animals helping humans to discover salt, such as the famous stag of the “Teinacher Hirschquelle” or the legend from Hall/Tyrol where hunters observed that chamois licked saline rocks as part of their diet (Ritz, Reference Ritz1996). The salt production in Lunenburg has seized in the 1980’s due to poor economic feasibility, however, the German Salt Museum is preserving the great history of salt in the city as well as everything one wants to know about salt. Although the brine is not commercially collected for salt production anymore, there is still a faucet in the German Salt Museum were the brine is accessible today and used for educational purposes.
Here we report on the stress resistance of two organisms, isolated from the Lunenburg brine, Haloarcula (Har.) sp. NS06 as well as Halorubrum (Hrr.) sp. AS12. We tested the response of these two isolates against outer space relevant stress factors namely desiccation, UV-C and X-ray radiation, exposure to heavy ions (He, Ar and Fe) as well as to different concentrations of perchlorate.
Material and methods
Sampling and optimum growth conditions
The cultures tested here were isolated from Lunenburg brine samples taken in September 2022 (Figure 1) and a detailed description of the sampling campaign, enrichment of the cultures as well as a detailed analysis of the brine is given by Runzheimer et al. (Reference Runzheimer, Schwab, Engel, Schaudinn, Laue, Rebrosova, Beblo-Vranesevic and Leuko2025). Cultures were routinely grown in Artificial Seawater for halophiles Medium (ASW, J457) containing 195.00 g/L NaCl, 35.00 g/L MgCl2 x 6 H2O, 50.00 g/L MgSO4 x 7 H2O, 5.00 g/L KCl, 1.00 g/L NaNO3, 0.50 g/L CaCl2 x 2 H2O, 0.05 g/L KH2PO4, 0.03 g/L NH4Cl, 0.05 g/L yeast extract and the pH was adjusted to 7.4 using 1M Tris base. The medium was autoclaved for 15 min at 121°C and following autoclaving filter-sterilize 8% (w/v) NaHCO3 and 25% (w/v) sodium pyruvate solutions were added to the medium aseptically. For agar plates, 15 g/L agar were added to the medium before autoclaving.

Figure 1. (A) Location of the city of Lunenburg in Germany; (B) A typical salt pan, used for heating the brine and harvesting the crude salt and (C) the faucet located in the salt museum used for show panning of the salt and the location to collect brine samples. (Webpage: Deutsches Salzmuseum | Startseite neu).
To determine the optimum NaCl concentrations, both strains were grown in liquid for 14 days with the following NaCl concentrations: 10%, 15 %, 20%, 25% and 30%. The optimum temperature was determined by incubating the strains at 30°C, 37°C, 42°C and 50°C for 7 days in liquid culture. It was determined that both strains grow best in ASW medium containing 20% NaCl, and at 37°C for AS12 and at 42°C for NS06.
Sample preparation
One colony was used to enrich precultures before every experiment. Three separate main cultures were inoculated with 1% from this preculture and grown for 7 days. Twenty mL were centrifuged for 10 min at 4,000 xg at room temperature and the resulting pellet was washed three times within 10 mL TN buffer (20% NaCl, 0.1 M Tris, pH 7.4). For each experiment, the OD600nm was adjusted to 0.2 (approx. 107 cells/mL for Har. sp. NS06 and approx. 108 cells/mL for Hrr. sp. AS12). Survival was determined by plating 50 µL of serial diluted cells onto respective agar plates and evaluating colony forming units (CFU) formation after 14 days of incubation at the respected optimum growth temperature. To protect the plates from drying during prolonged incubation time, an open Petri dish containing two water-soaked wipes was added to the top of the bag and sealed. Significant differences were compared using a Student’s t test.
Desiccation tolerance
Samples were prepared as described above and 50 µL were transferred into a 96 well plate. The 96 well plate was placed in a desiccator containing silica gel. The average temperature during the experiment was 21°C with a humidity of 10%. Cells were reactivated following 7, 14, 21, 28, 35 and 42 days by adding 200 µL TN buffer to the wells and gently pipetting up and down several times until the sample was completely resuspended. Survival was determined as previously described.
UV-C radiation
Cells were prepared as described above and additionally diluted 1:100 to mitigate a possible protective effect by self-shading. In this experiment, a 365/254 nm Vilber UV Lamp was used and a fluence rate of 133 µW/cm2 at 254 nm was determined with a UV-X Digital Radiometer (UVP Ultra-Violet Products, Cambridge, UK). Twenty mL of cells were exposed within a petri dish at room temperature while the suspension was constantly stirred with a magnetic stirrer to guarantee homologs exposure of the cells up to a fluence of 500 J/m2 as previously described by Moeller et al. (Reference Moeller, Reitz, Douki, Cadet, Horneck and Stan-Lotter2010). One hundred µL were taken at 100, 200, 300, 400 and 500 J/m2 and the survival was determined by evaluating CFU’s as previously described. F10 values, representing the dosage required to reduce the survival rate by one order of magnitude, are expressed in J/m2. These values were determined from the regression lines of the exponential slopes of the survival curves, as outlined by Harm (Reference Harm1980).
X-ray exposure
Cultures were harvested by centrifugation and the cell concentration adjusted as described before. Fifty µL were transferred to 0.2 mL PCR tubes and placed on the exposition table. An empty Eppendorf tube was used for defining the shielding effect and considered within the calculation of the final dose. Samples were irradiated at room temperature at a rate of 18.53 Gy/min until a final dose of 100, 250, 500, 750 and 1.000 Gy, respectively, was accumulated. Survival was determined by evaluating CFU formation after 14 days of incubation at the respected optimum growth temperature. D10 values, representing the dosage required to reduce the survival rate by one order of magnitude, are expressed in Gy, and were calculated as described above.
Exposure to heavy ions
Cultures were harvested by centrifugation and the cell concentration adjusted as described before. Two-hundred µL were transferred to 0.5 mL Eppendorf tubes and sent at ambient temperature to the Heavy Ion Medical Accelerator facility at the National Institute of Radiological Science in Chiba, Japan. Samples were exposed to He (2.24 keV/µm), Ar (95,6 keV/µm) and Fe (197,6 keV/µm) up to a dose of 500 Gy. Samples were returned at ambient temperature and were stored at 4°C upon return until further analysis. Survival was determined as previously described.
Growth in the presence of perchlorate
The impact of sodium and magnesium perchlorate on microbial growth was assessed by adding perchlorate concentrations of 0.5%, 1%, and 4% to standard cultivation medium and spectroscopically monitoring the growth of Hrr. sp. AS12 and Har. sp. NS06 over time. Perchlorate concentrations were chosen to meet the general findings of perchlorate concentrations of 0.4 to 0.6% on Mars (Hecht et al., Reference Hecht, Kounaves, Quinn, West, Young, Ming, Catling, Clark, Boynton, Hoffman, De Flores, Gospodinova, Kapit and Smith2009) and even higher concentrations, where previous research showed a significant impact on other organisms (Beblo-Vranesevic et al., Reference Beblo-Vranesevic, Huber and Rettberg2017; Zaccaria et al., Reference Zaccaria, de Jonge, Domínguez-Andrés, Netea, Beblo-Vranesevic and Rettberg2024).
For this, three biological replicates of 50 mL cultures of strains Har. sp. NS06 and Hrr. sp. AS12 were grown in ASW for seven days as described above. After incubation, the cultures were harvested, and the supernatant was discarded. The cells were resuspended in 10 mL of fresh ASW, and the OD600 was measured and adjusted to 0.2 for both strains. Perchlorate solutions were directly prepared in ASW to achieve final concentrations of 0.5%, 1%, and 4% in the final experimental setup. Final concentrations lead to final molarities of 35.59 mM (0.5%); 71.18 mM (1%), 284,72 mM (4%) for NaClO4 and 22,4 mM (0.5%), 44,8 mM (1%), and 179,2 mM (4%) for MgClO4.
The solutions were sterilized using a 0.2 µm filter, and prewarming the solutions before filtration was found to be beneficial for complete solving. In a 96-well plate, 30 µL of culture was mixed with 270 µL of perchlorate solution. Blank wells containing only the perchlorate solution were also prepared. The OD600 was measured every 30 min within a plate reader over a period of 125 h at 37°C (AS12) and 42°C (NS06). Extended incubation times led to the precipitation of salts in some samples. To analyze the data, the OD600 values of the samples were corrected by subtracting the values of the blanks. The mean and standard deviation were calculated and plotted against time to visualize the results.
Results
We tested several astrobiological-relevant parameters such as desiccation, UV-C and X-ray radiation, exposure to heavy ions as well as resistance against Perchlorates. and evaluated the response in the terms of survival of the tested organism. As previously stated, it is well established that halophilic Archaea are desiccation resistant and can endure for years in a dried state. Our experiments show that Har. sp. NS06 cannot be considered desiccation resistant since the strain did not survive desiccation for more than 14 days (Figure 2). No survival of this strain was measurable following desiccation for 21 days. In contrast, Hrr. sp. AS12 showed descent desiccation resistance (Figure 2). Although there was a significant decline in survival following 28 days of desiccation, the survival remained stable after this time and a -log3 decline in survival. No further decline in survival was observed for this strain even after desiccation for 42 days.
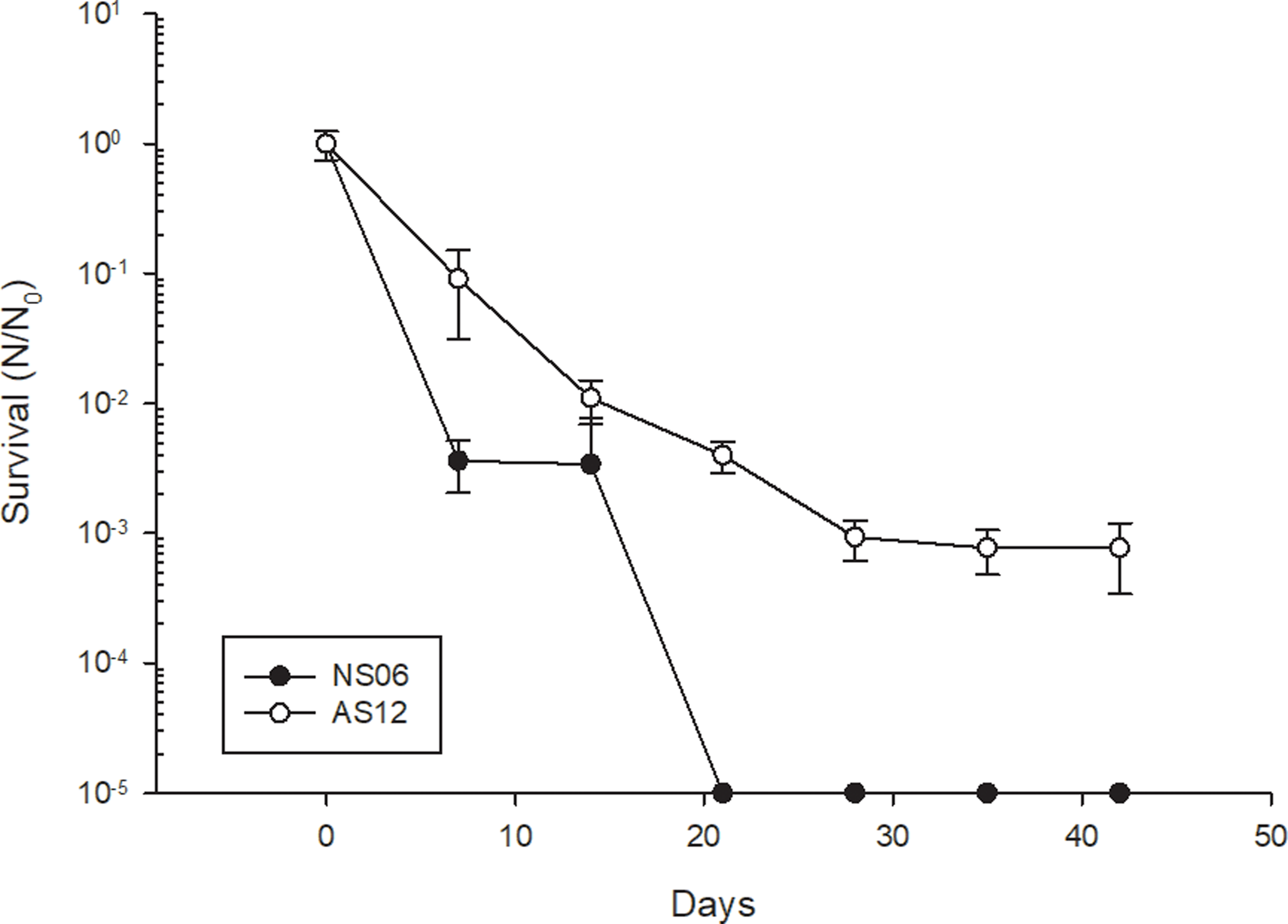
Figure 2. Survival of Har. sp. NS06 and Hrr. sp. AS12 to prolonged desiccation. Error bars indicate standard deviation (n=3).
In contrast, the results of the survival after irradiation with germicidal UV-C light or X-ray radiation revealed that Har. sp. NS06 is more resistant than Hrr. sp. AS12. Here we show that Har. sp. NS06 shows a significant higher tolerance against UV-C radiation when compared to Hrr. sp. AS12 (Figure 3A). The calculated F10 values were 194,9 (± 13,7) J/m2 for Har. sp. NS06 and 111,6 (± 6,4) J/m2 for Hrr. sp. AS12. No survival following exposure to 400 J/m2 was recorded for either strain; however, Hrr. sp. AS12 shows a significantly stronger decline in survival following exposure to 200 J/m2 compared to Har. sp. NS06. The difference in radiation resistance is even more pronounces when looking at the survival rate following exposure to X-ray radiation (Figure 3B). When exposed to up to 1 kGy of X-ray radiation, Har. sp. NS06 showed remarkable resistance with not even a -log1 reduction in survival. In contrast, Hrr. sp. AS12 displayed a significant sensitivity to this type of radiation which lead to a significant decline in survival after 500 Gy, supported by a D10 value of 228,2 (± 8,9) Gy. The calculation of the D10 value was not possible since the decline in survival was not sufficient following exposure to 1 kGy of radiation.
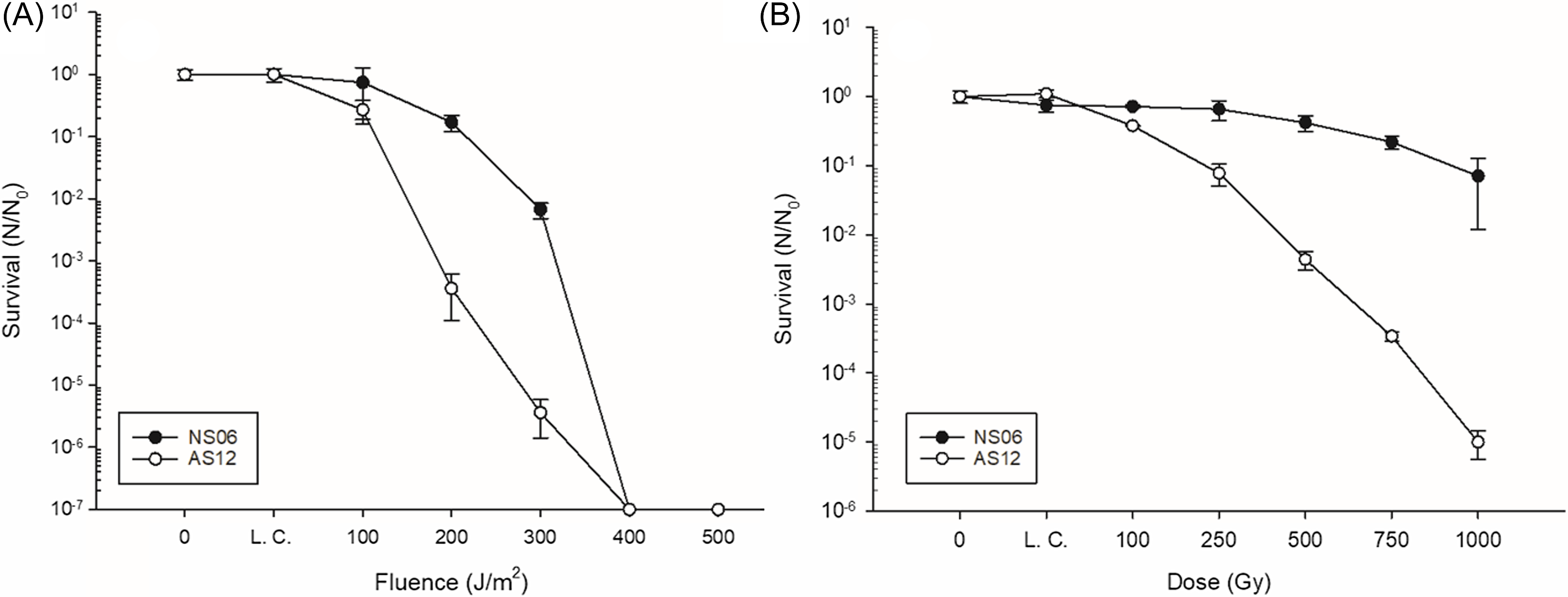
Figure 3. Survival of Har. sp. NS06 and Hrr. sp. AS12 following exposure to (A) UV-C and (B) X-ray radiation. L. C. stands for lab control. Error bars indicate standard deviation (n=3).
Exposure of both strains to heavy ions at the Heavy Ion Facility in Chiba showed very little effect on the strains (Figure 4). Although it seems that there is a strong decline in survival for Hrr. sp. AS12, this can’t be attributed to the effects of radiation since the laboratory control as well as the transport control showed a similar decline in survival. On the other hand, Har. sp. NS06 showed neglectable effects in survival due to storage and transportation and we did not see a significant decrease in survival following exposure to heavy ions. Only a radiation dose of 500 Gy Ar showed some decline in survival, however, that decline was calculated to not be significant.
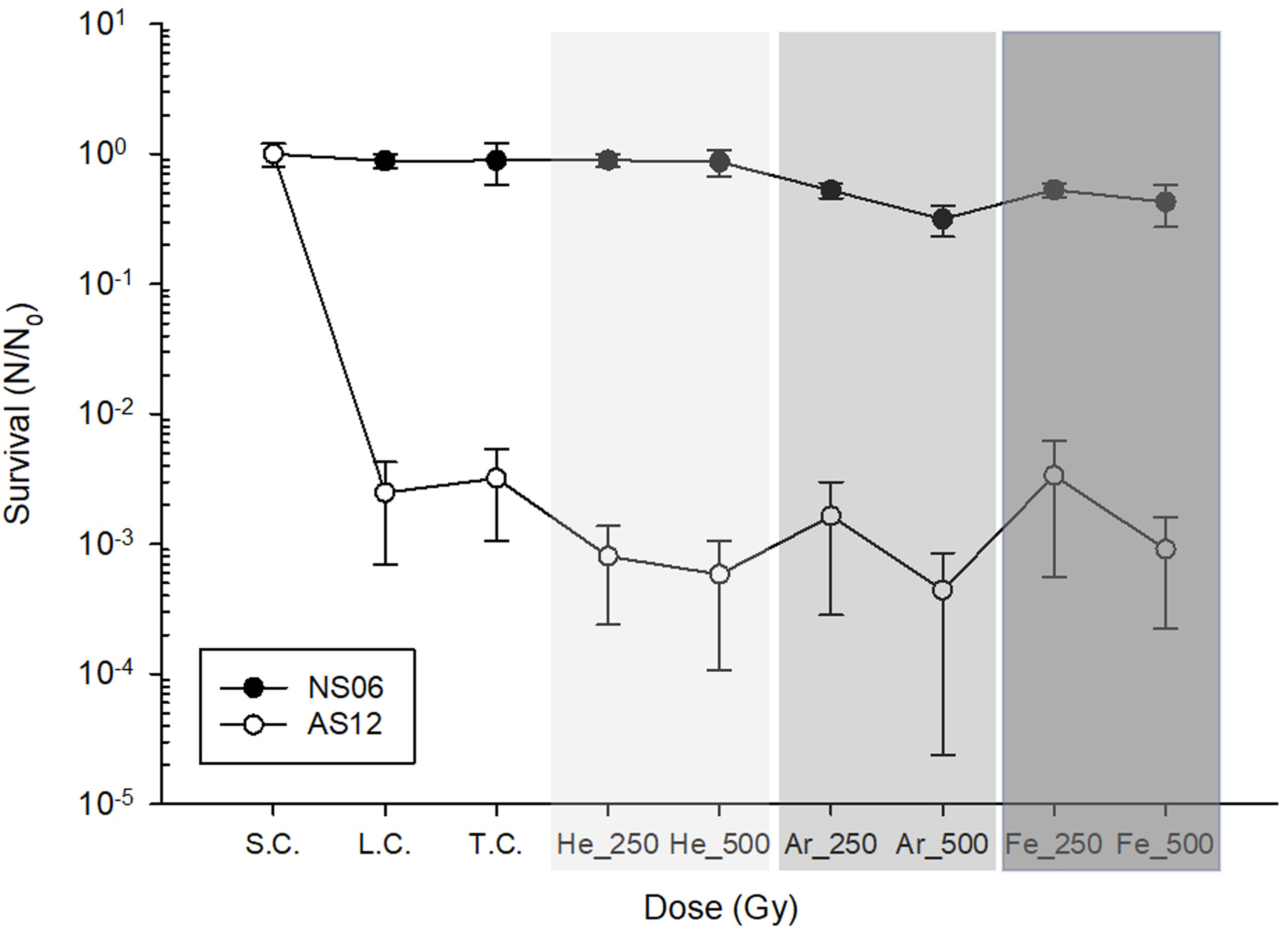
Figure 4. Survival of Har. sp. NS06 and Hrr. sp. AS12 following exposure to heavy ions up to a dose of 500 Gy. S.C. stand for initial cell concentration, L.C. stands for laboratory control; T.C. stands for transport control, were samples were transported to the HiMAC facility in Japan but have not been exposed to heavy ions. Different shades of gray indicate different ions. Error bars indicate standard deviation (n=3).
Figure 5 presents the growth behavior of Hrr. sp. AS12 and Har. sp. NS06 in absence and presence of Na- and MgClO4. In general, the growth behavior revealed to be faster for NS06 than for AS12, while still being slow reaching an OD600 of 0.037 (NS06) and 0.032 (AS12) after 125 hours of growth. Results show that NaClO4 is able to promote growth within both strains, while MgClO4 has only slightly beneficial effects on the growth. Cultures supplemented with 1% of NaClO4 were able to reach an optical density of 0.0868 (AS12) and 0.143 (NS06) which displays an ∼2.7 to ∼3.9 times higher OD than without perchlorate supplementation. However, while 0.5 and 1% of NaClO4 reveal increasing promoting effects, 4% seem to have no further beneficial effect on the growth behavior leading to the assumption of a potential saturation point of the promoting growth effect of NaClO4. Cultures grown with 1% MgClO4 revealed an OD of 0.0431 (AS12) and 0.0703 (NS06) which displays a 1.4 to 1.9 increased growth in comparison to no supplementation.
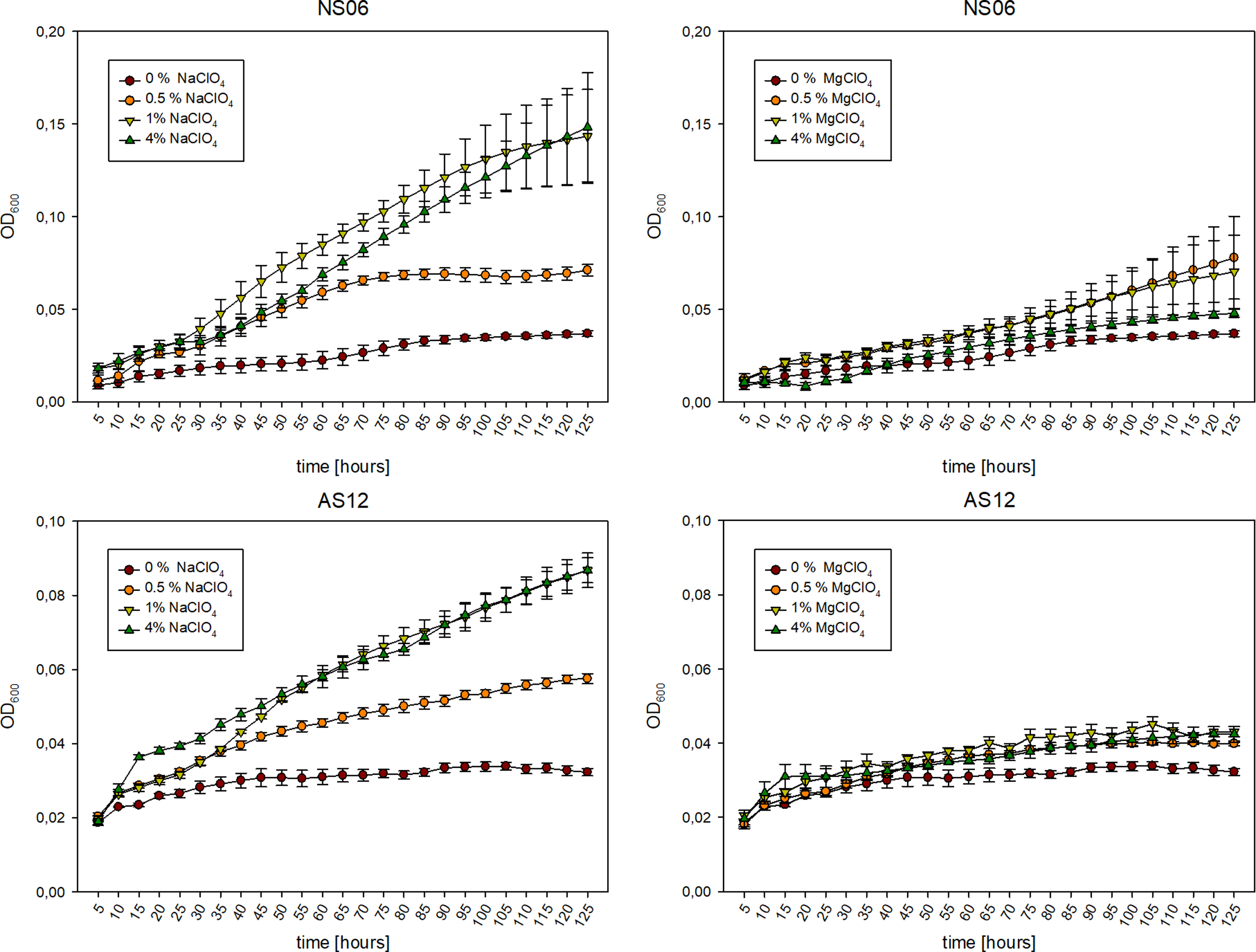
Figure 5. Growth of Har. sp. NS06 (top) and Hrr. sp. AS12 (bottom) over time (125 hours) in absence (0%, purple) and presence of 0.5 (orange), 1 (yellow) and 4%(green) of NaClO4 (left) and MgClO4 (right). NaClO4 supplementation revealed the promotion of growth of both isolates. Error bars indicate standard deviation (n=3).
Discussion
For future missions to find life on other planets, the limits of life and its adaptions to extreme conditions need to be understood. It is well established that halophilic Archaea are strong contestants in this field, since they are generally highly resistant to detrimental environmental factors such as desiccation, radiation or the presence of perchlorates (Oren, Reference Oren2014). In this study, the resistance of two novel halophilic isolates from brine samples collected in Lüneburg were tested against astrobiologically relevant stress factors. The true age and origin of these organisms cannot be determined at the moment, although it is intriguing to believe that they truly were entrapped during the evaporation of the Zechstein ocean 250 million years ago. There are several reports showing the isolation of halophilic organisms associated with ancient halite (Vreeland et al., Reference Vreeland, Rosenzweig and Powers2000; Stan-Lotter et al., Reference Stan-Lotter, Pfaffenhuemer, Legat, Busse, Radax and Gruber2002; Gruber et al., Reference Gruber, Legat, Pfaffenhuemer, Radax, Weidler, Busse and Stan-Lotter2004); however, there are some doubts about the true age of these organisms (Nickle et al., Reference Nickle, Learn, Rain, Mullins and Mittler2002). Halophilic Archaea have a distinct need for NaCl, with extreme halophilic organisms require between 3.4–5.1 M NaCl (DasSarma and DasSarma, Reference DasSarma and DasSarma2017), and most representatives will lyse when exposed to environmental conditions with low salt concentration. Therefore, a deposition after the formation of the evaporate via ground water seems unlikely as most halophilic Archaea would immediately lyse. Further research should focus on if these organisms have truly been deposited there 250 million years ago or if they were introduced during a later stage.
Although being isolated from the same environment, both strains show significant differences in their ability to survive adverse environmental conditions. The most interesting result is the difference in resistance to desiccation (Figure 2) of the two strains. While Hrr. sp. AS12 is somewhat desiccation resistant, Har. sp. NS06 did not survive desiccation for 21 days under the tested conditions. Hrr. sp. AS12 showed significant decline as well, yet, survival could still be determined after 42 days. Most halophilic Archaea tolerate desiccation well, which may be due to their polyploid nature (Ludt and Soppa, Reference Ludt and Soppa2019). For instance, it was previously shown that the survival rate of Haloferax volcanii after 12 days of desiccation was significantly higher when the cells contained 20 genome copies compared to cells only containing two copies because the cells can use genomic DNA as a storage polymer for phosphate (Zerulla et al., Reference Zerulla, Chimileski, Näther, Gophna, Thane Papke and Soppa2014). There are no previous reports about the desiccation of Har. spp., except one report by Mancinelli et al. (Reference Mancinelli, White and Rothschild1998) were it was shown that an isolate designated Har. sp. (G) was able to survive exposure to desiccation during the BIOPAN space mission. However, the cells were entrapped in artificial halite-gypsum crystals during exposure and it was later confirmed that the isolate is in fact a member of the genus Halorubrum, namely Hrr. chaoviator (Mancinelli et al., Reference Mancinelli, Landheim, Sanchez-Porro, Dornmayr-Pfaffenhuemer, Gruber, Legat, Ventosa, Radax, Ihara, White and Stan-Lotter2009). Interestingly, when exposing both strains to UV-C and X-ray radiation, a different pattern emerges.
Ionizing radiation (IR) as well as UV-C radiation can be major stress factors on Earth and on other planets as both types of radiation induce the formation of Reactive-Oxygen Species in organisms, which can directly lead metabolic interference as well as DNA lesions (Riley, Reference Riley1994; Jones and Baxter, Reference Jones and Baxter2017). Again, the results from the two isolates are significantly different, while Har. sp. NS06 displays a high resistance against X-ray radiation, Hrr. sp. AS12 (D10: 228,2 Gy) seems highly susceptible to this type of radiation. Previous research has shown that species like Halococcus morrhuae (D10: 2,67 kGy), Halobacterium salinarum NRC-1 (D10: 1,42 kGy) and Halococcus hamelinensis (D10: 1,95 kGy) can tolerate X-Ray irradiation well (Leuko and Rettberg, Reference Leuko and Rettberg2017). Similar to these findings, Har. sp. NS06 may have comparable radiation resistance to the previously tested organisms; however, further tests with higher doses are needed to verify this hypothesis. As for the UV-C resistance, Har. sp. NS06 shows significant better survival (F10: 194,9 J/m2) when compared to Hrr. sp. AS12 (F10 111,6 J/m2) (Figure 3A). Several previous studies have focused on the genera Halobacterium spp., Halococcus spp., and Natronomonas spp. (McCready et al., Reference McCready, Müller, Boubriak, Berquist, Ng and DasSarma2005; Moeller et al., Reference Moeller, Reitz, Douki, Cadet, Horneck and Stan-Lotter2010; Leuko et al., Reference Leuko, Neilan, Burns, Walter and Rothschild2011; Leuko et al., Reference Leuko, Domingos, Parpart, Reitz and Rettberg2015; Stantial et al., Reference Stantial, Dumpe, Pietrosimone, Baltazar and Crowley2016) but there are not many studies published investigating the UV-C resistance of representatives of the genera Halorubrum spp. and Haloarcula spp. (Wu et al., Reference Wu, McGenity, Rettberg, Simoes, Li and Antunes2022). Baxter and colleagues showed that Halorubrum salsolis, an isolate from the Great Salt Lake (Utah) is highly resistant against UV-C radiation (LD50 203 J/cm2), which is significantly more resistant than the isolate Hrr. sp. AS12 (Baxter et al., Reference Baxter, Eddington, Riddle, Webster and Avery2007). Other studies that exposed Haloarcula spp. or Halorubrum spp. to UV radiation are difficult to compare since Fendrihan and colleagues exposed Haloarcula japonica to the full spectrum of UV light (200–400 nm) and the cells were entrapped in halite crystals (Fendrihan et al., Reference Fendrihan, Berces, Lammer, Musso, Ronto, Polacsek, Holzinger, Kolb and Stan-Lotter2009), whereas Trigui and colleagues focused on the response of different Halorubrum strains to UV-B radiation (Trigui et al., Reference Trigui, Masmoudi, Brochier-Armanet, Maalej and Dukan2011).
One important defense mechanism against radiation is the internal salt concentration of halophilic. It has previously been showed that the high halides concentration in the cytoplasm of Hbt. salinarum NRC-1 is a major factor in protecting the DNA against such oxidative effects (Kottemann et al., Reference Kottemann, Kish, Iloanusi, Bjork and DiRuggiero2005; Kish et al., Reference Kish, Kirkali, Robinson, Rosenblatt, Jaruga, Dizdaroglu and DiRuggiero2009). It may be speculated at this point, if there is a difference in the internal salt concentration of both strains which leads to the observed results. As discussed for desiccation, the potential polyploidy of the organisms is of high interest, since several copies of the DNA would mitigate radiation-induced DNA damage. Another important factor in radiation resistance is the ability to efficiently repair DNA damage. Halophilic Archaea have highly effective and resilient mechanisms for repairing various types of damage (Kish and DiRuggiero, Reference Kish, DiRuggiero and Vreeland2012). Their genomes contain genes with evolutionary ties to both eukaryotic cells, such as yeast rad genes, and bacteria, including uvr genes (DasSarma et al., Reference DasSarma, Kennedy, Berquist, Ng, Baliga, Spudich, Krebs, Eisen, Johnson and Hood2001). Genes for different repair mechanisms such as, rad, dsb and uvr are readily available within both strains yet interestingly it seems that they do not possess the photolyase gene phr2. This may be due to the fact that these organisms have been trapped for an extended period of time underground, and a similar phenomenon was recently reported by Nag and colleagues, where they showed that isolates from the subsurface lack a robust photoreactivation system (Nag et al., Reference Nag, DasSarma, Crowley, Hamawi, Tepper, Anton, Guzmán and DasSarma2023). Interestingly, Har. sp. NS06 revealed two have to different copies of the rad gene within its primary chromosome, while AS12 showed only one copy. In addition, NS06 is having multiple plasmids, AS12 consists of only one primary chromosome and one plasmid (Runzheimer et al., Reference Runzheimer, Schwab, Engel, Schaudinn, Laue, Rebrosova, Beblo-Vranesevic and Leuko2025). This might also explain the lower sensitive of NS06 in comparison to AS12 in terms of radiation.
Besides UV-C and X-ray radiation, survival abilities of the strains against high atomic number and energy (HZE) particles were tested. X-rays and gamma rays are classified as low-LET (Linear Energy Transfer) radiation sources, with LET values ranging from less than 0.5 keV/μm for gamma rays to a few keV/μm for X-rays (Pouget and Mather, Reference Pouget and Mather2001). In contrast, HZE particles, such as helium, ferrous and argon ions, are highly ionizing and considered high-LET radiation sources. These particles are of particular interest to radiobiologists because they represent a significant source of radiation exposure in outer space (Horneck, Reference Horneck1994). Cosmic radiation’s heavy ions are hypothesized to impose the ultimate limit on the survival of organisms in space (Horneck et al., Reference Horneck, Baumstark-Khan, Reitz and Bitton2002). When exposing Har. sp. NS06 and Hrr. sp. AS12 to up to 500 Gy of He, Ar, and Fe particles, we could not determine a significant decrease in survival due to the radiation. Hrr. sp. AS12 showed a significant decline (−log3 reduction) in survival, however, this was observed in the transport control as well (Figure 4). It was previously shown that halophilic Archaea survive up to 1 kGy of HZE particle exposure with little effect on the survival and DNA integrity (Leuko and Rettberg, Reference Leuko and Rettberg2017). However, no data in regard to stress resistance towards ionizing irradiation is yet available for the genera Halorubrum and Haloarcula (Wu et al., Reference Wu, McGenity, Rettberg, Simoes, Li and Antunes2022). It would be of great interest to expose these two strains to higher HZE particle radiation to investigate if they behave in a similar way like other representatives of the halophilic Archaea.
Perchlorates display an interesting and important factor to study in terms of potential life on other moons and planets. In fact, perchlorates are abundant in space, while previous studies have reported a concentration of 0.4 to 0.6% of perchlorates on Mars, with Na2+ and Mg2+ being the predominant cations found on Mars (Hecht et al., Reference Hecht, Kounaves, Quinn, West, Young, Ming, Catling, Clark, Boynton, Hoffman, De Flores, Gospodinova, Kapit and Smith2009). Perchlorate salts are able to decrease the freezing point and may enable the presence of liquid brines on Mars and on other planets (Ligier et al., Reference Ligier, Poulet, Carter, Brunetto and Gourgeot2016; Chevier and Slank, Reference Chevrier and Slank2024) and thus potential life. Perchlorate are commonly toxic for life and especially microorganisms as past research has shown for example bacterial pathogens (Zaccaria et al., Reference Zaccaria, de Jonge, Domínguez-Andrés, Netea, Beblo-Vranesevic and Rettberg2024).
Our experiments have shown that Na- and MgClO4 are growth promoting on both haloarchaeal strains (Figure 5), In terms of haloarchaea, a study investigating the effect of perchlorates on Halobacterium sp. NRC-1 reported no growth after supplementation with 0.04 M (0.49%) NaClO4 (Laye and DasSarma, Reference Laye and DasSarma2018), while other studies revealed no negatively effect on growth of Halobacterium salinarum of 0.2 M (2.45%) (Oren et al., Reference Oren, Bardavid and Mana2014). Studies have already shown that haloarchaea (Haloarcula, Halomonas and Haloferax species) are able to use perchlorate as alternative electron acceptors within anaerobic growth (Okeke et al., Reference Okeke, Giblin and Frankenberger2002; Oren et al., Reference Oren, Bardavid and Mana2014). Promoted growth within this study may be either due to higher ionic concentrations of magnesium and sodium, or/and the metabolic use of perchlorate.
Conclusions
The desire of exploring and searching for life in extreme places has been a human endeavor for centuries. Here, we can add the brine of Lüneburg to such an environment, which can help us to increase the possibility of finding life on other planets. The two tested strains, Halorubrum. sp. AS12 and Haloarcula sp. NS06, showed a remarkable difference in survival following different environmental stress factors, leaving us with the question on why there is such a discrepancy. While Halorubrum sp. AS12 displayed a higher sensitivity to UV-C, X-ray, and heavy ion irradiation, Haloarcula sp. NS06 was more susceptible to desiccation. The growth rates of both strains increased when the medium was supplemented with perchlorates at concentrations similar to those found on Mars. Further research should look into the molecular differences between these two strains, elucidating potential differences or novel strategies to mitigate environmental stress situations. Our experiments contribute to our understanding of the stress resistance of haloarchaea, as the class Halobacteria still lacks important stress response data.
Data availability statement
The 16S rRNA data are available within the NCBI GenBank with submission numbers PV344486 and PV344487. Genome sequences are available within the BioProject Numbers PRJNA1236223 (Halorubrum sp. AS12) and PRJNA1236224 (Haloarcula sp. NS06).
Acknowledgments
The authors would like to thank the team of the German Salt Museum under the lead of Dr. Alexandra Hentschel. A special thanks goes to Jürgen Stenzel for his continuous help and support during our sampling campaigns. We would also like to remember Prof. Ralf Möller who initiated this cooperation, who sadly passed away way too young in 2024. K.R. and S.L were supported by the BMBF-VDI/VDEInnovation + Technik grant “MultiKulti “(Förderkennzeichen: 161L0285D) and by the German Aerospace Center (Deutsches Zentrum für Luft- und Raumfahrt, DLR) grant FuE-Project “ISS LIFE,” LFuW, TP 475). K.R. was supported by ESA via the ESA Contract No. 4000137602/22/NL/GLC/my for the Co-sponsored Ph.D., project I-2021-01758 (BioProtect-Bioinspired Shielding Material for Radiation Protection Purposes)
Competing interests
Both authors declare no conflict of interest.







