Introduction
Tools to assess animals’ affective states are important for animal welfare research. The cognitive bias task has revolutionised this aspect of animal welfare research by providing a validated method to assess the valence (positive or negative) of emotional states in many animal species (Harding et al. Reference Harding, Paul and Mendl2004; Paul et al. Reference Paul, Harding and Mendl2005; Mendl et al. Reference Mendl, Burman, Parker and Paul2009, Reference Mendl, Brooks, Basse, Burman, Paul, Blackwell and Casey2010a,Reference Mendl, Burman and Paulb), rather than focusing solely on arousal levels, as is often the case with other commonly used measures of animal welfare such as heart rate or glucocorticoid levels. Generally, in a cognitive bias task, animals are trained to discriminate between two stimuli, one associated with a reward and the other associated with the absence of a reward. Once the discrimination is learned, animals are presented with one or more ambiguous stimuli. The reaction towards these ambiguous stimuli, measured as the speed of approach (e.g. Mendl et al. Reference Mendl, Brooks, Basse, Burman, Paul, Blackwell and Casey2010a) or frequency of operant response (e.g. Harding et al. Reference Harding, Paul and Mendl2004), provides an estimate of whether the subject judges these cues positively (‘optimistic’) or negatively (‘pessimistic’), and is then used as a measure of affect. However, this depends on these cues remaining ambiguous. Since ambiguous cues are usually unreinforced, in studies involving repeated testing, animals may learn about the reinforcement contingencies. For example, Doyle et al. (Reference Doyle, Vidal, Hinch, Fisher, Boissy and Lee2010) trained sheep (Ovis aries) to discriminate between two locations and then tested their responses to nine ambiguous locations positioned between the training ones over nine sessions. The authors found a decline in the number of approaches to the ambiguous locations over time. More recently, Wilson et al. (Reference Wilson, Hall, Aviles-Rosa, Campbell, Arnott and Reeve2023) tested dogs (Canis familiaris) on a spatial cognitive bias task over five sessions and found that the animals were slower to approach the three ambiguous locations as the number of sessions increased, and that the number of times the dogs did not approach the ambiguous stimuli at all also increased with session number. The results of these two studies suggest that both sheep and dogs learned that the ambiguous cues were unrewarded. Hence, if cognitive bias tasks are applied repeatedly, results may be impacted by learning and may thus confound the effects of ongoing experimental treatments. It is thus important to find alternative methods for animal welfare research requiring repeated testing.
One such alternative is the successive negative contrast (SNC), based on the reaction to a sudden reduction in reward value in a (usually operant) task. The test comprises a pre-shift phase in which a group of animals (Experimental Group) receives a high-value reward for performing a task, followed by a post-shift phase in which they receive a low-value reward for performing the same task. The behaviour of the Experimental Group is compared to that of a Control Group which always receives the low-value reward. The unexpected reward devaluation elicits a negative emotional state of frustration in the experimental animals, and consequently a decrease in consummatory or operant behaviour to levels below those of the control animals — the successive negative contrast (SNC) phenomenon (e.g. Papini et al. Reference Papini, Mustaca and Bitterman1988; Flaherty et al. Reference Flaherty, Clarke and Coppotelli1996). Moreover, in a pioneer study exploring the relationship between sensitivity to reward loss and animal affect, Burman et al. (Reference Burman, Parker, Paul and Mendl2008) found that rats (Rattus norvegicus) facing the removal of enrichment objects (expected to induce negative affect) showed a more prolonged SNC response to reward loss in a runway task than rats maintained in enriched conditions. The authors concluded that animals in more negative affective states were more sensitive to the sudden decrease in reward value, and thus proposed sensitivity to reward devaluation as an indicator of animal emotion and welfare.
While it is reported to be well established in other mammalian species (e.g. rats, mice [Mus musculus], sheep, deer [Dama dama], monkeys [Macaca spp], and humans; for a review, see Papini Reference Papini2014), SNC has not been consistently reproduced in dogs, with previous studies producing conflicting results. Bentosela et al. (Reference Bentosela, Jakovcevic, Elgier, Mustaca and Papini2009) provided the first demonstration of SNC in dogs, testing owned dogs in a task where gazing at the experimenter was reinforced with food. For control animals, the food reward was dog dry food in all phases, whereas for experimental animals the reward was liver in the pre-shift phase (high-value) and dog dry food in the post-shift phase (low-value). Gaze duration decreased in dogs downshifted from liver to dry food below the level of dogs always reinforced with dry food. Pongrácz et al. (Reference Pongrácz, Hegedüs, Sanjurjo, Kővári and Miklósi2013) could not reproduce these findings using a two-object human pointing task: owned dogs shifted from sausage to dry food showed no differences in the number of correct choices and in the choice latency between phases. Subsequently, Riemer et al. (Reference Riemer, Ellis, Ryan, Thompson and Burman2016) replicated the protocol used by Bentosela et al. (Reference Bentosela, Jakovcevic, Elgier, Mustaca and Papini2009) with both owned and shelter dogs but failed to find differences in gaze duration and proportion of food rejected when the food reward was downshifted from sausage to dry food. Riemer and collaborators (Reference Riemer, Ellis, Thompson and Burman2018a,Reference Riemer, Ellis, Thompson and Burmanb) were the first to use non-social tasks to test SNC in dogs. Riemer et al. (Reference Riemer, Ellis, Thompson and Burman2018a) examined the effects of both food quality (sausage vs dry dog food) and food quantity (5 vs 1 piece of dry dog food) on the running speed of owned dogs in a runway task and observed no SNC for either condition. Riemer et al. (Reference Riemer, Ellis, Thompson and Burman2018b) tested owned dogs in a foraging task, in which food was provided in four ‘activity boards’. Following a reward downshift from commercial dog treats to dry dog food, subjects showed an SNC effect by switching significantly more often between boards compared to an ‘unshifted’ condition. Similar results were found by Dzik et al. (Reference Dzik, Cavalli, Iglesias and Bentosela2019) when owned dogs had to solve a commercial dog puzzle toy to obtain food rewards. Dogs downshifted from liver to dry food removed fewer puzzle pieces than dogs that always worked for dry food. However, another group of dogs for whom the high-value reward was sausage instead of liver did not show SNC. More recently, Dzik et al. (Reference Dzik, Carballo, Cavalli, Iglesias, Faragó, Kubinyi and Bentosela2024) conducted two SNC studies with owned dogs, one using a social task (based on Prongácz et al. Reference Pongrácz, Hegedüs, Sanjurjo, Kővári and Miklósi2013) and another using the non-social task used by Dzik et al. (Reference Dzik, Cavalli, Iglesias and Bentosela2019). Dogs downshifted from liver to dry food exhibited SNC in the former but not the latter.
In summary, the SNC literature in dogs has provided inconsistent results, with some studies or conditions finding SNC but not others. Critically, in all but two studies where SNC was not obtained, the authors observed no response reduction at all for the experimental animals following reward devaluation (Riemer et al. Reference Riemer, Ellis, Ryan, Thompson and Burman2016, Reference Riemer, Ellis, Thompson and Burman2018a in the food quality condition found a response reduction for the experimental animals, but not below that of the control animals). To date, most research in SNC on dogs has tended to focus on exploring potential methodological reasons for the inconsistent test results, such as the potential influence of the type of task (social vs non-social) or the familiarity of testing place, among others (for a complete and systematic overview of the methodological differences between studies, see Dzik et al. Reference Dzik, Carballo, Cavalli, Iglesias, Faragó, Kubinyi and Bentosela2024). Importantly, this research has focused on trying to understand what aspects of methodology explain the absence of a decrease in operant response following a decrease in reward value for the experimental animals. As for the study population, previous research (with the exception of Riemer et al. Reference Riemer, Ellis, Ryan, Thompson and Burman2016) has been carried out with dogs that live with a human family. For the SNC to prove a useful test of dog welfare it is critical to understand the extent to which the consistency in response varies between different populations of dogs. The goal of the present study was to investigate the suitability of the SNC as a test for assessing affective states in dogs. To that end, we conducted reward devaluation studies with three different dog populations, namely laboratory, shelter and owned dogs. These represent three of the four main contexts within which domestic dogs live under human care (i.e. laboratory, shelter, owned, and working dogs). For clarity, the inclusion of different populations aimed to ensure systematic diversity, not to investigate differences in affective states between them. To reduce the potential for confounding influences of human interaction associated with social tasks (see Riemer et al. Reference Riemer, Ellis, Ryan, Thompson and Burman2016, Reference Riemer, Ellis, Thompson and Burman2018a) and follow more closely the protocols used for other animal species, here, we used the non-social task implemented by Dzik et al. (Reference Dzik, Cavalli, Iglesias and Bentosela2019). Including dogs with different genetic and socialisation backgrounds, exposed to different housing and husbandry conditions, and tested in different contexts allows understanding if the SNC effect is robust. Our focus was on the inconsistency of the behaviour of dogs in the experimental condition, in particular the repeatedly reported absence of a response reduction, which is the reason why we focus on this condition, rather than on a comparison between experimental and control groups (for a systematic overview of comparisons, see Papini et al. Reference Papini2014 and also the Discussion here). Given the potential relevance for dog welfare research, it is crucial to understand whether it is worth pursuing SNC experiments for this purpose in dogs.
Materials and methods
Ethical statement
The dogs were neither food deprived nor exposed to any kind of stress as part of the research, all of which was non-invasive and took place in the dogs’ home environment; thus the study does not require a licence under Portuguese and European legislation. No human data were collected. The experiments with laboratory and shelter dogs were part of larger studies approved by the Animal Ethics Committee of ICBAS-UP (Laboratory dogs, Project 317/2019/ORBEA) and i3S Animal Welfare and Ethics Review Body (Shelter dogs, Internal reference 2021–29). Since the video recordings with owned dogs sometimes included the dog owner, approval was sought from the i3S Data Protection Officer. All dog owners gave their informed consent to their own and their dog’s participation in the study, including video recording.
Study animals and housing conditions
We aimed for a final sample of 36 dogs, 12 for each population (for demographics, see Table S1 in the Supplementary material). From our initially recruited subjects, three owned dogs and four shelter dogs were excluded because they: (a) showed no preference between rewards in the food preference test (two owned dogs); (b) showed no interest in any of the food types presented in the food preference test (one shelter dog); (c) were afraid of the experimenters (one shelter dog); (d) were afraid of the puzzle toy (two shelter dogs); or (e) were not interested in solving the puzzle (one owned dog). Therefore, additional subjects were recruited until achieving n = 12 per population. Our final sample thus comprised 12 laboratory Beagle dogs (six males, six females; two years old), 12 shelter dogs (one Bull terrier, eleven mixed-bred; eight males, four females; mean [± SD] age: 4.42 [± 2.27] years old), and 12 owned dogs (two Beagles, one Australian shepherd, one American Bully, eight mixed-breed; four males, eight females; mean [± SD] age: 5.58 [± 2.07] years old).
Laboratory dogs were housed at the kennel of ICBAS-UP. They were housed in pairs in communicating boxes with sliding doors, with each box comprising an interior and an exterior area (measuring 1.8 and 3.5 m2, respectively). The boxes were formulated in line in such a way as to ensure dogs did not have visual contact with conspecifics housed in the other boxes. Animals were leash-walked once a day for at least 30 min and had free access to an outdoor park area approximately between 0900 and 1700h. Dogs were individually fed twice a day (at 0900 and 1700h). Shelter dogs were housed at Plataforma de Acolhimento e Tratamento Animal (PATA), a municipal-owned animal shelter in the Porto region, Portugal. The dogs were kept individually or in pairs in pens with indoor and outdoor areas (measuring 4 m2 each), and were not routinely handled beyond the basic human interactions during feeding and kennel cleaning. The pens were situated in two parallel rows, with the indoor areas facing each other. They were individually fed once a day (around 0800h). Owned dogs lived with their owners, nine in apartments and three in houses with outdoor space and had full to almost full access to the different house rooms. These dogs were walked at least once a day, had daily interaction-dedicated time with their owners (in the form of training and/or playtime), and regular environmental enrichment activities. All owned dogs were fed twice a day, in the morning and in the evening.
Detailed methodology
All dogs were tested in quiet and familiar rooms: laboratory dogs in a room in the building where they were housed, shelter dogs in a room usually used for interactions with potential adopters and adjacent to the building housing the dogs, and owned dogs in a room at home chosen by the owners. Each dog was allowed to explore the room for around 5 min prior to starting the tests. Each dog was first subjected to a food preference test and, on a different day, to an unexpected reward loss test. The test area was prepared before the dog was brought in. At one end of the room, the experimenter (E) prepared the food bowls (food preference test) or puzzle toy (unexpected reward loss test), while a helper (H) held the dog at the opposite end of the room. H was a person unfamiliar to the dogs for the shelter population, but familiar for laboratory and owned dogs (these were a kennel caretaker and the owner, respectively). A camera mounted on a tripod recorded the entire test each time.
Daily feeding routine was maintained in the test days. All tests were conducted between 1000 and 1600h. Data were collected in 2019 for the laboratory dogs, and in 2023 for the shelter and owned dogs.
Food preference test
Apparatus
Five identical food bowls, each always employed for a specific food type, were used. Food types used were boiled cow liver, canned sausage, dog dry food and dry treats, and pieces of a similar size were always used.
Procedure
On each trial, E placed one piece of food in each bowl using a spoon (a different spoon was used for each food type, to avoid mixing the smells). H held the dog approximately 3 m away from the place where E prepared the bowls.
Stage 1: Side bias
In order to evaluate whether there was a side bias, we conducted six trials where both bowls contained a dry dog treat. For each trial, H held the dog by their collar or harness at a marked spot in the floor (with tape) and E brought one bowl in each hand close by, showing them to the dog, one at a time, while allowing them to smell. The bowl that was shown first was counterbalanced across trials in two different sets, with the exception that the same side was not repeated for more than two consecutive trials (LRRLRL and RLRLLR; L – left, R – right), and these sets were counterbalanced across dogs. While E was presenting the bowls, H held the dog to prevent them from picking up the food. After the dog had smelt each bowl, E took a step back and, while holding the bowls in their hands, separated them approximately 1 m (spreading their arms as much as possible) and bent their knees (in order for the bowls to be presented at the dogs’ head level, see Figure 1, left panel). Following a verbal signal by E, H released the dog and they were allowed to choose from the bowls (touching the bowl or coming to within 10 cm of it). E avoided eye contact with the dog during this period by looking towards the floor. Once the choice was made, the other bowl was moved away. After the dog finished eating, H brought them back to the start position by the collar or harness. The inter-trial interval lasted 30 s for all trials. A side bias was considered to exist if dogs chose the same side on all six test trials (e.g. Bolló et al. Reference Bolló, File, Topál and Kis2023). This was never the case for the participating subjects and all of them were then moved onto Stage 2.

Figure 1. Experimental setting for (a) the food preference test and (b), (c), (d) the unexpected reward devaluation test performed with laboratory, shelter, and companion dogs (n = 12 per population). Figure shows (a) Helper holding the dog and Experimenter holding the food bowls before the dog is released and allowed to make a choice, (b) Helper holding the dog while Experimenter puts the baited toy down on the floor; (c) dog is allowed to remove the cones to access food for a maximum duration of 3 min; (d) after the dog removes all the cones, Helper collects the dog and Experimenter takes the toy to refill it.
Stage 2: High-value vs high-value (liver vs sausage)
A comparison between two (potentially) high-value food types (boiled liver and sausage) was performed. In the first two trials (pre-test), dogs were allowed to eat from both bowls in order to become familiarised with the two available food types. The bowl that was shown first was counterbalanced across dogs. Immediately, thereafter, the test began. During the test, which comprised ten trials, once the dog selected one bowl, (s)he was allowed to eat the corresponding piece of food and the other bowl was moved away. The inter-trial interval lasted 30 s for all trials. The side at which each food type was located was counterbalanced across dogs. The bowl that was shown first was counterbalanced across trials in two different sets, with the exception that the same side was not repeated in more than two consecutive trials (RLLRRLRRLL and LRRLLRLLRR), and these sets were counterbalanced across dogs.
Stage 3: High-value (chosen) vs low-value (dry food)
The high-value food chosen in more trials in Stage 2 was compared to dry food (the brand regularly eaten by each dog) in this stage. The location of the high-value food (left or right) was reversed in this stage, i.e. if boiled liver was the reward chosen most often and it was presented on the right in Stage 2, then it would be presented on the left in Stage 3 with dry food on the right. All remaining methodological details were the same as in Stage 2, including the pre-test and test trials order sets, which were kept the same for each dog. For detailed examples of the food preference test, please refer to Table S2 in the Supplementary material.
One owned dog revealed preference for dry food over liver in Stage 3 and for liver over hot dog in Stage 2 and, hence, at this stage he was tested with dry food vs sausage.
The laboratory dogs were interested in neither liver nor sausage. This was likely due to these animals being unfamiliar with other food types apart from dry dog food. Hence, for them, we tested two different dry food brands as high- and low-value rewards (the dry food brands were chosen based on caretakers’ reports of preference, and only Stage 1 and Stage 3 were conducted for these animals).
Data analysis
The variable analysed was the number of trials in which the dogs chose each food type (for each stage). This was scored live by E. The food preference test served to establish, for each dog, a high-value and a low-value reward for later use in the unexpected reward devaluation task.
Unexpected reward devaluation test
Apparatus
The apparatuses used were two Trixie Dog Activity© (https://www.trixie.de/en) interactive toys, which the dogs had not seen before. Each toy consisted of a round base of 29 cm in diameter, with seven round-shaped depressions containing seven plastic removable cones (six arranged in a circle, and the seventh located in the middle, each weighing 35 g). All cones had a small hole to release the smell of food hidden underneath (Figure 1, right panel). One toy was smeared with the high-value reward (previously determined), while the other was smeared with the low-value reward (usually the dry food the dog usually ate, soaked in water, in order to distribute the smell evenly).
Procedure
To begin, H held the dog by the collar or harness in a fixed position (marked on the floor with tape, approximately 3 m from the place where the toy would be placed). Then, E carried the toy baited with the high-value reward, put the toy down on the floor, moved away and said ‘now’ to signal H to release the dog. For the first trial, the toy had three cones partially removed, in order to facilitate the resolution of the task. Dogs were free to interact with the toy, and once the trial ended (either after 3 min had elapsed or the dog had removed all cones), E came back and took the toy to refill it. The same procedure was repeated for the subsequent three trials, but this time no cone was partially removed. This comprised the pre-shift phase. In cases where, during the first trial, the dog struggled with solving the task, or did not approach the toy at all, H walked the dog to the toy at the end of the trial (after 3 min had elapsed) and lifted the cones to show how the apparatus worked. No help was provided for the following trials.
Then, the dog was given a 20-min interval during which (s)he was taken outside the room for a walk. This interval was included to avoid an effect of sensorial carryover across the phases (i.e. the sensorial properties of the food type used in the pre-shift phase affecting the perception of the characteristics of the new food type; Ferris et al. Reference Ferris, Kempton and Muir2003; Dzik et al. Reference Dzik, Cavalli, Iglesias and Bentosela2019). On return, a post-shift phase of three trials with the low-value reward was conducted, following the same procedure as the pre-shift phase except that for this phase, trials lasted 3 min regardless of the number of cones removed. Finally, after 1 min, there was a re-shift phase of one trial with the high-value reward, in order to control for the effects of satiety or fatigue on the behaviour. Inter-trial interval was 1 min for all phases, during which the toy was taken away and refilled outside the dog’s view. All durations were measured by E via a stopwatch.
Data analysis
The variables measured were: (1) number of cones removed; (2) number of rewards eaten; and (3) latency (s) until all cones were removed. These were scored live by E. Each variable was analysed per trial (1–8) and per phase (pre-shift, post-shift, re-shift). The values per phase refer to the average of the four trials from the pre-shift phase, to the average of the three trials from the post-shift phase, and to the one trial from the post-shift phase.
Statistical analysis
The three response variables were analysed by phase (pre-shift, post-shift, re-shift) for each population (laboratory, shelter, and owned dogs). None of the variables was normally distributed (Shapiro-Wilk: P < 0.05), hence non-parametric tests were used.
Friedman tests were conducted to analyse the effect of phase on the number of cones removed, the number of rewards eaten, and the latency to remove all cones. Where effects were found, post hoc Wilcoxon signed-rank tests were then used for pair-wise comparisons, with Bonferroni corrections applied for multiple comparisons.
Results
Establishing preferences
Laboratory dogs
All these showed that they preferred an occasionally fed dry food brand over the regularly eaten dry food brand; hence the former was established as the high-value reward and the latter as the low-value reward for all animals.
Shelter dogs
Seven animals preferred liver over sausage and five preferred sausage over liver, and all 12 preferred liver or sausage over their regular dry food. Hence, liver and sausage were established as the high-value reward for these seven and five dogs, respectively, and dry food as the low-value reward for all of them.
Owned dogs
Nine animals preferred liver over sausage and two preferred sausage over liver, and all these eleven dogs preferred liver or sausage over dry food. Hence, liver and sausage were established as the high-value reward for these nine and two dogs, respectively, and dry food as the low-value reward for all of them. One owned dog preferred dry food over liver and, in turn, liver over sausage. For this dog, dry food was established as the high-value reward and sausage as the low-value reward.
Reaction to changes in reward value
Figure 2 depicts the results for the three dog populations.
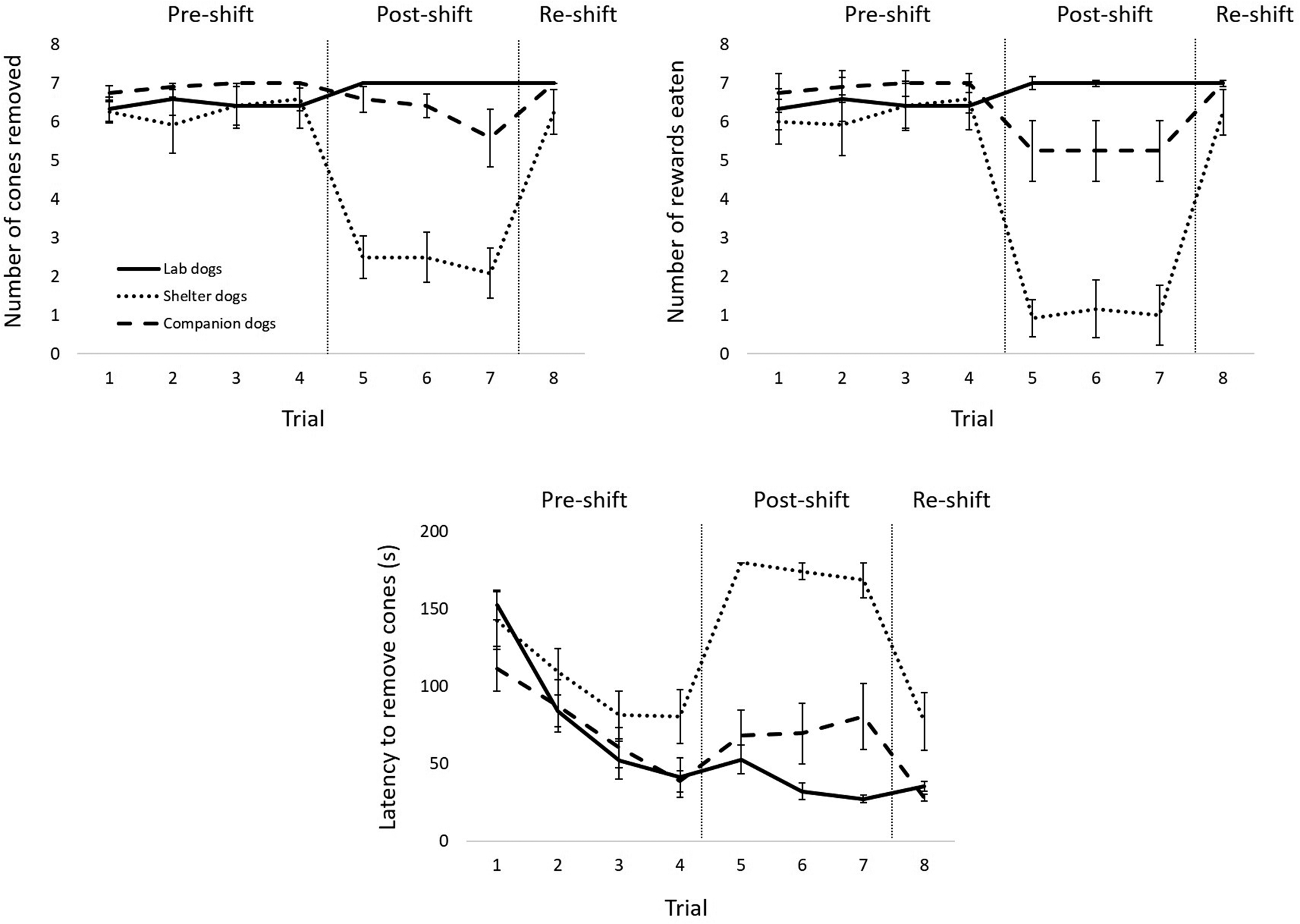
Figure 2. The top left panel shows the mean (± SEM) number of cones removed across trials by the laboratory, shelter, and companion dogs. The top right panel shows the mean (± SEM) number of rewards eaten across trials by the laboratory, shelter, and companion dogs. The bottom panel shows the mean (± SEM) latency to remove all cones across trials for the laboratory, shelter, and companion dogs. Vertical lines indicate the different phases (pre-shift, post-shift, re-shift).
Number of cones removed
We found statistically significant effects of phase on the number of cones removed for laboratory dogs (χ2[2] = 8.00; P = 0.018; Figure 2, top left panel, solid line) and shelter dogs (χ2[2] = 17.07; P < 0.001, Figure 2, top left panel, dotted line), but not for owned dogs (χ2[2] = 2.80, ns, Figure 2, top left panel, dashed line). Post hoc tests showed no significant differences between phases for laboratory dogs (P = ns for pre- vs post-shift phases, post- vs re-shift phases, and pre- vs re-shift phases), but significant differences between pre- and post-shift phases (W= –3.06; P = 0.002) and between post- and re-shift phases (W = 2.98; P = 0.003) for shelter dogs.
Number of rewards eaten
We found statistically significant effects of phase on the number of rewards eaten for laboratory dogs (χ2[2] = 8.00; P = 0.018, Figure 2, top right panel, solid line) and shelter dogs (χ2[2] = 19.19; P < 0.001, Figure 2, top right panel, dotted line), but not for owned dogs (χ2[2] = 2.80, ns; Figure 2, top right panel, dashed line). Post hoc tests showed no significant differences between phases for laboratory dogs (ns for all comparisons), but significant differences between pre- and post-shift phases (W= –3.06; P = 0.002) and between post- and re-shift phases (W = 2.94; P = 0.003) for shelter dogs.
Latency to remove all cones
We found statistically significant effects of phase on the latency to remove all cones for laboratory dogs (χ2[2] = 15.17; P < 0.001; Figure 2, bottom panel, solid line), shelter dogs (χ2[2] = 13.00; P = 0.002; Figure 2, bottom panel, dotted line), and owned dogs (χ2[2] = 15.17; P < 0.001; Figure 2, bottom panel, dashed line). Post hoc tests showed significant differences between pre- and post-shift phases (W = –2.98; P = 0.003) and between pre- and re-shift phases (W = –3.06; P = 0.002) for laboratory dogs, between pre- and post-shift phases (W = 2.50; P = 0.013) and post- and re-shift phases (W = –2.70; P = 0.007) for shelter dogs, and between pre- and re-shift phases (W = –3.06; P = 0.002) for owned dogs.
Individual differences
The average data reflected the individual data for laboratory and shelter dogs, but not for owned dogs. For the latter, individual data revealed that three of these animals showed a decrease in the number of cones removed and in the number of rewards eaten from the pre- to the post-shift phase and a subsequent increase from the post- to the re-shift phase, as opposed to the remaining nine animals, for whom the values remained the same across phases. As for the latency to remove all cones, the former three dogs showed an increase from the pre- to the post-shift phase and a subsequent decrease in the re-shift phase, while the latter nine animals displayed a decrease across phases (see Figure 3; refer also to Figure S1; Supplementary material).
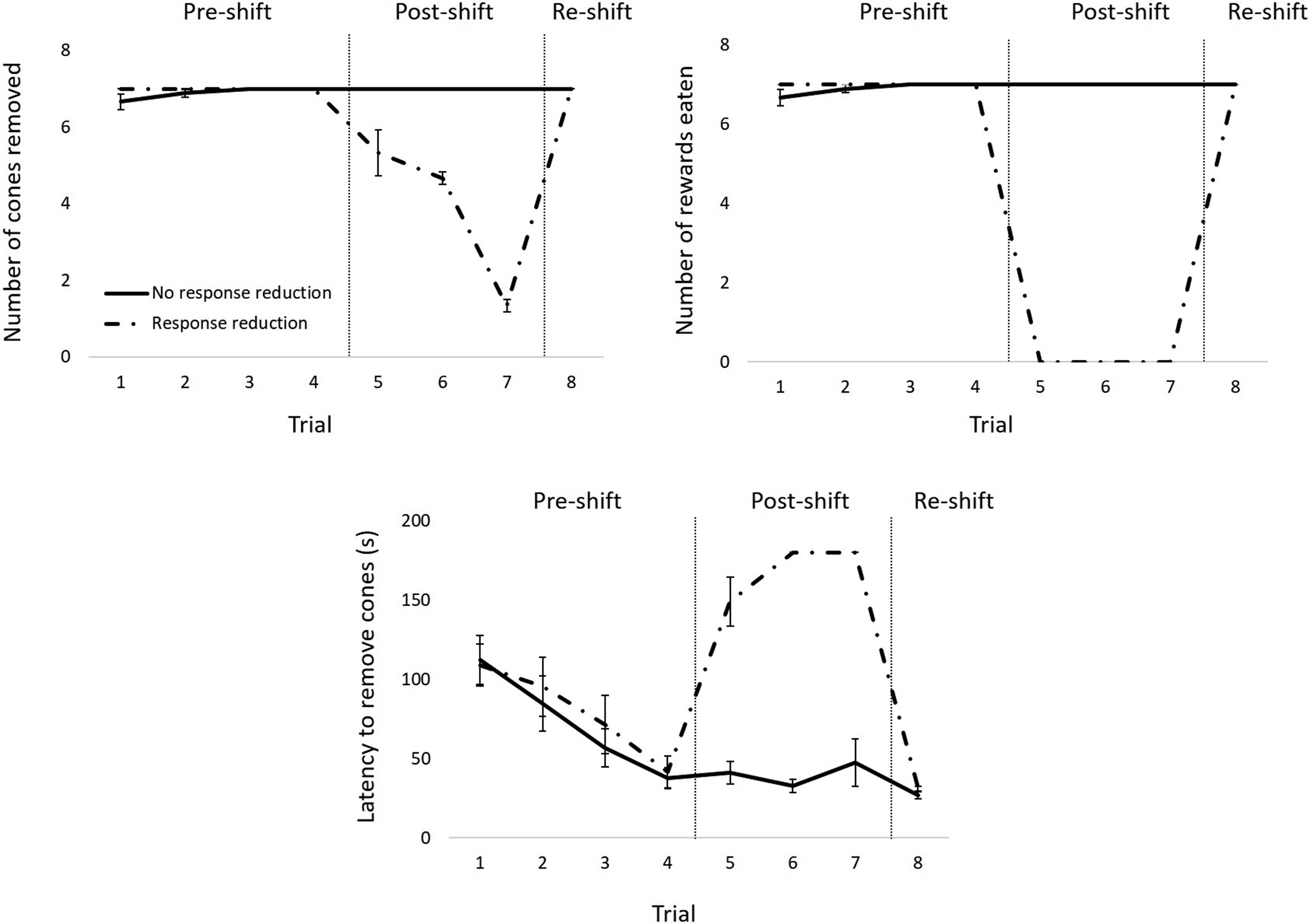
Figure 3. Results for companion dogs averaged for the animals that showed a response reduction in the post-shift phase (interrupted lines) and the animals that did not show a response reduction in the post-shift phase (solid lines). The top left panel shows the mean (± SEM) number of cones removed across trials. The top right panel shows the mean (± SEM) number of rewards eaten across trials. The bottom panel shows the mean (± SEM) latency to remove all cones across trials. Vertical lines indicate the different phases (pre-shift, post-shift, re-shift). Please note that data-points with no error bars refer to points for which there was no variability.
Discussion
The goal of the current study was to examine whether the SNC is a suitable test for evaluating dogs’ affective states. To that end, we tested three different dog populations (laboratory, shelter and owned dogs), to assess the extent to which behaviour in the task varied across these groups with different genetic, housing and socialisation backgrounds, and different experimental contexts. Our findings showed that shelter dogs removed fewer cones, ate fewer rewards and took longer to complete the task (remove all the cones) in the post-shift phase compared to both the pre- and re-shift phases. For the populations of laboratory and owned dogs, we found no differences in the number of cones removed and in the number of rewards eaten across phases, but the latency to remove all cones decreased from the pre- to the post- and re-shift phases for laboratory dogs, and from the pre- to the re-shift phase for owned dogs.
The absence of a response reduction in the post-shift phase for laboratory and owned dogs suggest no SNC for these animals, whereas the decreased latency to complete the task likely reflects an increase in proficiency across the test. In contrast, the shelter dogs showed a reduction in the number of cones removed in the post-shift phase, accompanied by a consistent decrease in the number of rewards eaten and an increase in time to complete the task (latency to remove all cones). However, the average data for owned dogs masked what happened at the individual level: whereas nine owned dogs showed no response reduction, the remaining three reacted similarly to the shelter dogs. The response reduction shown by these three owned dogs and all shelter dogs is consistent with SNC; however, a control group working for the low-value reward throughout the entire test would be needed to confirm the presence of the effect. By definition, SNC is said to be present when the response reduction shown by ‘downshifted’ subjects drops to levels below those of ‘unshifted’ animals (e.g. Flaherty et al. Reference Flaherty, Clarke and Coppotelli1996; Papini Reference Papini2014). On the other hand, all laboratory dogs and nine owned dogs failed to show SNC, as evidenced by the fact that they continued to respond at the same level after the reward downshift, in other words showed no change in behaviour following changes in reward value.
The present findings are in line with results of previous studies, showing that SNC is not consistently observed in dogs (Bentosela et al. Reference Bentosela, Jakovcevic, Elgier, Mustaca and Papini2009; Prongácz et al. Reference Pongrácz, Hegedüs, Sanjurjo, Kővári and Miklósi2013; Riemer et al. Reference Riemer, Ellis, Ryan, Thompson and Burman2016, Reference Riemer, Ellis, Thompson and Burman2018a,Reference Riemer, Ellis, Thompson and Burmanb; Dzik et al. Reference Dzik, Cavalli, Iglesias and Bentosela2019, Reference Dzik, Carballo, Cavalli, Iglesias, Faragó, Kubinyi and Bentosela2024). This raises the question of whether SNC in itself is a condition for the test to be valid as a tool to assess dogs’ affective states. Most of the discussion surrounding SNC in dogs has focused on trying to identify factors not related to animal welfare to explain the absence of the phenomenon under certain circumstances, such as methodological differences between studies, or species differences such as dogs being natural scavengers and/or being exposed to intermittent reinforcement schedules throughout life (e.g. Dzik et al. Reference Dzik, Carballo, Cavalli, Iglesias, Faragó, Kubinyi and Bentosela2024). However, some authors have proposed that the absence of SNC can indeed reflect highly positive affective states of the tested animals (Riemer et al. Reference Riemer, Ellis, Thompson and Burman2018a,Reference Riemer, Ellis, Thompson and Burmanb, see also Rosas et al. Reference Rosas, Callejas-Aguilera, Escarabajal, Gómez, de la Torre, Agüero, Tobeña, Fernández-Teruel and Torres2007). It is unclear from the existing literature what a “lower sensitivity to reward loss” of animals in more positive affective states (Burman et al. Reference Burman, Parker, Paul and Mendl2008) operationally means in an SNC test — whether a less pronounced SNC, an absence of SNC, or both. The body of research into SNC in dogs has failed thus far to address the question of whether the presence of an SNC effect is a condition for interpreting the background affective states, nor has it attempted to correlate response to the reduction in reward value with other established measures of affective state, such as cognitive bias.
It is possible that the differences between populations found in the present study reflect differences in affective states. Sheltering is known to cause chronic stress and lead to decreased welfare in dogs (e.g. Taylor & Mills Reference Taylor and Mills2007; Righi et al. Reference Righi, Menchetti, Orlandi, Moscati, Mancini and Diverio2019). As said above, the consistent response reduction in the post-shift phase observed for shelter dogs is consistent with SNC for this population. This could thus reflect less positive affective states in these animals as compared with the other two populations, for which no consistent response reduction was observed. However, any such conclusion would require that the study design included control groups.
When reflecting on the usefulness of the SNC as a test of animal welfare, its practicality should also be considered. In the present study, the unexpected reward devaluation task proved easy to implement in various contexts, ranging from dog owners’ houses to shelter and university facilities, required little space and few human resources, and was run with low-budget equipment. Moreover, the puzzle toy used proved highly intuitive to solve, even for dogs who were not used to these types of environmental enrichment toys. A less positive aspect is the duration of the test, which lasted around 1 h per animal (including the 20-min break). The necessity for a food preference test makes the entire experimental procedure demanding. The preference test proved long (around 45 min without breaks) and physically and mentally demanding for the experimenters (especially if testing more than one dog per day), and for the dogs (some dogs became less willing to co-operate as the test carried on, being more easily distracted, and some actually laid down in between trials). Including a food preference test requires two sessions per dog and on different days, to avoid fatigue and/or satiety to carry over to the SNC test. Alternatively, paired-stimulus preference tests could be used (e.g. Vicars et al. Reference Vicars, Miguel and Sobie2014; Waite & Kodak Reference Waite and Kodak2023). This type of test would reduce the number of trials on top of addressing side bias, which could significantly reduce the time and effort invested at this stage in future studies. Still, time and effort invested in this first phase may be lost. In the present study, we conducted food preference tests with 43 dogs, but five could not complete the unexpected reward devaluation test afterwards because they either showed no preference between rewards, were afraid of the puzzle toy, or were not interested in solving it. Considering two other dogs that did not collaborate in the food preference test (due to fear or lack of interest in the food offered), this study faced a 16% subject exclusion rate. Other studies reported the exclusion of subjects for similar reasons and in higher proportions: 32% in Riemer et al. (Reference Riemer, Ellis, Thompson and Burman2018a), 41% in Dzik et al. (Reference Dzik, Cavalli, Iglesias and Bentosela2019), and 40% in Dzik et al. (Reference Dzik, Carballo, Cavalli, Iglesias, Faragó, Kubinyi and Bentosela2024). A weakness of the SNC test thus seems to be a somewhat high risk for drop-outs due to dogs’ motivation and/or fear issues. To minimise this risk, researchers could attempt to identify subjects who may not co-operate, either by asking owners or caretakers about perceived fear and food motivation or pre-testing the animals with a different problem-solving task.
Studies on SNC in dogs have not always assessed preferences among the food types used as rewards in the test (Dzik et al. Reference Dzik, Carballo, Cavalli, Iglesias, Faragó, Kubinyi and Bentosela2024) and have not always relied on individual preferences to choose the rewards to be used for each animal (e.g. Pongácz et al. Reference Pongrácz, Hegedüs, Sanjurjo, Kővári and Miklósi2013; Riemer et al. Reference Riemer, Ellis, Ryan, Thompson and Burman2016; Dzik et al. Reference Dzik, Cavalli, Iglesias and Bentosela2019). The results of the present study revealed that the preferred reward for owned and shelter dogs varied among animals. Interestingly, one owned dog preferred their regular dry food to sausage and liver. Moreover, despite laboratory dogs being tested with dog dry food only (two different commercially available brands), these were the animals that showed the strongest preference for the high-value reward, measured as the number of trials on which dogs chose the high-value reward over the low-value reward in the food preference test. These results clearly indicate that reward value is highly individual and highlight the importance of testing individual food preferences to define the rewards to be used in SNC tests (for the importance of testing individual food preferences in positive-reinforcement dog training, see also Bremhorst et al. Reference Bremhorst, Bütler, Würbel and Riemer2018). Reward value should not be extrapolated from one study to another or from some dogs to others (even within the same population). The discrepancy in the hedonic value of the high- and low-value rewards has been suggested as one of the most important aspects for the exhibition of an SNC effect (e.g. Amsel Reference Amsel1992). In line with this, Dzik and collaborators (Reference Dzik, Cavalli, Iglesias and Bentosela2019) found SNC in dogs when using liver as the high-value reward, but not when using sausage. In the present study, when comparing the number of trials on which dogs chose the high-value reward over the low-value reward in the food preference test, we found that, on average, the proportion of high-value reward choices was 0.9 for laboratory dogs, 0.6 for shelter dogs and 0.7 for owned dogs (for individual data refer to Table S3; Supplementary materials). If the difference in value between the two rewards determined SNC, the effect should have emerged for laboratory dogs as they showed the strongest preference for the high-value reward, and this was not the case.
For the shelter and laboratory dogs, all individuals within the group responded similarly: when the reward value decreased, all shelter dogs showed a reduction in response whereas no laboratory dog did. In contrast, among the owned dogs, individual differences emerged regarding how dogs reacted to the reduction in reward value. To our knowledge, of the many SNC studies in owned dogs, ours is the first to report different outcomes for different subjects. Although we standardised the owned dog sample as far as possible by recruiting dogs with similar levels of enrichment, differences emerged. These results suggest that methodological variations (the focus of most research, to date, e.g. Dzik et al. Reference Dzik, Carballo, Cavalli, Iglesias, Faragó, Kubinyi and Bentosela2024) may not be the main reason for the lack of consistency in dog behaviour in SNC tests. In the present study, all owned dogs were exposed to exactly the same problem-solving task, in a familiar room in their home and with the owner always present during the test. Regardless, some of the animals showed a response reduction whereas others did not.
Finally, there are a number of limitations relating to the present study to consider. In contrast to other studies on the topic, which restricted feeding from 4 to 18 h preceding the tests (Bentosela et al. Reference Bentosela, Jakovcevic, Elgier, Mustaca and Papini2009; Riemer et al. Reference Riemer, Ellis, Ryan, Thompson and Burman2016, Reference Riemer, Ellis, Thompson and Burman2018a,Reference Riemer, Ellis, Thompson and Burmanb; Dzik et al. Reference Dzik, Cavalli, Iglesias and Bentosela2019, Reference Dzik, Carballo, Cavalli, Iglesias, Faragó, Kubinyi and Bentosela2024), here, all study dogs were fed their normal morning meal on test days. This may have reduced the value of the dry food for our dogs as compared to the dogs from the other studies. Moreover, breed composition was not standardised across groups, but these were representative of the respective populations (at least in the Portuguese context), with Beagles being the most common laboratory dog breed, shelter dogs comprising mainly mixed breed animals and owned dogs including different breeds and breed crosses. Additionally, the familiarity with the experimenters was not homogenous across the different dog populations. Laboratory and owned dogs were tested with their caretakers and their owners as handlers, respectively, whereas for shelter dogs the handler was an unfamiliar person. Critically, for the latter, no equivalent existed regarding familiarity with a human; the shelter where these animals were housed employed several caretakers and they spent little time with each animal. This may have contributed to exaggerating the contrast between shelter dogs and the other two populations, with social reward attenuating the response reduction following reward devaluation in dogs tested with familiar humans (see Bentosela et al. Reference Bentosela, Jakovcevic, Elgier, Mustaca and Papini2009 and Pongrácz et al. Reference Pongrácz, Hegedüs, Sanjurjo, Kővári and Miklósi2013). Finally, by definition, SNC involves comparing the behaviour of ‘downshifted’ subjects with ‘unshifted’ subjects (e.g. Papini et al. Reference Papini, Mustaca and Bitterman1988; Flaherty et al. Reference Flaherty, Clarke and Coppotelli1996), such that the SNC effect is said to occur if the responses of the ‘downshifted’ subjects fall below those of ‘unshifted’ subjects. The fact that the present study, like Prongácz et al. (Reference Pongrácz, Hegedüs, Sanjurjo, Kővári and Miklósi2013), did not include control groups that worked for the low-value reward throughout the entire test means that we cannot establish with conviction that the decrease in response after reward downshift displayed by shelter dogs and three owned dogs is a true SNC effect. This would require comparing the response of dogs who have experienced the reward downshift with that of dogs who have only experienced the low-value reward. However, the present study design allows us to confirm the absence of SNC for the laboratory dogs and the remaining nine owned dogs following the absence of a decrease in response after downshifting the reward value: there can never be a regular SNC effect if there is no decrease in response whatsoever (a reverse SNC can still be present, however, it is the regular SNC effect that has been proposed as an indicator of animals’ affective states; for a detailed review on regular, reverse and null SNCs, see Papini et al. Reference Papini2014).
Animal welfare implications
The present study investigated the suitability of the successive negative contrast as a test for assessing affective states in dogs. This has important implications for animal welfare research in general, and dog welfare research in particular, because at present we lack validated methods for assessing animal affective states that can deal with repeated testing.
We found the entire procedure to be demanding and time consuming (especially given the food preference test used), and prone to high subject exclusion rates. However, it proved low-budget and relatively easy to implement in different experimental contexts and with different dog populations. In line with previous studies in dogs, we found that some subjects did not display the SNC effect. Importantly, we highlight the need for clarifying whether the presence of an SNC effect is a condition for interpreting the background affective states of animals in an SNC task. This is a critical contribution for research over the use of this test as a tool for assessing animal welfare.
Conclusion
Although the present study does not allow us to confirm an SNC effect for dogs that showed a response reduction after reward downshift, it allows establishing the absence of the effect for the animals that did not show a response reduction. The current findings corroborate previous studies in demonstrating that SNC is not consistently found in dogs and expands upon them by suggesting that this may be related to the dogs rather than to the method. Despite not being able to pinpoint what explains the conflicting results, the fact that SNC does not replicate consistently across contexts and populations limits its usefulness as a tool to measure dog welfare.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/awf.2025.29.
Acknowledgements
Firstly, we would like to thank Ana Margarida Pereira, Joana Guilherme-Fernandes, Margarida Guedes, Miriam Casaca, and Sofia Morais for helping with data collection. We would also like to thank PATA for collaborating in this study, and DTC Social for helping to establish the first contact with PATA. We also thank all dog owners who volunteered for participation with their companion folks. Thanks are also due to António Mira Fonseca for contributing towards the study with laboratory dogs. Finally, a dedicated acknowledgement to Alexandra Protopopova’s and Camila Cavalli’s research groups for the fruitful early discussion of our data, as well as to all researchers who approached after our oral presentation at ISAE 2023 for the valuable shared experiences and unpublished results of SNC studies in different animals. The study was supported by the Portuguese Foundation for Science and Technology (FCT/MCTES) through projects UIDB/04033/2020 (https://doi.org/10.54499/UIDB/04033/2020) and UIDP/50006/2020 (https://doi.org/10.54499/UIDP/50006/2020).
Competing interests
None.




