Introduction
Boreal forest soils are typically covered by a thick layer of surface accumulated organic matter known as the forest floor (Prescott et al. Reference Prescott, Maynard and Laiho2000), which stores over 20% of the total ecosystem carbon in Canada’s managed boreal forests (Kurz et al. Reference Kurz, Shaw, Boisvenue, Stinson, Metsaranta and Leckie2013). The majority of soil biological processes occur in the forest floor, which is also habitat for many soil fauna (Coleman et al. Reference Coleman, Crossley and Hendrix2004). Oribatid mites are an abundant faunal group in Canada’s boreal forest floor, where they are considered keystone detritivores, initiating litter decomposition and nutrient cycling while maintaining forest floor structure (Behan-Pelletier Reference Behan-Pelletier1999; Coleman et al. Reference Coleman, Crossley and Hendrix2004). Soil mites play a major role in regulating soil organic matter decomposition, including through the consumption of litter and fungal mycelia, which generates frass. This contributes to particulate organic matter and creates favourable conditions for increased microbial biomass, leading to production of mineral-associated organic matter (Angst et al. Reference Angst, Potapov, Joly, Angst, Frouz, Ganault and Eisenhauer2024). Oribatid mites have also been shown to influence microbial communities by transporting microorganisms in their gut or on their body surface (Angst et al. Reference Angst, Potapov, Joly, Angst, Frouz, Ganault and Eisenhauer2024), by stimulating fungal growth in leaf litter (Kun Reference Kun2015), and by influencing fungal community composition (Janoušková et al. Reference Janoušková, Kohout, Moradi, Doubková, Frouz, Vosolsobě and Rydlová2018), among other pathways.
The recent invasion of nonnative earthworm species into the northern boreal forests of North America may threaten this carbon reservoir (Langor et al. Reference Langor, Cameron, MacQuarrie, McBeath, McClay and Peter2014). Earthworms, similar to oribatid mites, are detritivorous fauna (Frelich et al. Reference Frelich, Hale, Scheu, Holdsworth, Heneghan, Bohlen and Reich2006; Eisenhauer Reference Eisenhauer2010) and have long been classified as “ecosystem engineers” for their substantial influence on soil structure and associated biogeochemical processes, including litter decomposition (Jones et al. Reference Jones, Lawton and Shachak1994; Lavelle Reference Lavelle1997). Earthworms can increase soil organic matter decomposition rates throughout the soil profile when introduced into previously earthworm-free ecosystems, and they can also increase microbial activity and alter endemic biotic communities (Wardle Reference Wardle2002; Bohlen et al. Reference Bohlen, Scheu, Hale, McLean, Migge, Groffman and Parkinson2004b; Frelich et al. Reference Frelich, Hale, Scheu, Holdsworth, Heneghan, Bohlen and Reich2006). The rate and extent of earthworm-induced alterations depend on the earthworm ecological group(s) present (Bohlen et al. Reference Bohlen, Scheu, Hale, McLean, Migge, Groffman and Parkinson2004b) – namely, epigeic (litter-dwelling), endogeic (mineral-dwelling), and anecic (litter- and mineral-dwelling) earthworms, as defined by Bouché (Reference Bouché1977). Although anecic species on their own have the largest impacts on soil properties through bioturbation of the mineral and organic layers, the presence of multiple ecological groups can cause an even greater extent of alterations (Hale et al. Reference Hale, Frelich, Reich and Pastor2008; Cameron et al. Reference Cameron, Knysh, Proctor and Bayne2013a; Huang et al. Reference Huang, González and Zou2020). Most similar to oribatid mites in ecological function and habitat are the epigeic earthworm species (2–5 cm long), which often lead the invasion front of nonnative earthworms into new environments (Hendrix Reference Hendrix2006; Eisenhauer Reference Eisenhauer2010). Lastly, the magnitude of the impacts caused by an earthworm invasion within an ecosystem depends largely on land use, with earthworm invasion posing a higher risk of significantly altering undisturbed forests than already-managed agricultural soils (Bohlen et al. Reference Bohlen, Pelletier, Groffman, Fahey and Fisk2004a).
Oribatid mites are sensitive to soil perturbation and can be used as indirect indicators for the impacts of nonnative earthworms on soil biological parameters (Dunger and Voigtländer Reference Dunger and Voigtländer2005; Gutiérrez López et al. Reference Gutiérrez López, Jesús Lidón, Trigo Aza, Fernández García and Díaz Cosín2009). However, for boreal forest soils, such interactions have been studied only in laboratory experiments (Cameron et al. Reference Cameron, Knysh, Proctor and Bayne2013a, Reference Cameron, Proctor and Bayne2013b). To date, few field studies have assessed the influence of nonnative earthworms on northern boreal soils, and none has focused on impacts on microarthropod communities (Cameron et al. Reference Cameron, Bayne and Clapperton2007; Cameron and Bayne Reference Cameron and Bayne2009; Lejoly et al. Reference Lejoly, Quideau and Laganière2021). The current, ongoing earthworm invasion into the Canadian boreal forest poses a unique opportunity to study the impacts of nonnative earthworms on endemic soil biological communities and associated biogeochemical processes at a landscape scale (Frelich et al. Reference Frelich, Hale, Scheu, Holdsworth, Heneghan, Bohlen and Reich2006; Blouin et al. Reference Blouin, Hodson, Delgado, Baker, Brussaard and Butt2013). The aim of the present study was to assess the current state of earthworm activity in a boreal stand of trembling aspen, Populus tremuloides Michaux (Salicaceae), in northern Alberta by achieving the following objectives: (1) to assess the physical extent of and the earthworm ecological groups present in the invasion; (2) to determine the effects of the earthworm invasion on stand characteristics, including forest floor thickness, soil properties, and understorey vegetation; and (3) to determine the effects of the earthworm invasion on other soil biodiversity, specifically oribatid mite communities. Based on the current state of knowledge on earthworm invasion, we predicted that: (1) earthworms will have invaded further into the stand at higher densities than previously documented for the area (Cameron et al. Reference Cameron, Bayne and Clapperton2007) and potentially would include additional taxa that have been detected in Alberta (Reynolds Reference Reynolds2022); (2) higher earthworm densities or multiple functional groups will have reduced forest floor thickness and understorey vegetation diversity and will have altered soil properties (Hale et al. Reference Hale, Frelich and Reich2005a; Eisenhauer et al. Reference Eisenhauer, Partsch, Parkinson and Scheu2007; Addison Reference Addison2009); and (3) higher earthworm densities or multiple functional groups will have altered oribatid mite abundance, diversity, and community structure (Eisenhauer et al. Reference Eisenhauer, Partsch, Parkinson and Scheu2007; Burke et al. Reference Burke, Maerz, Milanovich, Fisk and Gandhi2011).
Materials and methods
Study area and site selection
The study area was located south of Wolf Lake Provincial Recreational Area (54.6484° N, –110.9815° W), in the Central Mixedwood Natural Subregion of Alberta’s boreal forest region (Natural Regions Committee 2006). Central mixed-wood forests are composed of trembling aspen–dominated canopy and common understorey plants, including low bush cranberry, Viburnum edule (Michaux) Rafinesque (Adoxaceae), rose, Rosa acicularis Lindley (Rosaceae), wild sarsaparilla, Aralia nudicaulis Linnaeus (Araliaceae), dewberry, Rubus pubescens Rafinesque (Rosaceae), bunchberry, Cornus canadensis Linnaeus (Cornaceae), and Canada buffaloberry, Shepherdia canadensis (Linnaeus) Nuttall (Elaeagnaceae). The climate is characterised by long, cold winters and cool, short summers, with mean monthly temperatures ranging from –15.1 °C to 17.4 °C and an average of 116 frost-free days each year. Mean annual precipitation is 421 mm, with 319 mm falling as rain (Environment Canada 2010). Soils in the area are mostly orthic grey luvisols, developed over moderately to strongly calcareous glacial till (Alberta Agriculture and Forestry 2017). To control for site variation, one aspen stand covering an approximate area of 0.03 km2 and with consistent canopy type, slope aspect, and minimal topographic changes (≤ 2% slope) was selected. The selected stand was close to Wolf Lake, Alberta sites used in previously published nonnative earthworm studies (Cameron et al. Reference Cameron, Bayne and Clapperton2007, Reference Cameron, Knysh, Proctor and Bayne2013a; Cameron and Bayne Reference Cameron and Bayne2009).
Field sampling
Field sampling occurred in August 2016. First, earthworm invasion was extensively investigated by conducting preliminary earthworm surveys every 20 m, using two transects (10 m apart) extending from the forest edge (∼10 m from the road) to the forest interior (∼350 m from the road). These surveys led to the identification of two 400-m2 areas: (1) an area with lower earthworm density 300 m from the road that was occupied by only epigeic species and was characterised by the presence of distinct litter and fermented and humified organic soil horizons, and (2) an area with higher earthworm density 150 m from the road that was occupied by multiple epigeic and endogeic earthworm species and was characterised by the obvious mixing of mineral and organic soil horizons with many casts present. Four 4-m2 subplots (Fig. 1) were established just beyond each corner boundary of the 400-m2 plots, with a distance of at least 20 m between collection points, as documented in Eisenhauer et al. (Reference Eisenhauer, Partsch, Parkinson and Scheu2007) and Straube et al. (Reference Straube, Johnson, Parkinson, Scheu and Eisenhauer2009), which assessed impacts of earthworm invasion on soil microarthropods in a deciduous forest stand. A soil pit was dug in the middle of each 400-m2 area and was described according to the Canadian system of soil classification (Soil Classification Working Group 1998). This description confirmed that soils belonged to the orthic grey luvisolic subgroup.
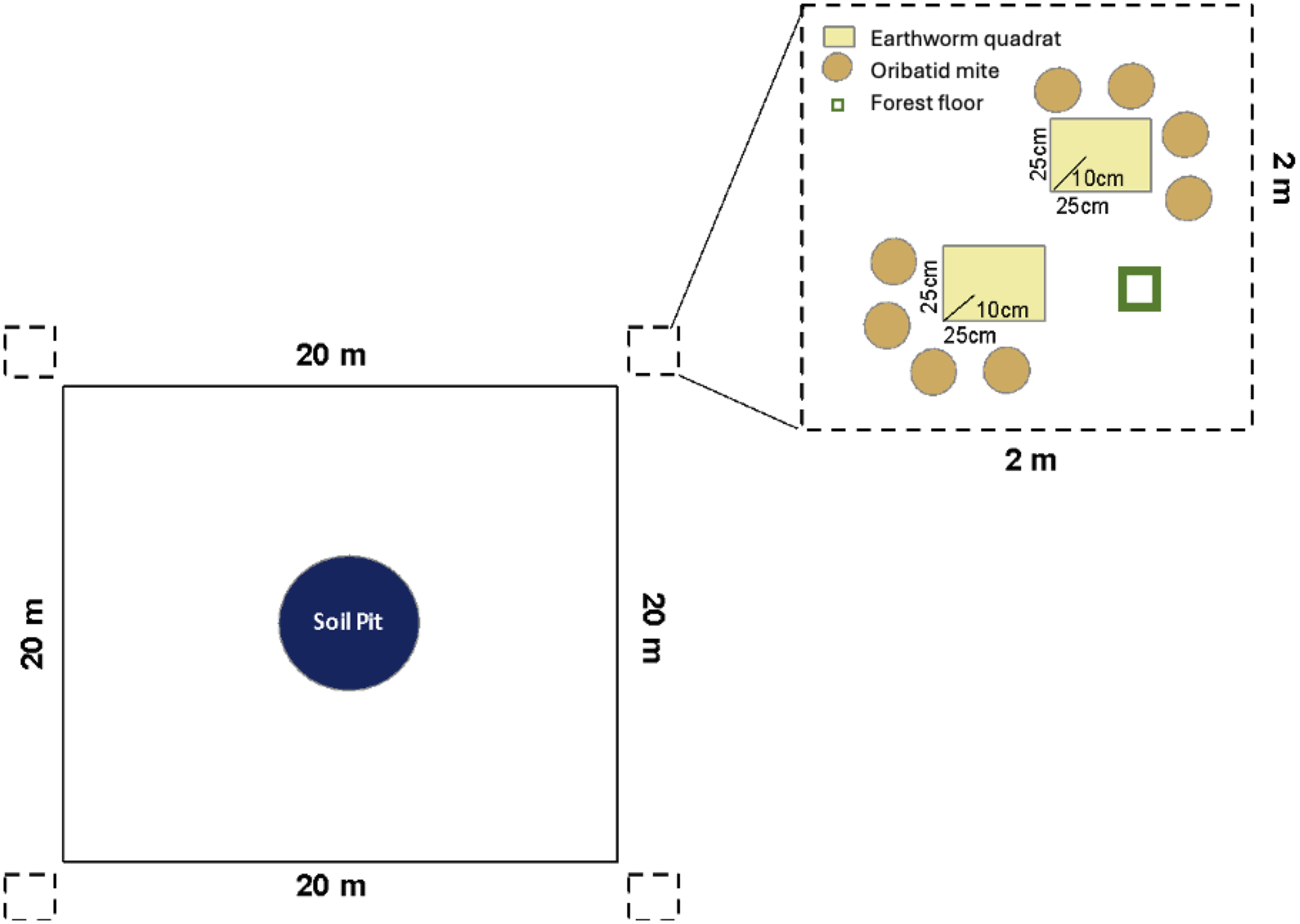
Figure 1. Schematic diagram of field sampling for each earthworm invasion area (400 m2). The central blue circle shows the location of the soil pit, with the four sampling subplots (dashed boxes) established 20 m from each other. In each subplot, earthworms were sampled in two 25-cm × 25-cm quadrats, samples for oribatid mites were obtained from four samples around each earthworm quadrat, and the entire forest floor was sampled using a 10-cm × 10-cm template.
At each of the four subplots, the presence and distribution of earthworm species were assessed by excavating two 25-cm × 25-cm quadrats to a depth of 10 cm, per Cameron and Bayne (Reference Cameron and Bayne2009), which had previously shown this to be a sufficient size. A liquid extraction was performed on all excavated earthworm quadrats to collect specimens found below 10 cm using hot mustard solution (“hot” meaning spicy or irritating; Lawrence and Bowers Reference Lawrence and Bowers2002). Earthworm specimens were handsorted in the excavated soil and from the liquid extraction, then fixed in formalin, and stored in 90% isopropyl alcohol until identification. In addition, oribatid mites were sampled using a modified version of the Alberta Biodiversity Monitoring Institute terrestrial protocol for arthropod extraction from organic soils (Alberta Biodiversity Monitoring Institute 2014; McAdams et al. Reference McAdams, Quideau, Swallow and Lumley2018). In each of four locations around each earthworm quadrat, four subsamples were collected using a 7.5-cm-diameter metal soil corer to a depth of 7.5 cm and were composited to obtain one 500-cm3 sample. This resulted in a total of two earthworm quadrats and eight oribatid mite samples per subplot in each invasion area (Fig. 1). A total of eight earthworm quadrats were excavated, and 32 oribatid mite samples were collected at each of the two areas under study (the lower earthworm density and higher earthworm density areas). Within each of the four subplots per area, the forest floor was also sampled using a 10-cm × 10-cm quadrat for bulk density and moisture content assessment. Thickness of the forest floor was measured at the four corners of the template to estimate the volume of forest floor removed. Understorey vegetation richness was determined before all soil sampling by identifying understorey vegetation species within a 1-m2 area without trees (Johnson et al. Reference Johnson, Kershaw, MacKinnon and Pojar1995).
Laboratory analyses: specimen identification and soil properties
Earthworm specimens were fixed with formalin and preserved in 90% isopropyl alcohol before counting and identification to the genus and species levels as possible (Reynolds Reference Reynolds1977). Identified specimens were separated into two groups: mineral-burrowing (Apporectodea spp.) and litter-dwelling (Dendrobaena octaedra) earthworms.
Within seven days of field collection, soil invertebrates were extracted into containers containing absolute ethanol on a modified Tullgren funnel (Crossley and Blair Reference Crossley and Blair1991) and separated into two size fractions using stacked 53-µm and 300-µm sieves. Oribatid mite specimens that were no smaller than 300 µm were identified to species level using Walter et al. (Reference Walter, Latonas, Byers and Lumley2014). When compound microscopy was necessary for identification, specimens were cleared in 85% lactic acid, mounted on slides using polyvinyl alcohol, and oven-dried at 55–60 °C. All specimens and residuals were deposited in the Alberta Biodiversity Monitoring Institute Terrestrial Invertebrates collection at the University of Alberta, Edmonton, Alberta, Canada.
Bulk density and gravimetric moisture content were calculated by weighing forest floor samples before and after oven-drying at 65 °C for 48 hours (Kalra and Maynard Reference Kalra and Maynard1991). Additional soil properties were determined from the air-dried forest floor samples used for oribatid mite extraction. For carbon and nitrogen analyses, samples were finely ground with a ball mill and analysed through dry combustion using a Thermo FLASH 2000 model combustion Elemental Analyzer (ThermoFisher Scientific, Waltham, Massachusetts, United States of America). The pH values were determined in a 0.01-M CaCl2 solution (1:4 air-dried soil:CaCl2 solution ratio) with an Accumet XL200 meter (ThermoFisher Scientific; Kalra and Maynard Reference Kalra and Maynard1991).
Statistical analyses
All variables were analysed, using R statistical software, version 6.3.2 (R Core Team 2020), for differences between (1) lower-density + single-species earthworm invasion and (2) higher-density + multiple-species earthworm invasion. Selected variables included bulk density, moisture content, forest floor thickness, pH, total organic carbon, total nitrogen, carbon:nitrogen ratio, understorey vegetation richness, and oribatid soil mite community characteristics, including oribatid abundance and species richness. Oribatid mite abundance was represented as the number of individuals of each species, and oribatid species richness was represented as the total number of different species per composite (500-cm3) sample.
Before statistical analysis, data were checked for normality and homogeneity assumptions using the Shapiro–Wilk and Levene’s tests, respectively. Data were transformed using inverse transformation when necessary to meet normality criteria (total organic carbon, total nitrogen). The effects of earthworm invasion on mite abundance, mite richness, and soil and vegetation characteristics were determined by one-way analysis of variance, with earthworm density as a factor and subplot as block, except for bulk density, moisture content, forest floor thickness, and understorey vegetation richness, where only one measurement per subplot was available and, therefore, one-way analysis of variance was performed without blocks.
Nonmetric multidimensional scaling was performed using the vegan package (Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre and Mcglinn2019) to visualise oribatid mite composition on all oribatid mite samples (n = 64). Species count data were used after Hellinger transformation, adequate for zero-inflated datasets, using the Bray–Curtis distance measurement (Legendre and Gallagher Reference Legendre and Gallagher2001; Anderson and Willis Reference Anderson and Willis2003). Additionally, all site and soil characteristics were considered for vectors in the nonmetric multidimensional scaling using the envfit function from the vegan package. Following nonmetric multidimensional scaling, a permutational multivariate analysis of variance was conducted, using the adonis function from the vegan package, with subplot serving as block, to identify differences in community composition between the two earthworm density areas.
Results and discussion
Earthworm distribution
Our first objective was to assess the physical extent of the invasion and the earthworm ecological groups present in the invasion, predicting that earthworms will have invaded further into the stand at higher densities than previously documented for the area (Cameron et al. Reference Cameron, Bayne and Clapperton2007) and that they potentially would include additional taxa that had been detected in Alberta (Reynolds Reference Reynolds2022). Our preliminary investigation of earthworm invasion along transects showed apparent nonlinear invasion and no obvious invasion front or leading edge from the road to the interior forest, unlike what was observed in field studies in Minnesota, United States of America (Hale et al. Reference Hale, Frelich and Reich2005a; Schlaghamerský et al. Reference Schlaghamerský, Eisenhauer and Frelich2014). In the lower earthworm density area 300 m from the road, one earthworm species, Dendrobaena octaedra, was found, with an average of 27 ± 11 adults per square metre (adults/m2) and 26 ± 11 juveniles per square metre (juveniles/m2; Table 1). These densities are higher than Cameron et al. (Reference Cameron, Bayne and Clapperton2007) documented for Dendrobaena in interior forest sites (300–500 m from road; mean relative densities of 4.5 ± 2.9 adults/m2 and 2.5 ± 2.2 juveniles/m2) in the Alberta boreal forest. In the higher-density earthworm area 150 m from the road, D. octaedra was also found (20 ± 13 adults/m2 and 54 ± 30 juveniles/m2), together with Aporrectodea spp. (Crassiclitellata: Lumbricidae) at a density of 58 ± 30 adults per square metre and 40 ± 17 juveniles per square metre, thereby corresponding to a higher total earthworm density compared to the lower-density area. Although D. octaedra has been observed in more remote and interior forests, Aporrectodea spp. are common bait species that are usually found near boat launches (Cameron et al. Reference Cameron, Bayne and Clapperton2007; Cameron and Bayne Reference Cameron and Bayne2009). All Aporrectodea species detected in Alberta to date are endogeic (Reynolds Reference Reynolds2022). Cameron et al. (Reference Cameron, Bayne and Clapperton2007) detected Aporrectodea spp. only at boat launches except for a single plot on one road transect, where one adult and two juveniles were detected 2 m from the forest edge along a road. Although we predicted that densities would have increased since Cameron et al.’s (Reference Cameron, Bayne and Clapperton2007) survey, we were not expecting to not be able to locate a control area with no earthworm species in interior forest nor to detect endogeic species at such distance (150 m) from the road. We did not detect any anecic species, which are also known to occur in Alberta (Reynolds Reference Reynolds2022). We suggest continued monitoring to assess the invasion front of Aporrectodea spp. and to document earthworm species and densities because other studies indicate that they have not yet peaked (McLean and Parkinson Reference McLean and Parkinson2000; Migge Reference Migge2001; Eisenhauer et al. Reference Eisenhauer, Partsch, Parkinson and Scheu2007). In a study in a lodgepole pine, Pinus contorta var. latifolia Engelmann (Pinaceae), forest in Alberta’s Rocky Mountain natural region, field densities of the epigeic species, D. octaedra, were as high as 3349 individuals per square metre, with a mean of 854 individuals per square metre (McLean and Parkinson Reference McLean and Parkinson2000). At a later stage of invasion, endogeic species such as Aporrectodea caliginosa were found at a density of 125 individuals per square metre (Migge Reference Migge2001). These densities are higher than we observed in the present study, suggesting that as the current invasion continues to spread throughout the aspen stand, earthworm densities of all ecological groups, and specifically of the endogeic and anecic species groups, will likely increase (Eisenhauer et al. Reference Eisenhauer, Partsch, Parkinson and Scheu2007).
Table 1. Mean earthworm densities (adults/m2 and juveniles/m2) and standard errors in parentheses for adult and juvenile specimens of epigeic and other (endogeic/anecic) species detected in lower earthworm density and higher earthworm density areas.

Soil characteristics and understorey vegetation
Our second objective was to assess the effects of the earthworm invasion on the forest floor, understorey vegetation, and soil properties. As predicted, both forest floor and surface vegetation were altered by earthworm invasion in the higher-density area and were characterised by significantly (P < 0.05) thinner forest floors with higher bulk density and a trend (P < 0.07) towards lower moisture content, as compared to the lower-density area (Table 2; Supplementary material, Table S1). Similar changes to the soil habitat were observed after a multiple-species invasion in Minnesota (Hale et al. Reference Hale, Frelich, Reich and Pastor2008). The decrease in forest floor thickness that we observed is consistent with previous observations of most studies (e.g., Hale et al. Reference Hale, Frelich, Reich and Pastor2005b; Eisenhauer et al. Reference Eisenhauer, Partsch, Parkinson and Scheu2007) and could be due to both the higher total earthworm density and the activity of multiple species, including the increased feeding and burrowing activities of D. octaedra and Aporrectodea spp. that increase litter turnover or litter–mineral soil bioturbation (Hale et al. Reference Hale, Frelich, Reich and Pastor2005b; Wironen and Moore Reference Wironen and Moore2006; Addison Reference Addison2009; Lejoly et al. Reference Lejoly, Quideau and Laganière2021). Bioturbation by earthworms would also explain the lower total organic carbon (P < 0.001), lower total nitrogen (P < 0.001), higher bulk density, and trend towards lower moisture content of the forest floor in the higher earthworm density area because mineral particles typically are denser with much lower water-holding capacity, total organic carbon content, and total nitrogen content compared to organic soil layers (Lavelle Reference Lavelle1997). The combination of thinner forest floors and lower total organic carbon content suggests that carbon stocks in the forest floor might decrease with earthworm invasion. This hypothesis is supported by Cameron et al. (Reference Cameron, Shaw, Bayne, Kurz and Kull2015), which estimated that the combined effects of invasive earthworms and wildfires could reduce forest floor carbon stocks in northern Alberta by 50–94%.
Table 2. Site characteristics of the lower earthworm density and higher earthworm density areas. Means and standard errors (in parentheses) are displayed. Different letters indicate significant differences between the lower earthworm density and higher earthworm density areas according to the analysis of variance with statistical significance set at P-value < 0.05, which can be found in Supplementary material, Table S1.
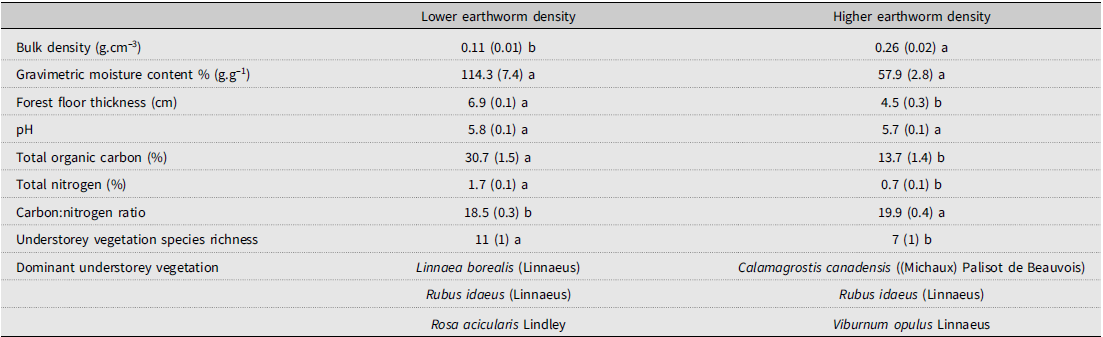
In addition, the carbon:nitrogen ratio of the forest floor was significantly higher in the higher-density area (P < 0.001; Table 2; Supplementary material, Table S1). Wironen and Moore (Reference Wironen and Moore2006) also found an increase in carbon:nitrogen in the litter layer following earthworm invasion in southern Quebec. This may be due to the addition of more recalcitrant soil organic matter from deeper soil horizons or to a change in the understorey vegetation composition, or it could result from earthworm feeding on more palatable compounds, leaving behind woodier debris such as leaf stems (Curry and Schmidt Reference Curry and Schmidt2007). Lastly, as Lejoly et al. (Reference Lejoly, Quideau, Laganière, Karst, Martineau and Swallow2023) reported following earthworm invasion, increased fungi:bacteria ratios may have also contributed to the increased levels of carbon compared to nitrogen due to the higher carbon:nitrogen ratios generally found in soil fungal biomass compared to soil bacterial biomass.
Understorey vegetation species richness was significantly lower in the higher-density area (P < 0.001; Table 2; Supplementary material, Table S1), where the dominant species was Calamagrostis canadensis ((Michaux) Palisot de Beauvois) (Poaceae). Contrastingly, the area with the lower density of earthworms had sparse amounts of grassy vegetation and was dominated by twinflower, Linnaea borealis (Linnaeus) (Caprifoliaceae), red raspberry, Rubus idaeus (Linnaeus) (Rosaceae), and rose, R. acicularis Lindley. Craven et al. (Reference Craven, Thakur, Cameron, Frelich, Beauséjour and Blair2017) found that vegetation diversity significantly declined with increasing earthworm richness, although plant species richness did not significantly change in their meta-analysis. The differences in understorey vegetation observed in the present study could again be linked to bioturbation, with mixing of the forest floor into the mineral soil layer altering soil nutrient and microbial dynamics and resulting in a grass-dominated environment. Costello and Lamberti (Reference Costello and Lamberti2009) found increased nitrogen turnover due to invasive earthworms, which could facilitate further increase in general nutrient cycling linked to the microbial community, with a potential shift from fungal dominance to the bacterial dominance seen in rapid cycling grasslands (de Vries et al. Reference de Vries, Hoffland, van Eekeren, Brussaard and Bloem2006). However, because we also observed a higher carbon:nitrogen ratio in the higher earthworm density area, which is typically associated with fungal dominance, other mechanisms may be in play, including that earthworms, which have been documented as seed predators, may alter plant communities through preferentially selecting for seed size (Cassin and Kotanen Reference Cassin and Kotanen2016).
Oribatid mite community
Our third objective was to determine the effects of the earthworm invasion on oribatid mite communities. A total of 940 oribatid mite individuals were extracted from the forest floor samples, and 24 species were identified from 18 families. Mite abundance was 25% (149 individuals) lower in the higher earthworm density area compared to the lower earthworm density area (P = 0.13; Fig. 2A; Supplementary material, Table S1), which is similar to the decrease in abundance observed by Cameron et al. (Reference Cameron, Proctor and Bayne2013b) in their laboratory studies investigating the effects of earthworm invasion on microarthropods in the forest floor. Although the change in abundance that we observed was not calculated as significant, we suggest continued monitoring of the study area: McLean and Parkinson (Reference McLean and Parkinson1998) observed greater losses in mite abundance as earthworm invasion progressed, and Gutiérrez López et al. (Reference Gutiérrez López, Ramajo Matesanz, Jesús Lidón and Díaz Cosín2003) and Migge-Kleian et al. (Reference Migge-Kleian, McLean, Maerz and Heneghan2006) also found the presence of invasive earthworms to negatively affect the abundance of microarthropods, including oribatid mites. Mite species richness more strongly decreased (34%, P < 0.001) in the higher earthworm density area than did mite abundance (Fig. 2B; Supplementary material, Table S1). Gutiérrez López et al. (Reference Gutiérrez López, Ramajo Matesanz, Jesús Lidón and Díaz Cosín2003), Burke et al. (Reference Burke, Maerz, Milanovich, Fisk and Gandhi2011), and Ferlian et al. (Reference Ferlian, Eisenhauer, Aguirrebengoa, Camara, Ramirez-Rojas and Santos2018) reported a decrease in oribatid mite richness and diversity in the presence of earthworms, but McLean and Parkinson (Reference McLean and Parkinson1998) noted potentially positive effects at moderate densities. Of the 24 species of mites that we identified in the present study, 16 (67%) were present in both areas. The area with a higher earthworm density contained no unique species, but eight species (33%) found in the area with lower earthworm density were unique and were not found in the higher-density area, including six additional species found only as singletons and doubletons (Table 3). All of the unique species are found in upland forest habitats, and most of these species are associated with mid- to old-growth forests (Alberta Biodiversity Monitoring Institute 2023) that contain an intact and substantial forest floor. Battigelli et al. (Reference Battigelli, Spence, Langor and Berch2004) found in their forest harvest study that removal of forest floor reduced the number of rare oribatid mite species. This suggests that the significant loss of forest floor depth that occurred in the higher-density earthworm area may have had a similar impact on rare oribatid mite species in the present study. Although these rarer species may have already existed at lower abundances in the lower-density area, several species are relatively common in the boreal (e.g., Phthiracarus boresetosus (Oribatida: Phthiracaridae), Quatrobelba montana (Oribatida: Damaeidae)), suggesting that the presence of D. octaedra only may have had similar effects as the presence of multiple ecological earthworm groups. Cameron et al. (Reference Cameron, Proctor and Bayne2013b) observed that oribatid mite species richness and abundance did not differ between treatments with only D. octaedra and treatments with both D. octaedra and Lumbricus terrestris (Crassiclitellata: Lumbricidae) (anecic species). The rarer mite species may be highly sensitive to earthworm influence. Migge (Reference Migge2001) reported a similar diversity loss in aspen litter after a multiple-species earthworm invasion.
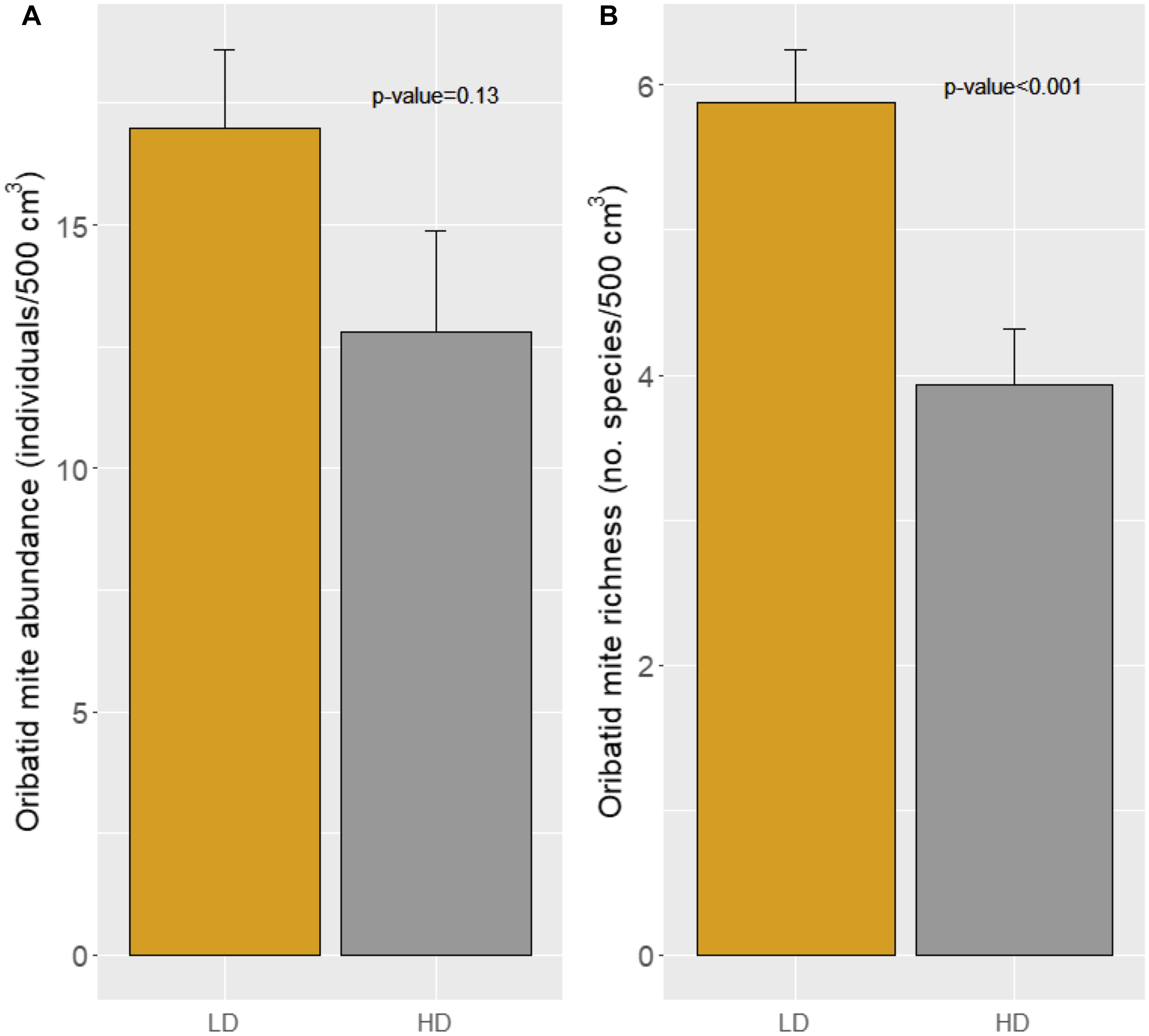
Figure 2. A, Mean oribatid mite abundance and B, species richness per 500 cm3 in the lower earthworm density (LD) and in the higher earthworm density (HD) invasion areas. Each error bar corresponds to one standard error. Differences between invasion areas are displayed above each comparison with the corresponding P-value, obtained from a one-way analysis of variance with earthworm density as factor and subplot as block.
Table 3. Mean oribatid mite species abundance (number of individuals/500 cm3) in the lower-density and higher-density earthworm invasion areas. Values in parentheses represent one standard error.
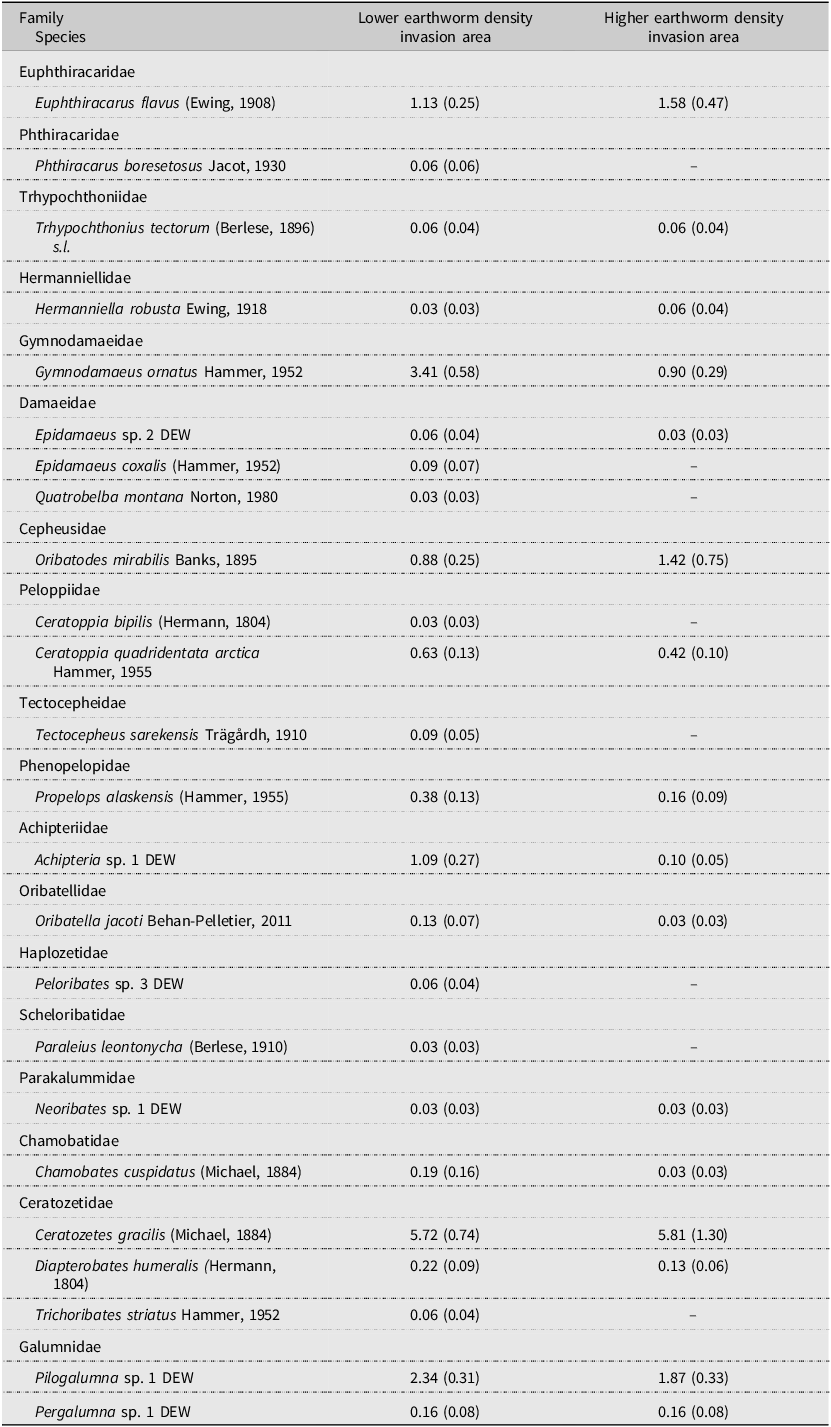
In the present study, the lower-density and higher-density earthworm areas had almost the same dominant oribatid mite species in similar abundances, excluding Gymnodamaeus ornatus Hammer, 1952 (Oribatida: Gymnodamaeidae). The two most dominant species were Ceratozetes gracilis (Michael, 1884) (Oribatida: Ceratozetidae) and Pilogalumna sp. 1 DEW (Oribatida: Galumnidae). Euphthiracarus flavus (Ewing, 1908) (Oribatida: Euphthiracaridae) was also observed at similar densities in both areas (Table 3). In the higher earthworm density area, the abundance of G. ornatus was notably lower (28) than that in the lower-density area (109), corresponding to a reduction by 75%. Gymnodamaeus ornatus is associated most strongly with mature to old-growth deciduous forest habitats (Alberta Biodiversity Monitoring Institute 2023). Intactness models indicate that it is one of the oribatid mite species most sensitive to disturbance in old-growth deciduous and mixed-wood forests in Alberta (Alberta Biodiversity Monitoring Institute 2020, 2022). These habitats typically have an intact, stable forest floor, and the decrease in abundance of G. ornatus is likely due to the changes to the forest floor that we observed in the higher earthworm density area. Further work is needed to assess Gymnodamaeus ornatus and other species found unique to the lower-density area to understand which underlying forest floor factors have the most impact, because earthworms changed not only the forest floor thickness but also the soil properties and understorey vegetation.
The oribatid mite community composition differed between the lower-density and higher-density areas, as revealed by the nonmetric multidimensional scaling and permutational multivariate analysis of variance (P < 0.001; Fig. 3; Supplementary material, Table S2). The results align with Huhta et al.’s (Reference Huhta, Räty, Ahlroth, Hänninen, Mattila, Penttinen and Rintala2005) conclusions that earthworms were the main explanatory variable for differences in oribatid mite community structure. As shown with environmental vectors, the low earthworm density area was positively correlated with total organic carbon and total nitrogen contents, as well as with mite species richness and forest floor thickness, whereas the high earthworm density area was positively correlated with bulk density and carbon:nitrogen ratio. Our results indicate that the presence of species in the Aporrectodea genus in the higher-density area caused a substantial increase in forest floor bulk density, which may have negatively affected mite communities (Table 2). Moreover, because the abundance of oribatid mites is typically lower in mineral soil (Straube et al. Reference Straube, Johnson, Parkinson, Scheu and Eisenhauer2009), the inclusion of mineral particles into the forest floor from earthworm bioturbation may have contributed to the decrease in forest floor species richness observed in this study.
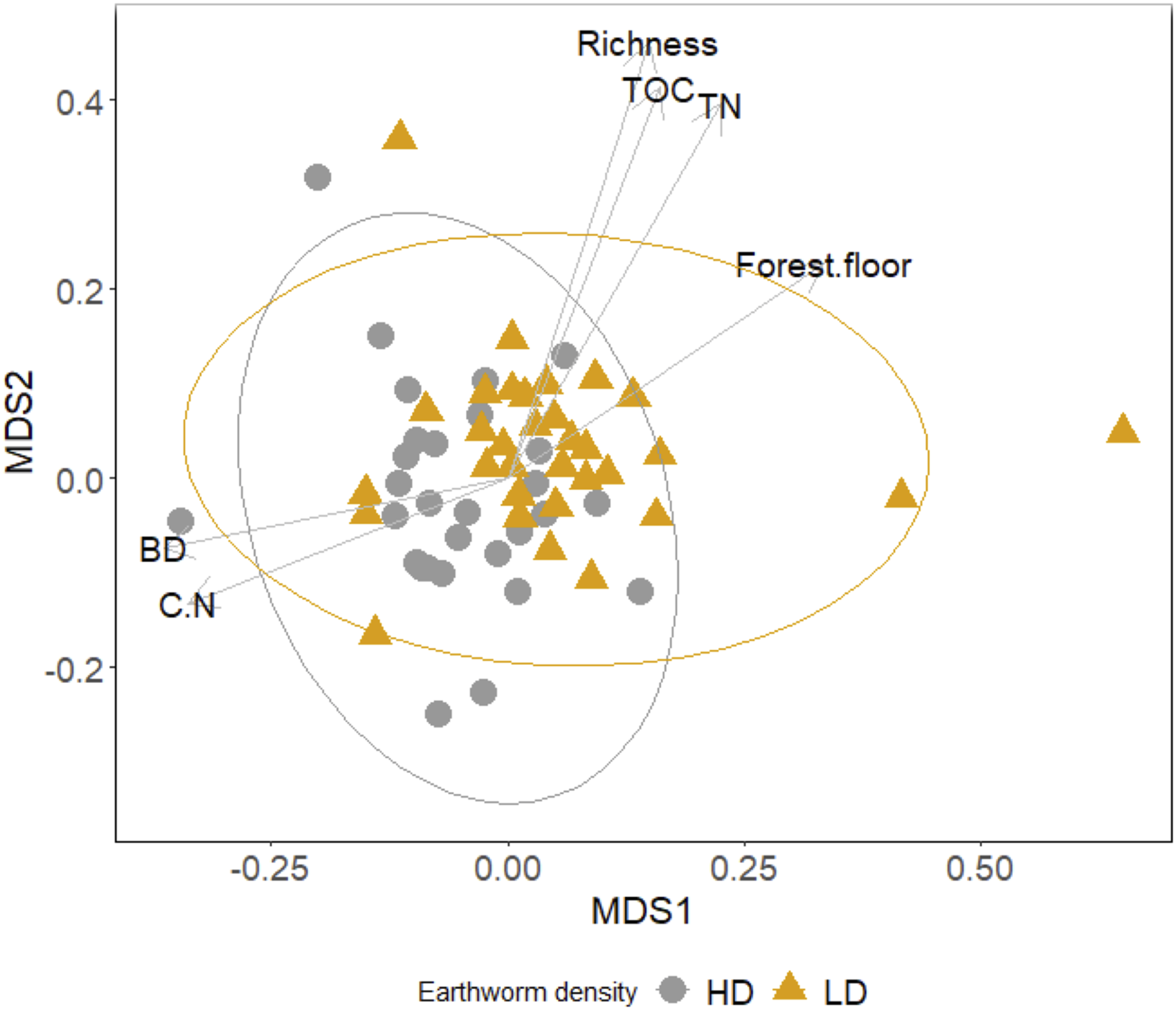
Figure 3. Nonmetric multi-dimensional scaling (NMDS) ordination using Hellinger-transformed oribatid mite abundance data in the lower earthworm density (LD) and higher earthworm density (HD) invasion areas with a final stress of 18.9% and nonmetric fit R 2 = 0.96. Vectors correspond to environmental variables strongly associated with the mite abundance distribution (P-value < 0.05) including: mite species richness (Richness), total organic carbon (TOC), total nitrogen (TN), carbon to nitrogen ratio (C.N), bulk density (BD), and forest floor thickness (Forest.floor). The permutational multivariate analysis of variance revealed significant differences between the lower earthworm density and higher earthworm density areas (F: 5.4; P-value: 0.001).
Because we were unable to locate a control area with absence of earthworms, we cannot determine if the documented mite abundance, species richness, and community composition observed in the lower-density area are a result of the presence of D. octaedra alone or if these are actually representative of the oribatid mite communities in the noninvaded forest. Other studies (cited below) suggest that increases in oribatid mite abundance in the early stages of invasion or in the presence of D. octaedra are a result of greater habitat heterogeneity. This concept has been discussed in terms of the intermediate disturbance hypothesis, where intermediate levels of disturbance allow maximum coexistence and diversity for all species (Connell Reference Connell1978). To corroborate this, McLean and Parkinson (Reference McLean and Parkinson1998) observed greater habitat heterogeneity in the forest floor shortly after D. octaedra invasion (three months), which resulted in increased oribatid mite abundances. Even deeply burrowing species such as O. tyrtaeum have resulted in greater microarthropod abundances when present at low densities (Straube et al. Reference Straube, Johnson, Parkinson, Scheu and Eisenhauer2009). Conversely, when present at high densities, the same earthworm species negatively affected microarthropod abundance when compared to an earlier assessment in the same forest stand (Eisenhauer et al. Reference Eisenhauer, Partsch, Parkinson and Scheu2007). Based on this, the density of the invading species may be more influential than the ecological group to which the invading species belongs when considering the intermediate disturbance hypothesis (Eisenhauer et al. Reference Eisenhauer, Partsch, Parkinson and Scheu2007; Straube et al. Reference Straube, Johnson, Parkinson, Scheu and Eisenhauer2009; Cameron et al. Reference Cameron, Knysh, Proctor and Bayne2013a). For example, Cameron et al. (Reference Cameron, Knysh, Proctor and Bayne2013a) observed a greater change in microarthropod abundances at high densities of D. octaedra than at low densities of Lumbricus terrestris. Other studies have suggested that even low levels of habitat disturbance, such as those caused by low densities of D. octaedra, negatively influence oribatid mite communities (Maraun et al. Reference Maraun, Salamon, Schneider, Schaefer and Scheu2003). Further work to understand the wide range of effects on soil-dwelling microarthropods at different stages of earthworm invasion may help to determine at what time since invasion and at what density of earthworms the intermediate disturbance hypothesis would be observed. In-field experiments to examine this hypothesis and other mechanisms associated with earthworm disturbance will likely become more challenging as earthworms continue to expand their range, making noninhabited reference conditions more difficult to locate or access.
Conclusion
It is apparent that earthworm invasion into previously earthworm-free forests can cause drastic changes to oribatid mite communities and forest floor characteristics. The overall increased earthworm density and the presence of Aporrectodea genus in the high earthworm density area in the present study resulted in a dramatic loss of mite species G. ornatus (–75%), decreased species richness (–34%) and abundance (–25%), and induced a shift in the overall mite community composition. These observations were associated with significant changes to forest floor characteristics, including increased bulk density, decreased moisture content, lower carbon and nitrogen content, decreased forest floor thickness, and changes in plant community composition. These substantial changes are likely to affect litter decomposition and long-term carbon dynamics in boreal soils. Dedicated work to monitor the extent of earthworm invasion and of changes to soil fauna communities across broader spatial and temporal scales, including in noninvaded zones, is recommended to further document the impacts and implications for the boreal forest of this ongoing invasion.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.4039/tce.2025.13.
Acknowledgements
Support for this work came from the National Science and Engineering Research Council (NSERC) of Canada through a Discovery Grant [RGPIN-2014-04693] to Sylvie A. Quideau. The authors thank the Alberta Biodiversity Monitoring Institute and the Royal Alberta Museum, both of Edmonton, Alberta, for providing support towards necessary training, identification, and verification of oribatid mite specimens.
Competing interests
The authors declare that they have no competing interest.








