This past decade has witnessed intense effort to identify biomarkers that would significantly enhance the prevention, diagnosis and treatment of psychiatric disorders, ultimately leading us closer to ‘personalised medicine’. While biomarkers are widely used in other clinical disciplines, psychiatry has yet to develop reliable biological diagnostic and therapeutic tools. These challenges result from various factors including the heterogeneity of clinical presentations, high rates of comorbidity, pathophysiological complexity and individual variability in treatment responses.
The female reproductive transitions present a promising opportunity to study psychiatric biomarkers. The hormonal changes across menstruation, pregnancy, parturition and perimenopause can have substantial effects on the mental health of those at risk, and individual markers could emerge from our new understandings of these transitions. Illnesses at times of reproductive hormonal transition present a unique biological trigger that is not available in other psychiatric conditions. For these reasons, the search for biomarkers in conditions such as perinatal mood and anxiety disorders, premenstrual dysphoric disorder (PMDD) and perimenopausal mood disorders may also help in the search for biomarkers associated with psychiatric disease at other times, where the biological link is less obvious. Key factors under investigation include genetic, epigenetic, hormonal, immunological and neural circuit dynamics. (Of note, research in this area has focused largely on biological women, with little known about how either biology or symptom presentation may differ for those whose biological gender does not match their gender identity. This paper will use the terms appropriate to the population of each study; by and large, this will mean women.) This review summarises current knowledge on potential biomarkers and will focus on six conditions (though individual sections will discuss only those conditions for which we have evidence).
-
(a) Antenatal/perinatal/postpartum depression (AND/PND/PPD): there are many conflicting definitions of these terms. Some studies use them interchangeably, while others distinguish between antenatal (only during pregnancy), postpartum (only after delivery) and perinatal (both periods included). The DSM-5 definition is ‘with peripartum onset’, i.e., symptoms should have started either during pregnancy or within the first 4 weeks following delivery.1 These conflicting definitions are often inconsistent with both clinical experience (in which women can present with depression at any point in pregnancy or in the first postpartum year) and with biological evidence (particularly genetic evidence, which finds familial risk only for depression that begins within 6–8 weeks after childbirth).Reference McEvoy, Osborne, Nanavati and Payne2 In this paper, we will use the term ‘antenatal’ when the data pertain specifically to the antenatal period, ‘postpartum’ when they pertain specifically to the postpartum period and ‘perinatal’ when the distinction is unclear or when the data span both periods.
-
(b) Perinatal anxiety: distinct from PND, characterised by heightened and often debilitating worry and anxiety during pregnancy and the postpartum period. While anxiety could also be subject to the same distinctions listed above for depression, most data span both pregnancy and postpartum and we will use ‘perinatal’ unless the data are exclusive to one period or the other.
-
(c) Perinatal obsessive–compulsive disorder (pOCD): onset or exacerbation of obsessive–compulsive disorder (OCD) symptoms during pregnancy or in the first year of postpartum. While OCD could also be subject to the same distinctions listed above for depression, most data span both pregnancy and postpartum and we will use ‘perinatal’ unless the data are exclusive to one period or the other.
-
(d) Postpartum psychosis (PPP): a rare but severe psychiatric emergency; an affective psychosis that occurs shortly after childbirth.
-
(e) PMDD: a severe form of premenstrual syndrome (PMS) occurring during the luteal phase of the menstrual cycle.
-
(f) Perimenopausal depression: a form of depression that presents in the perimenopausal period (the transitional years leading to menopause), characterised by the core symptoms of major depressive disorder (MDD), often accompanied by the physical and cognitive symptoms common to the menopausal transition. Other psychiatric illnesses can also be exacerbated in this period, but due to limited literature we will focus on depression only.
Early research on biomarkers of reproductive psychiatric illness focused on genetics, but this literature has yielded little conclusive information. Several twin and family studies show evidence for heritability in PPD, and candidate gene studies have focused on hormonal regulation (e.g. oestrogen receptors) and neurotransmitter systems (e.g. serotonin, dopamine), for example, polymorphisms in oestrogen receptor genes (ESR1 and ESR2) and the serotonin transporter gene.Reference McEvoy, Osborne, Nanavati and Payne2,Reference Couto, Brancaglion, Alvim-Soares, Moreira, Garcia and Nicolato3 These studies tend to have positive findings for symptoms that arise shortly after childbirth and negative findings for symptoms arising at other times. In addition, a model using 116 gene transcripts, with a focus on oestrogen-responsive genes, predicted PPD with an accuracy of 88%.Reference Mehta, Newport, Frishman, Kraus, Rex-Haffner and Ritchie4 Similarly, genetic studies on PMDD indicate a potential hereditary component, with positive associations again with oestrogen and serotonin receptor candidate genes, although these findings lack consistent replication.Reference McEvoy, Osborne, Nanavati and Payne2
More recently, biomarker research in this area has moved beyond genetics to explore blood-based epigenetic and protein markers as well as neuroimaging markers. While there are numerous interacting systems that could yield such markers, this paper will focus on those that have yielded the most comprehensive data thus far – epigenetic markers, neuroactive steroids (NASs), the immune system and inflammatory pathways and markers discernible through brain imaging. While the animal literature is rich, and we make reference to some fundamental older studies, we will primarily confine our remarks to human studies since 2010. Because this literature is vast, we make no claim to be comprehensive, but rather have chosen studies that we feel are most illuminating for our purposes. We have also focused on those studies that track endogenous biomarkers and have not considered studies of how these markers may change in response to treatment.
Epigenetic markers
Epigenetic mechanisms are increasingly recognised as important to pathophysiology in both physical and mental disease. These mechanisms serve as dynamic and adaptive regulators of gene expression, and provide a real-life glimpse into how environmental elements shape health. Epigenetic mechanisms include DNA methylation, histone modification and chromatin remodelling, and epigenetic changes have been implicated in a wide range of diseases, from diabetes to autoimmune disease to cancer. Understanding such mechanisms can help us to unravel aetiology – and understanding how to reverse them, when maladaptive, can lead to novel drug discovery. Epigenetic mechanisms are increasingly being recognised as important contributors to the pathophysiology of PPD, although data on other reproductive psychiatric disorders is limited.
AND
There is some evidence of epigenetic changes in AND. In a series of studies that began with animal research and extended into women, the Payne and Kaminsky group identified altered DNA methylation at two loci at the heterochromatin protein 1, binding protein 3 (HP1BP3 – associated with oestrogen signalling) and tetratricopeptide repeat domain 9B (TTC9B – associated with synaptic plasticity) as predictors of AND.Reference Osborne, Clive, Kimmel, Gispen, Guintivano and Brown5,Reference Payne, Osborne, Cox, Kelly, Meilman and Jones6 Their study included blood samples from four prospectively collected cohorts, with DNA methylation data available from the first, second and/or third trimesters, depending on the cohort. Research has also linked epigenetic modifications in the nuclear receptor subfamily 3 group C member 1 (NR3C1 – associated with glucocorticoid signalling) gene to AND, with a positive correlation between increased methylation at CpG 4 and antenatal depressive symptoms.Reference Castro, Gardini, Iliadis, Ehlert, Kallak and Skalkidou7 Another study found that DNA methylation changes in T-lymphocyte genes, particularly within a CD3 gene CpG cluster, were associated with AND.Reference Robakis, Lee, Werner, Liu, Miller and Wylie8
In addition, placental DNA methylation analysis identified 16 CpGs associated with AND, including two CpGs linked to the expression of ADAM23 (a disintegrin and metalloproteinase domain-containing protein 23) and CTDP1 (CTD phosphatase subunit 1) – genes implicated in neurodevelopment and neuropsychiatric disorders.Reference Tesfaye, Chatterjee, Zeng, Joseph and Tekola-Ayele9 These findings suggest that prenatal depression may induce epigenetic changes in the placenta, potentially influencing fetal brain development.
PPD
A growing body of evidence suggests that DNA methylation variations can serve as predictive biomarkers of PPD. The Payne and Kaminsky biomarkers at TTC9B and HPIBP3, mentioned above under AND, were first studied for PPD. The group found that DNA methylation at two loci on these genes remains stable throughout pregnancy and significantly predicts PPD (area under the curve (AUC) 0.87).Reference Guintivano, Arad, Gould, Payne and Kaminsky10 These initial studies were carried out in women who were euthymic in pregnancy and went on to develop PPD. Subsequent studies in independent cohorts provided further validation of the predictive value of these markers, and identified that the two genes in question were involved in oestrogen signalling (HP1BP3) and synaptic plasticity (TTC9B).Reference Osborne, Clive, Kimmel, Gispen, Guintivano and Brown5,Reference Payne, Osborne, Cox, Kelly, Meilman and Jones6
Several studies have also examined epigenetic changes in the oxytocin receptor (OXTR, associated with oxytocin receptor) gene. Bell et alReference Bell, Carter, Steer, Golding, Davis and Steffen11 revealed an association between higher methylation levels at CpG site (−934) and increased PPD risk in women who were euthymic in pregnancy, particularly in those carrying the rs53576 GG genotype. Kimmel et alReference Kimmel, Clive, Gispen, Guintivano, Brown and Cox12 identified a significant association between PPD and an intronic region of OXTR located proximal to an oestrogen receptor binding region; CpGs in the region interacted with adverse life events to mediate the risk for PPD. Notably, adverse life events was associated with significantly increased DNA methylation in women who did not develop PPD.Reference Kimmel, Clive, Gispen, Guintivano, Brown and Cox12 This finding was replicated in an independent cohort; moreover, DNA methylation in the region was negatively correlated with oestradiol (E2) ratios, and the interaction between E2 and OXTR methylation was positively associated with the ratio of allopregnanolone (ALLO) to progesterone (PROG). Interestingly, OXTR DNA methylation patterns have also been identified as potential epigenetic biomarkers of childhood adversity, with associations observed between PPD and mother–child bonding.Reference Robakis, Zhang, Rasgon, Li, Wang and Roth13 Furthermore, lower OXTR methylation was associated with increased sensitivity to postnatal depressive symptoms in Latina mothers.Reference Rodriguez, Smith, Harris, Nephew, Santos and Murgatroyd14
Other studies identified additional epigenetic markers of PPD. One study identified positive correlation of NR3C1 methylation at CpG 2 with postpartum depressive symptoms.Reference Castro, Gardini, Iliadis, Ehlert, Kallak and Skalkidou7 Another study investigated inflammatory markers of PPD and identified that lower TNF-α expression in late pregnancy was associated with PPD in patients who had high methylation at the FOXP3 (forkhead box P3) Treg-cell-specific demethylated region (TSDR). This suggests that discrimination-induced stress may enhance hypothalamic-pituitary-adrenal (HPA) activity, leading to immune suppression and altered inflammatory gene regulation.Reference Sluiter, Incollingo Rodriguez, Nephew, Cali, Murgatroyd and Santos15
PND
One study found that persistent depressive symptoms occurring during the perinatal period (either prenatally, postpartum or both) were associated with higher OXTR methylation within exon 3 and lower methylation in the intergenic region (IGR) between the oxytocin (OXT) and vasopressin (AVP) genes.Reference King, Robins, Chen, Yerko, Zhou and Nagy16
Perinatal anxiety
Two of the studies covered in the PPD section also investigated epigenetic changes in perinatal anxiety.Reference Robakis, Lee, Werner, Liu, Miller and Wylie8,Reference Sluiter, Incollingo Rodriguez, Nephew, Cali, Murgatroyd and Santos15 One study observed that DNA methylation changes in T-lymphocyte genes, particularly within a CD3 gene CpG cluster, were associated with antenatal anxiety.Reference Robakis, Lee, Werner, Liu, Miller and Wylie8 The other study identified lower TNF-α expression in late pregnancy was associated with postpartum anxiety symptoms in those with elevated methylation at the FOXP3 TSDR.Reference Sluiter, Incollingo Rodriguez, Nephew, Cali, Murgatroyd and Santos15
pOCD
The Payne and Kaminsky group examined the epigenetic markers they identified for PPD in pOCD. They found that increased methylation at one of these same markers, TTC9B, along with increased methylation at the membrane-spanning 4A gene family (MS4A7), predicted OCD symptom exacerbation during pregnancy with moderate accuracy. Incorporating a methylome-wide approach, they further identified a DNA methylation proxy of the ratio of monocytes to non-monocytes, which improved the accuracy of the prediction model (AUC 0.75, 95% CI: 0.59–0.91). While promising, the small sample size (N = 24) and balanced case–control distribution may overestimate predictive performance. Further validation in larger, diverse cohorts is needed to confirm clinical applicability and explore whether these epigenetic changes are causally linked to pOCD.Reference Kaminsky, Osborne, Guglielmi, Jones, Grenier and Clark17
PMDD
A study using a comparative whole-genome RNA-seq analysis between the mouse ventral hippocampus and lymphoblastoid cell lines from women with and without PMDD identified shared epigenetic biomarkers across species and cell types, including epigenetic modifiers of the ESC/E(Z) complex (ESC/E(Z):Extra Sex Combs/Enhancer of Zeste), a key regulator in the response to ovarian steroids.Reference Marrocco, Einhorn, Petty, Li, Dubey and Hoffman18
Summary
Overall, this latest research into epigenetic markers in reproductive psychiatric disorders highlights DNA methylation on hormone-responsive genes as potential biomarkers for reproductive psychiatric illness. Nevertheless, challenges still remain, particularly regarding tissue specificity, sample size limitations, disease specificity and the need for longitudinal validation. Subsequent studies must include multi-omics approaches to identify robust, reproducible epigenetic markers across diverse populations. Research is currently ongoing to examine these markers in PMDD and PPP. In addition, exploring tissue-specific DNA methylation differences – more specifically in brain tissue versus peripheral blood – will be critical in determining whether epigenetic changes are secondary to systemic inflammation or directly contribute to neuropsychiatric symptoms. Furthermore, given the role of oestrogen signalling in reproductive mood disorders, studying how hormonal fluctuations regulate such epigenetic signatures would provide valuable mechanistic insights. Integrating epigenetic findings with NAS and immune system data under a multi-omics framework may yield a more comprehensive understanding of reproductive psychiatric pathophysiology.
Neuroactive steroids
NASs play a critical role in women’s health, with significant fluctuations throughout reproductive life stages. These naturally occurring metabolites of cholesterol are synthesised either in the brain or in the periphery (adrenal glands and ovaries). They act on the central nervous system by modulating behaviour and neurotransmitter function, increasing levels of serotonin and counteracting some effects of cortisol in the brain.Reference Tuem and Atey19 NASs regulate the HPA axis and stress response.Reference Sze and Brunton20 They act on several receptors, primarily gamma-aminobutyric acid (GABA)-A,; Reference Tuem and Atey19,Reference Sze and Brunton20 the receptor for the primary inhibitory neurotransmitter in the central nervous system. Research highlights the roles of various NASs, such as pregnanolone, dehydroepiandrosterone (DHEA) oestradiol (E2) and testosterone, in mood and anxiety symptoms related to reproductive transitions, with a significant focus on allopregnanolone (ALLO), a metabolite of progesterone (PROG).Reference McEvoy, Payne and Osborne21 ALLO and pregnanolone are positive allosteric modulators of the GABA-A receptor, producing anxiolytic and sedative effects, while their isomers, isoallopregnanolone and epipregnanolone, act as negative modulators, reducing GABAergic neurotransmission.Reference Schüle, Nothdurfter and Rupprecht22
NAS synthesis varies during key reproductive stages – menstruation, the perinatal period and perimenopause. PROG and ALLO exhibit low levels during the follicular phase, increase throughout the luteal phase and then decline rapidly in the late luteal phase before menstruation.Reference Hantsoo and Payne23,Reference McEvoy and Osborne24 In the perinatal period PROG and ALLO rise steadily to supraphysiological levels during pregnancy and drop sharply after delivery.Reference McEvoy, Payne and Osborne21 The highly variable transition to menopause includes increased E2Reference Hale, Zhao, Hughes, Burger, Robertson and Fraser25 and decreased PROG in the luteal phaseReference Hale, Zhao, Hughes, Burger, Robertson and Fraser25 and fluctuations in ALLO.Reference McEvoy and Osborne24 Psychiatric conditions during these reproductive transitions are linked to changes in NASs. While there is some limited literature on the roles of other NASs – such as pregnanolone, allotetrahydroDOC, DHEA, DHEA-S and testosterone – in mood and anxiety disorders related to reproductive transitions,Reference McEvoy and Osborne24 the majority of the evidence currently available focuses on the metabolites of PROG, and we have therefore focused this section on those molecules. In perimenopausal depression, we also briefly consider the role of changes in E2, as there is a more substantial literature in this area.
AND
A study of 96 third-trimester pregnant women found that those with elevated depression scores (Montgomery–Åsberg Depression Rating Scale – self-rating version; MADRS-S ≥ 13) had significantly lower ALLO levels and a negative correlation with concurrent self-reported depression scores,Reference Hellgren, Åkerud, Skalkidou, Bäckström and Sundström-Poromaa26 while another study of 14 women linked lower PROG and combined ALLO and pregnanolone levels in the second trimester to increased negative emotional reactions to stress.Reference Crowley, O’Buckley, Schiller, Stuebe, Morrow and Girdler27
Our group found no significant association between concurrent depressive symptoms and PROG and ALLO levels during pregnancy in women with a prior mood disorder (n = 60).Reference Osborne, Gispen, Sanyal, Yenokyan, Meilman and Payne28 Similarly, in another study (n = 111) we found no significant relationship between ALLO levels and depression during pregnancy.Reference Standeven, Osborne, Betz, Yenokyan, Voegtline and Hantsoo29 Another study in 284 pregnant women found no relationship between ALLO and concurrent mood symptoms during pregnancy,Reference Hellgren, Comasco, Skalkidou and Sundström-Poromaa30 but did find that those who were homozygous for the minor allele in a polymorphism on the AKR1C2 gene (the gene for a key enzyme in the PROG metabolic pathway) had lower levels of ALLO and a steeper rise in depressive symptoms across pregnancy.
PPD
While at least one older study found no significant differences in postpartum ALLO levels between women with and without PPD at 9 weeks or 6 months postpartum,Reference Epperson, Gueorguieva, Czarkowski, Stiklus, Sellers and Krystal31 our group identified both cross-sectional and predictive relationships between NASs and postpartum depressive symptoms. We reported that higher ALLO levels at week 6 postpartum were associated with worse mood symptomsReference Standeven, Osborne, Betz, Yenokyan, Voegtline and Hantsoo29 and found that lower ALLO levels in euthymic women during the second trimester predicted postpartum-onset PPD, with each additional ng/mL decreasing the odds of developing PPD by 63%.Reference Osborne, Gispen, Sanyal, Yenokyan, Meilman and Payne28 Similarly, a subsequent study found that participants with a one-unit increase in PROG as well as in the isoallopregnanolone/pregnanolone ratio at the third trimester (T3) had higher odds of developing PPD, while those with a one-unit increase in the log pregnanolone/PROG ratio had lower odds.Reference Osborne, Etyemez, Pinna, Alemani, Standeven and Wang32 Conversely, another found no differences in either pregnancy or postpartum levels of pregnanolone, ALLO, pregneolone or their ratios to PROG between those with and without PPD.Reference Deligiannidis, Sikoglu, Shaffer, Frederick, Svenson and Kopoyan33 Recent clinical trials with synthetic ALLO also support a role for GABAergic NASs in PPD.Reference Meltzer-Brody, Colquhoun, Riesenberg, Epperson, Deligiannidis and Rubinow34 These findings suggest that it may not be the absolute levels of NASs that matter, but rather the dynamics of their withdrawal postpartum and changes in GABA-A receptor sensitivity, which may contribute to psychiatric symptoms. This underscores the importance of precise timing in evaluating potential biomarkers.
PND
Another study of 75 women found that those at risk for depression during pregnancy or the postpartum (The Edinburgh Postnatal Depression Scale (EPDS) >10 or a history of PND or MDD) had elevated levels of 5-alpha-dihydroprogesterone (5α-DHP) and 5-beta-dihydroprogesterone (5β-DHP) (the intermediary molecules between PROG and, respectively, ALLO and pregnanolone) and ALLO compared to healthy controls,Reference Deligiannidis, Kroll-Desrosiers, Tan, Dubuke and Shaffer35 while the same group showed that at-risk women also had higher PROG and pregnanolone levels, with pregnanolone positively associated with Hamilton Rating Scale for Depression (HAMD-17) and Hamilton Anxiety Rating Scale (HAM-A) scores, and lower GABA levels negatively associated with these scores.Reference Deligiannidis, Kroll-Desrosiers, Tan, Dubuke and Shaffer35
In light of this inconclusive evidence in AND, PPD and PND, some researchers have also explored ratios of NASs to their precursors in the PROG metabolic pathway. The Deligiannidis groupReference Deligiannidis, Kroll-Desrosiers, Tan, Dubuke and Shaffer35 found significant differences in the ratio of ALLO to 5α-DHP among participants with PND, those at risk and healthy controls, and in the ratio of pregnanolone to 5β-DHP between healthy controls and at-risk women, although no differences were noted between healthy controls and those with PND. In addition, another study reported a correlation between increased ratios of ALLO to PROG and pregnanolone to PROG with concurrent depressive symptoms during the first and second trimesters compared to healthy controls in a diverse population.Reference Wenzel, Pinna, Eisenlohr-Moul, Bernabe, Tallon and Nagelli36
A recent meta-analysis of 13 studies (2509 women, including 849 with peripartum depressive symptoms (PDSs), encompassing several of the studies listed above) examined the relationship between ALLO blood concentrations and PDSs. Overall, no significant differences in ALLO levels were observed between women with and without PDSs at any time point during the second or third trimesters or postpartum (p > 0.05). However, subgroup analyses revealed higher ALLO levels in women with PDSs at gestational weeks 21–24 and 25–28. Methodological factors, including sample type and analytical techniques, influenced the results. These findings underscore the challenges posed by heterogeneous time points, cohorts and methods in understanding the role of NASs like ALLO in PDSs.Reference Schoretsanitis, Osborne, Sundström-Poromaa, Wenzel, Payne and Barbui37
Perinatal anxiety
Our group found a correlation between low ALLO levels in the second trimester and subsequent postpartum anxiety,Reference Osborne, Betz, Yenokyan, Standeven and Payne38 while higher ALLO levels were associated with increased concurrent anxiety symptoms at 6 weeks postpartum.Reference Standeven, Osborne, Betz, Yenokyan, Voegtline and Hantsoo29 Our recent study broadened this scope to explore the entire PROG metabolic pathway in women with and without clinically significant anxiety, excluding those with comorbid depression. We found a significant moderating effect of anxiety group on the relationship between PROG and ALLO. In particular, when compared to healthy controls, women with anxiety exhibited a more pronounced shift from the third trimester to postpartum week 6 in metabolism towards the end-points of the PROG metabolic pathway.Reference Etyemez, Miller, Voegtline, Özdemir, Standeven and Santovito39 We also found that women with at least one minor allele at AKR1C2 rs1937863 (the gene for a key enzyme in the PROG metabolic pathway) had higher concentrations of 5α-DHP and a lower ratio of ALLO:5α-DHP at T3.
The variability in these results may stem from differences in study designs, including how anxiety is defined and timed, the presence of comorbid psychiatric disorders and the methods used for quantifying NASs.
PMDD
There is a substantial body of literature on fluctuations in NASs throughout the menstrual cycle and their role in the symptoms of PMDD. The primary NASs whose fluctuations have been studied during the menstrual cycle and are relevant to PMDD include PROG, its metabolite ALLO and E2. Ovarian steroid hormone levels may not differ between women with PMDD versus without;Reference Nguyen, Reuter, Gaikwad, Rotroff, Kucera and Motsinger-Reif40 rather, symptom onset in PMDD may be connected with an abnormal response caused by altered sensitivity of central nervous system receptors to normal hormonal fluctuations.Reference Dubey, Hoffman, Schuebel, Yuan, Martinez and Nieman41 Researchers have proposed the Dimensional Affective Sensitivity to Hormones across the Menstrual Cycle (DASH-MC) framework, suggesting that ALLO mediates hormonal effects on mood. This model links atypical responses to E2 and PROG fluctuations with distinct affective changes, offering a transdiagnostic approach to hormone sensitivity in psychiatric disorders.Reference Peters, Schmalenberger, Eng, Stumper, Martel and Eisenlohr-Moul42 Some evidence shows that PMDD symptoms start with the increase in PROG and E2 levels during the luteal phase, worsen in the luteal phase as hormone levels decline and improve with the start of menstruation.Reference Cunningham, Yonkers, O’Brien and Eriksson43 A recent study did report, however, that PROG had a distinct pattern in PMDD, featuring an early leftward shift that begins in the periovulatory phase followed by a rapid decrease during the mid to late luteal phase.Reference Hamidovic, Mumford, Schisterman, Davis and Soumare44 Other studies lend support to a difference in hormone levels. Elevated ALLO levels and ALLO/PROG ratios in women with PMDD compared to those without PMDD have also been observed, with a curious inverse relationship between ALLO levels and symptom severity.Reference Girdler, Straneva, Light, Pedersen and Morrow45 Another study found that isoallopregnanolone potentially alleviates negative mood symptoms by antagonising ALLO’s effects on the GABA-A receptor.Reference Bixo, Johansson, Timby, Michalski and Bäckström46 Brain activity in emotion-processing regions during the late luteal phase has been shown to correlate with the isoallopregnanolone/ALLO ratio in PMDD, while the opposite was observed in controls.Reference Stiernman, Dubol, Comasco, Sundström-Poromaa, Boraxbekk and Johansson47
Furthermore, PMDD patients show altered sensitivity to ALLO across the menstrual cycle, with changes in saccadic eye velocity (SEV).Reference Timby, Bäckström, Nyberg, Stenlund, Wihlbäck and Bixo48 This study aimed to examine how PMDD patients respond to intravenous ALLO across the menstrual cycle, using SEV as a measure of GABA-A receptor activity. PMDD patients exhibited a decreased SEV response in the follicular phase compared to the luteal phase, while controls showed the opposite pattern. These findings suggest that PMDD patients have altered sensitivity to ALLO, which is influenced by the menstrual cycle.Reference Timby, Bäckström, Nyberg, Stenlund, Wihlbäck and Bixo48
There is also evidence of disrupted ALLO–GABA-A receptor interactions, contributing to PMDD symptoms and increased stress sensitivity, particularly because of dysregulation of the HPA axis during the luteal phase.Reference Hantsoo and Epperson49 A randomised study showed that blocking 5-alpha reductase (which converts PROG to ALLO) reduced PMDD symptoms, supporting the idea that NAS regulation is involved in PMDD.Reference Martinez, Rubinow, Nieman, Koziol, Morrow and Schiller50
PMDD also involves disrupted autonomic nervous system regulation, with lower heart rate variability (HRV) and altered stress responses.Reference Hamidovic, Davis, Soumare, Naveed, Ghani and Semiz51 Baseline ALLO levels significantly predicted peak high frequency of the heart rate (HF-HRV) changes in the PMDD group, suggesting its role in modulating stress responses in PMDD.Reference Hamidovic, Davis, Soumare, Naveed, Ghani and Semiz51 Another study investigated the relationship between neuroactive PROG metabolites, such as ALLO, and HPA axis function in women with PMDD. While there were no differences in diurnal cortisol secretion or dexamethasone suppression between PMDD patients and healthy controls, higher ALLO levels in PMDD patients were associated with blunted nocturnal cortisol levels compared to controls with lower ALLO. This suggests that diurnal cortisol secretion in PMDD may be influenced by luteal phase ALLO levels, potentially explaining inconsistencies in previous studies of HPA axis function in PMDD.Reference Segebladh, Bannbers, Moby, Nyberg, Bixo and Bäckström52
The studies on PMDD inevitably point toward the contribution of fluctuations of the levels of such hormones, including PROG and its metabolite ALLO, to causation and exacerbation of PMDD. The key findings point towards the altered responsiveness of the central nervous system toward such fluctuations of the hormones, and not toward abnormalities of the levels of the hormones, and this may be contributing to the severity of the symptoms, more significantly in the luteal phase. Also, impaired interaction of ALLO with the GABA-A receptor, increased sensitivity to stress and dysfunction of the autonomic nervous system, more significantly in the luteal phase, are contributing factors to PMDD’s pathogenesis, and targets of treatment could be modulation of NAS action and the HPA axis.
Perimenopausal depression
As women transition to menopause, ovarian function gradually declines, particularly marked by decreased E2 and PROG levels. This weakens these hormones’ negative feedback on the pituitary gland, leading to increased follicle-stimulating hormone (FSH) and luteinising hormone.Reference Randolph, Zheng, Sowers, Crandall, Crawford and Gold53 E2 fluctuations are linked to significant changes in FSH, resulting in more menstrual cycles without follicular development and reduced PROG levels.Reference Joffe, de Wit, Coborn, Crawford, Freeman and Wiley54 Changes in PROG and its metabolites during the perimenopausal period are believed to contribute to mood fluctuations, with studies linking NAS variability to depression, anxiety and irritability.Reference Gordon, Girdler, Meltzer-Brody, Stika, Thurston and Clark55,Reference Schweizer-Schubert, Gordon, Eisenlohr-Moul, Meltzer-Brody, Schmalenberger and Slopien56 and mixed evidence on whether differences in absolute levels are important.Reference Joffe, de Wit, Coborn, Crawford, Freeman and Wiley54,Reference Han, Xia, Zheng, Zhong, Zhao and Wang57,Reference Süss, Willi, Grub and Ehlert58 There is also considerable evidence concerning changes in E2 at the menopausal transition, with studies indicating correlation between higher depressive symptoms or mood fluctuations and increased E2 variabilityReference Joffe, de Wit, Coborn, Crawford, Freeman and Wiley54,Reference Willi, Süss, Grub and Ehlert59 or a sudden drop in E2 levels.Reference Gordon and Sander60,Reference Schmidt, Ben Dor, Martinez, Guerrieri, Harsh and Thompson61
Summary
In summary, NASs are crucial for mood regulation, with levels of PROG and its metabolites in particular fluctuating across reproductive stages. While NAS dysregulation is implicated in perinatal affective disorders, PMDD and perimenopausal mood changes, inconsistencies in research findings highlight the need to focus on the dynamics of NAS fluctuations and their impact on GABA-A receptor function, rather than absolute levels. Future research should prioritise longitudinal studies with frequent NAS sampling, careful attention to timing of symptom onset, investigation of subunit-specific GABA-A receptor modulation and exploration of interactions with other neurotransmitter systems and the HPA axis. Standardised protocols and clinical trials are crucial for clarifying the role of NASs in these disorders and developing targeted interventions to improve women’s mental health.
Immunological biomarkers
The immune system has a major influence on homeostasis in the brain and mood modulation through different cytokines, chemokines and other immunological mechanisms. Recent research has revealed that inflammation and immune signalling can directly affect neurotransmitter systems, neuroplasticity and synaptic strength, and hence brain dysfunction, and a solid body of evidence links increased inflammation to depression outside of reproductive transitions.Reference Miller and Raison62
Among these key immunological players, cytokines and chemokines represent a group of small signalling proteins produced by immune cells and have a central function in cell communication during immune responses such as inflammation.Reference Miller, Berk, Bloch, Briquet-Laugier, Brouillon and Cuthbert63 These findings hint at a possible role of immune markers in regulating mood during the menopausal transition, but the nature of this relationship is not fully understood. Elevated inflammatory markers, such as interleukin-6 (IL-6), may contribute to neuroinflammation, further disrupting neurotransmitter pathways and possibly affecting the HPA axis function that regulates the response to stress and mood. Further, more research is needed to determine whether active immune dysregulation is a causal risk factor in mood disturbances or merely a measure of the biological response to the hormonal changes.Reference Łoś and Waszkiewicz64 In addition to cytokine signalling, certain psychiatric disorders may be associated with the peripheral release of extracellular vesicles, including exosomes and microvesicles, which influences intercellular communication. Extracellular vesicles contain bioactive molecules such as cytokines, microRNAs, proteins and lipids, which influence the function and behaviour of recipient cells. In psychiatric disorders, extracellular vesicles were reported to be involved in both neuroinflammation and neurodegeneration by transporting pro-inflammatory factors and microRNAs through the blood brain barrier (BBB), subsequently affecting synaptic connectivity in the brain immune subset.
Another very important immune-modulating pathway involved in psychiatric disorders is the kynurenine (KYN) pathway. Tryptophan (Trp) is either metabolised to serotonin or down the KYN pathway into neuroprotective or neurotoxic metabolites. In an inflammatory environment, inflammatory cytokines such as IFN-γ, interleukin-1β (IL-1β) and tumour necrosis factor-alpha (TNF-α) stimulate indoleamine 2,3-dioxygenase (IDO), which degrades Trp towards the KYN metabolites, thus reducing serotonin levels and potentially causing depressive symptoms.Reference Osborne, Payne and Osborne65 The KYN can be further converted into the neurotoxic quinolinic acid (QUIN), which activates N-methyl-D-aspartate (NMDA) receptors, or into the neuroprotective kynurenic acid (KYNA), an NMDA antagonist with anti-inflammatory effects.Reference Badawy66 Dysregulation of this pathway, influenced by inflammatory cytokines, has been implicated in depression, anxiety and psychosis, particularly during the perinatal period in women, suggesting that these metabolites along the KYN pathway may be candidates for biomarker studies of reproductive psychiatric disorders.Reference Osborne and Monk67
The following sections will delve into the specific immunological biomarkers identified in each of these disorders, summarising key research studies that have contributed to our current understanding.
AND
Biancardi et alReference Bianciardi, Barone, Lo Serro, De Stefano, Giacchetti and Aceti68 found higher levels of IL-6 and TNF-α in depressed women with a history of trauma during the second trimester compared to depressed women without a trauma history, suggesting that trauma prior to the pregnancy may worsen immune dsyregulation and be related to depressive symptoms during pregnancy. In contrast, another study illustrated the opposite correlation between increased levels of TNF-α and depressive symptoms in the third trimester, indicating dynamic immune changes during pregnancy and in patients with AND.Reference Buglione-Corbett, Deligiannidis, Leung, Zhang, Lee and Rosal69 In addition, Leff Gelman et alReference Leff Gelman, Mancilla-Herrera, Flores-Ramos, Saravia Takashima, Cruz Coronel and Cruz Fuentes70 reported positive correlations between depressive symptoms and cytokines such as interleukin 2 (IL-2), IL-6, TNF-α, Th2-related mediators interleukin 13 (IL-13), interleukin 10 (IL-10) and interleukin 9 (IL-9) and the T-helper 17 (Th17)-associated cytokine interleukin 17A (IL-17A) during the third trimester. Furthermore, a study by Miller et alReference Miller, Sakowicz, Roy, Yang, Sullivan and Grobman71 reported that higher levels of pro-inflammatory cytokines such as IL-1β, interleukin 23 (IL-23) and interleukin 33 (IL-33) in cerebrospinal fluid (CSF) were correlated with an increased risk for AND, suggesting that these inflammatory markers in the brain may play a more significant role in AND than peripheral inflammation alone. Interestingly, this study found little correlation between levels in CSF and those in the periphery. Most other studies have focused on the periphery alone, and it is unknown how correlations found in the periphery – even when associated with illness – may be correlated with action in the central nervous system.
Besides cytokine studies, several studies have shown that the KYN pathway is also influenced by inflammatory activity in AND. They found that prenatal Trp levels and the KYN/Trp ratio moderate the association between IL-6 levels and AND. Moreover, levels of IL-6, QUIN, TNF and KYN in the second trimester showed strong individual accuracy in predicting depression risk and severity in the third trimester, making them potential biomarkers for AND.Reference Nazzari, Molteni, Valtorta, Comai and Frigerio72 In addition, a longitudinal study by Sha et al found that elevated levels of inflammatory cytokines (IL-6 and TNFα) and alterations in markers of Trp metabolism (QUIN and KYN) during pregnancy can accurately predict depressive symptoms during pregnancy.Reference Sha, Madaj, Keaton, Escobar Galvis, Smart and Krzyzanowski73 Another recent study by the same group reported that low expression of critical KYN pathway enzymes such as quinolinate phosphoribosyltransferase (QPRT) and 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase (ACMSD) in placenta samples was associated with increased levels of plasma cytokines and a dysregulated KYN metabolite pattern, which were linked to depressive symptoms, suggesting the role of inflammation and KYN pathway enzymes as possible therapeutic targets in peripartum depression.Reference Sha, Escobar Galvis, Madaj, Keaton, Smart and Edgerly74 In addition, some of the studies have shown that elevated C-reactive protein (CRP) levels, alongside lower Trp levels, were correlated with AND.Reference Lahti-Pulkkinen, Girchenko, Robinson, Lehto, Toffol and Heinonen75,Reference Saadat, Zhang, Hyer, Padmanabhan, Woo and Engeland76 In contrast, women with depressive symptoms in the second trimester showed lower CRP levels.Reference Blackmore, Moynihan, Rubinow, Pressman, Gilchrist and O’Connor77
PPD
Recent research has identified key immunological biomarkers associated with PPD, particularly focusing on cytokines, the KYN pathway and extracellular vesicles.Reference Modzelewski, Oracz, Iłendo, Sokół and Waszkiewicz78,Reference Zhu, Jin and Tang79 As reviewed by Osborne and Monk,Reference Osborne and Monk67 numerous studies have found elevated pro-inflammatory markers such as IL-6 and TNF-α associated with increased risk of PPD, while a few studies have reported decreased cytokine levels linked with PPD symptoms, suggesting that cytokine–cortisol interactions may vary among individuals with PPD.Reference Corwin, Pajer, Paul, Lowe, Weber and McCarthy80 In addition, one study reported that prenatal Trp levels and the KYN/Trp ratio moderated the association between IL-6 levels and PPD.Reference Nazzari, Molteni, Valtorta, Comai and Frigerio72 Two studies have shown that low levels of anti-inflammatory cytokines, particularly IL-10, increase susceptibility to PPD. This highlights the protective role of anti-inflammatory states against mood disturbances.Reference Bränn, Papadopoulos, Fransson, White, Edvinsson and Hellgren81,Reference Ono, Yu, Obara, Ishikuro, Murakami and Kikuya82 Some researchers have attempted to consolidate such findings by developing composite inflammation scores; these have been able to predict the onset of PPD, but less effectively than a clinical history of prior depression.Reference Bränn, Papadopoulos, Fransson, White, Edvinsson and Hellgren81 The same research team conducted metabolic profiling studies, revealing variations in immune-related metabolic pathways associated with PPD. They identified distinct metabolic profiles connected to postpartum depressive symptoms, indicating that disruptions in lipid metabolism and immune-related metabolites may play a key role in the development of PPD.Reference Bränn, Malavaki, Fransson, Ioannidi, Henriksson and Papadopoulos83 Furthermore, another study by Min et alReference Min, Li and Ying84 found a positive correlation between elevated Th17 cells and related cytokine IL-17A with both PPD and anxiety, which further supports the role of Th17-mediated inflammation in these conditions. Our own group has explored the connection between cellular immune dysfunction and PPD. We found that defects in regulatory T-cells are linked to immune dysregulation and contribute to PPD,Reference Osborne, Gilden, Kamperman, Hoogendijk, Spicer and Drexhage85 and that altered mRNA communication in extracellular vesicles during pregnancy, tied to reduced autophagy, is also associated with subsequent PPD, suggesting that molecular signalling pathways involving immune cells may further contribute to the disorder.Reference Osborne, Payne, Sherer and Sabunciyan86
Research on the KYN pathway has also provided insights into underlying pathways of PPD. Sha et alReference Sha, Achtyes, Nagalla, Keaton, Smart and Leach87 have shown that hormonal changes, especially higher E2 levels, were linked to a pro-inflammatory profile and neurotoxic KYN metabolites, while PROG was associated with an anti-inflammatory profile, shedding light on the hormonal and immune interactions influencing PPD. In addition, another study has explored the role of the KYN pathway and kynurenic aminotransferase (KAT) alleles in PPD following caesarean section in Chinese women; their findings indicate that postpartum depressive symptoms (PDSs) are associated with decreased KAT activity and an increased QUIN/KYNA ratio, although no significant relationship was found between KAT alleles and PDSs.Reference Quan, Wang, Duan, Ma, Yu and Yang88
In summary, the current literature emphasises the complex role of cytokine profiles, immune cell activity, metabolic pathways and molecular signalling in the etiology and progression of PPD. These findings suggest that immune-related biomarkers are likely to be the key to the identification of women at risk of PPD and may provide new targets for treatment. However, methodological variations and small sample sizes are notable across these studies, with effect sizes differing depending on the demographic characteristics and immune markers measured.
PND
In our own study, we examined the role of pro-inflammatory cytokines in depression across the perinatal period and found increased levels of IL-6 and macrophage inflammatory protein-1 α, chemokine (C-C motif) ligand 3 (MIP-1α/CCL3) beginning in the third trimester and continuing through the early postpartum in more depressed women. This study also found that women with higher pro-inflammatory cytokine values were more likely to be Hispanic, but less likely to be African American or overweight/obese compared to women with lower pro-inflammatory cytokine values, suggesting differential patterns in cytokine levels by race, ethnicity and overweight/obesity status.Reference Osborne, Yenokyan, Fei, Kraus, Moran and Monk89
Small sample sizes, heterogeneity of mood assessment tools and cytokine detection techniques in these studies affect generalisability and consistency across findings. These findings highlight how inflammatory changes during pregnancy may contribute to PND, with immune dysregulation potentially persisting into the postpartum period. Understanding the trajectory of these immune alterations is of key importance to the discovery of biological mechanisms influencing mood disturbance during the perinatal phase.
Perinatal anxiety
Several studies have reported positive associations between high levels of pro-inflammatory cytokines such as IL-6, C-X-C motif chemokine ligand 8 (IL-8/CXCL-8) and TNF-α and/or a composite pro-inflammatory score with higher anxiety symptoms during pregnancy and postpartum.Reference Osborne, Yenokyan, Fei, Kraus, Moran and Monk89,Reference Mancuso, Ross, Accortt, Coussons-Read, Okun and Irwin90 Notably, Sherer et alReference Sherer, Voegtline, Park, Miller, Shuffrey and Klein91 found a negative correlation, where lower levels of pro-inflammatory cytokines were associated with higher anxiety. This finding is particularly relevant as it examines anxiety independently of depression, suggesting that there may be distinct immune mechanisms underlying perinatal anxiety versus comorbid anxiety and depression. In addition, population differences may be important to these differential findings.Reference Furtado, Van Lieshout, Van Ameringen, Green and Frey92 Furthermore, some studies have found that a pro-inflammatory profile is accompanied by elevated levels of anti-inflammatory cytokines (IL-13, IL-10), possibly as a compensatory response,Reference Karlsson, Nousiainen, Scheinin, Maksimow, Salmi and Lehto93 whereas other studies have linked increased levels of inflammation with increased cortisol concentrations in participants with higher levels of anxiety and depressive symptoms.Reference Weis, Yuan, Walker, Gibbons and Chan94 Notably worthy of mention is that only a few studies have been conducted in populations with anxiety alone, without comorbid depression – so findings may be partially explained by that discrepancy.
In addition, studies exploring chemokine profiles in perinatal anxiety have revealed elevated levels of pro-inflammatory chemokines, such as IL-8/CXCL-8, monocyte chemoattractant protein 1 chemokine (C-C motif) ligand 2 (MCP-1)/CCL2 and macrophage inflammatory protein 1 beta chemokine (C-C motif) ligand 4 (MIP-1β)/CCL4, as well as an increase in composite pro-inflammatory chemokine score in women with moderate to severe anxiety, suggesting that chemokine profiles may play a crucial role in immune dysregulation and mood disturbances,Reference Camacho-Arroyo, Flores-Ramos, Mancilla-Herrera, Cruz, Hernández-Ruiz and Diaz95 although some studies have not found differences.Reference Sherer, Voegtline, Park, Miller, Shuffrey and Klein91 We conducted a study in perinatal anxiety without comorbid depression in Pakistan and showed a distinct pattern of cytokine and chemokine profiles associated with perinatal anxiety, differing from those found in other populations, indicating that population-level factors may influence immune responses.Reference Etyemez, Mehta, Tutino, Zaidi, Atif and Rahman96 Specifically, elevated chemokine activity across pregnancy in highly anxious women was observed. Our group has also developed a composite factor score by performing principal component analysis (PCA) and clustering innate immune markers as well as chemokine markers with anxiety symptoms in a longitudinal framework, enabling us to assess immune patterns across multiple time points in this population.Reference Etyemez, Mehta, Tutino, Zaidi, Atif and Rahman96 Few studies in perinatal anxiety have moved beyond cytokines and chemokines to consider dysregulation in immune cells; one such study found that increases in the cytotoxic to helper T-cell ratio and altered balance between Th17 and Treg cells were associated with perinatal anxiety symptoms.Reference Sherer, Voegtline, Park, Miller, Shuffrey and Klein91
PPP
Research suggests that immune dysregulation, particularly alterations in cytokine levels and Trp metabolism, plays a vital role in the development of PPP.Reference Bergink, Gibney and Drexhage97 Bergink et alReference Bergink, Burgerhout, Weigelt, Pop, de Wit and Drexhage98 found distinct immune changes in first-onset PPP, including elevated MCP-1/CCL2 and reduced anti-inflammatory glucocorticoid receptor alpha (GR-α) compared to healthy postpartum and non-postpartum controls. In addition, higher IL-1β levels were observed in healthy postpartum women, indicating immune shifts specific to the postpartum period that may contribute to PPP’s pathophysiology. Furthermore, they reported significantly elevated T-cells (both T-effector and T-regulatory cells) in healthy women postpartum, whereas patients with PPP lacked this elevation. Instead, they had increased monocyte levels and upregulated immune-related monocyte genes compared to both postpartum and non-postpartum controls. Their findings suggest a balanced interplay between pro-inflammatory and anti-inflammatory responses, indicating that the immune system adapts during the postpartum period to maintain equilibrium and prevent excessive inflammation.Reference Bergink, Burgerhout, Weigelt, Pop, de Wit and Drexhage98 Similarly, Sathyanarayanan et alReference Sathyanarayanan, Thippeswamy, Mani, Venkataswamy, Kumar and Philip99 also showed elevated IL-6 and uniquely elevated IL-8/CXCL-8 in the PPP group, suggesting a specific role of IL-8/CXCL-8 in PPP development. In addition, studies on Trp metabolism found imbalances in neurotoxic and neuroprotective metabolites, with increases in 3-hydroxykynurenine (3HK) and decreases in KYNA, suggesting immune activation as a potential etiology for neuropsychiatric symptoms in PPP.Reference Veen, Myint, Burgerhout, Schwarz, Schütze and Kushner100
PMDD
Recent evidence suggests that the inflammatory response, which may be triggered by hormonal fluctuations, could contribute to the mood disturbances, fatigue and physical symptoms commonly seen in PMDD and PMS.Reference Tiranini and Nappi101 Barone et alReference Barone, Ho, Osborne, Eisenlohr-Moul, Morrow and Payne102 presented elevated levels of the pro-inflammatory markers IL-8/CXCL-8 and TNF-α during the luteal phase in a PMDD group compared to controls. Also, they found positive association between premenstrual symptom severity with higher levels of IL-8/CXCL-8, highlighting the potential role of inflammation in the manifestation of PMDD symptoms. Despite all these results, evidence in this area is still limited, and further studies are needed to better understand the underlying immunological mechanisms of PMDD.
Perimenopausal depression
Perimenopausal depression is a significant psychiatric illness that arises in the context of the menopausal transition, characterised by mood fluctuations, anxiety and cognitive symptoms. Growing evidence suggests that hormonal disturbances, neuroinflammation and oxidative stress contribute to its development.Reference Liang, Kow, Yusof, Tham, Ho and Lee103 One of the earliest immunological changes observed in perimenopausal depression is an increase in pro-inflammatory cytokines. As reviewed by Liang et al,Reference Liang, Kow, Yusof, Tham, Ho and Lee103 there have been many studies reporting elevated levels of interleukin-8 (IL-8), TNF-α, IL-1β and IL-6 in women experiencing perimenopausal depression. These cytokines are important mediators of neuroinflammation, synaptic dysfunction and neuronal apoptosis, all contributing to mood disorders.Reference Liang, Kow, Yusof, Tham, Ho and Lee103 In addition, Matthews et alReference Matthews, Schott, Bromberger, Cyranowski, Everson-Rose and Sowers104 also suggested that perimenopausal women with higher depressive symptoms exhibit increased fibrinogen and plasminogen activator inhibitor type 1 (PAI-1), both of which are markers of systemic inflammation and hypercoagulability. These findings highlight an increased cardiovascular risk associated with perimenopausal depression, further linking immune dysregulation to broader health concerns. Furthermore, decreased E2 levels favour a pro-inflammatory state, which in turn enhances IDO activity, leading to a shunt of Trp metabolism through the KYN pathway. This yields an overproduction of QUIN, a neurotoxic metabolite, that hyperstimulates NMDA receptors, resulting in glutamatergic excitotoxicity, oxidative stress and neurodegeneration. Concurrently, the levels of KYNA, a neuroprotective metabolite, are reduced, further destabilising the neuroprotection versus neurotoxicity balance, potentially leading to mood disturbances, cognitive impairment and increased vulnerability to depression during perimenopausal transition.Reference Liang, Kow, Yusof, Tham, Ho and Lee103 Due to this strong link among immune dysregulation, KYN metabolism and neuroinflammation, targeting these pathways could provide novel therapeutic opportunities for the prevention and treatment of perimenopausal depression.
Summary
In summary, the studies reviewed here strongly support the role of differential patterns of immunological biomarkers in reproductive psychiatric disorders. Among all mentioned disorders, there is convincing evidence of emerging biomarkers including cytokines, extracellular vesicles and the KYN pathway metabolites. Methodological inconsistency, small sample size and variation in the design of studies restrict generalisation. Large-scale longitudinal studies with multi-omics approaches that track sequential changes in immune biomarkers should be the top priority in future research to clearly establish the bidirectional relationship among hormonal fluctuations, immune dysregulation and symptoms. Research in relatively unexplored conditions, such as pOCD, will also be critical for bridging the gaps. The advancement of clinical application of immunological biomarkers opens new perspectives for personalised treatment that can help improve the outcomes of women’s mental health across life stages.
Neuroimaging biomarkers
Research into epigenetic, endocrinological and immunological markers of psychiatric illness in women is expanding, but studies examining the underlying neurocircuitry remain limited. Understanding normal physiological variations in the central nervous system throughout the female lifespan is important for elucidating the development of psychiatric illness in women. While specific imaging biomarkers have not yet been characterised, the field is promising, and the current literature provides valuable guidance for future studies. Given the unique challenges associated with imaging studies in pregnant individuals, it may take time before clinically predictive biomarkers are identified. However, characterising the neuroimaging features of these conditions may be the first step in the development of biomarkers. This section describes the imaging modalities used in studying peripartum depression, anxiety, psychosis, PMDD and perimenopause, along with recent key findings.
AND, PND and PPD
Imaging studies in pregnant women are limited, with most data focusing on PPD. Recent research suggests that pregnancy induces significant, lasting changes in brain structure, function and neural plasticity, which are generally considered adaptive to the reproductive transition.Reference Pritschet, Taylor, Cossio, Faskowitz, Santander and Handwerker105
There are no studies focusing exclusively on the antenatal period. For PPD, recent structural neuroimaging studies report abnormal changes in grey matter volume, cortical thickness, white matter integrity and network connectivity. A comparison of 29 women with PPD and 23 healthy postpartum women found a correlation between EPDS scores and cortical and subcortical differences in PPD, with greater cortical thickness in frontal and visual areas.Reference Yang, Jiang, Ma, Xiao, Liu and Ren106 Conversely, decreased cortical thickness in women with PPD in the right inferior parietal lobule was reported in another study comparing 21 PPD women to 18 controls.Reference Li, Chu, Che, Dong, Shi and Ma107 Additionally, increased grey matter volume in the left dorsolateral prefrontal cortex (DLPFCL) and the right anterior insula and increased surface area in several regions, including the DLPFC and insula, was found in women with postpartum depressive symptoms versus healthy controls.Reference Li, Chu, Che, Dong, Shi and Ma107,Reference Chen, Li, Zhang, Liu, Wang and Xu108 Recent studies have shifted focus from individual brain regions to examining the combined regions of the default mode network (DMN) and reward network. A few studies have used diffusion tensor imaging (DTI) techniques in PPD and have reported altered white matter integrity of the cortical thalamic circuits in PPD women compared to healthy controls.Reference Long, Zhou, Zhang, Li, Wang and Meng109,Reference Silver, Moore, Villamarin, Jaitly, Hall and Rothschild110
While imaging is largely confined to postpartum, there are some studies that conduct imaging postpartum but consider depressive symptoms during both pregnancy and postpartum. One recent study found that the severity of both AND and PPD symptoms, along with antepartum ALLO concentrations, predicted volume changes in brain regions within the DMN and reward network involved in emotion regulation and cognition among postpartum women.Reference Hare, Barber, Shaffer and Deligiannidis111
While research on structural neuroimaging is limited, functional magnetic resonance imaging (fMRI) has extensively identified brain regions linked to PPD symptoms. There are no studies confined to the antenatal period. Deligiannidis et alReference Deligiannidis, Sikoglu, Shaffer, Frederick, Svenson and Kopoyan33,Reference Deligiannidis, Fales, Kroll-Desrosiers, Shaffer, Villamarin and Tan112 found altered functional connectivity within areas of the DMN and in corticocortical and corticolimbic regions in PPD compared to healthy controls. In addition, they noted a relationship between postpartum ALLO concentrations and resting-state functional connectivity (RSFC) in the DMN for PPD.Reference Deligiannidis, Fales, Kroll-Desrosiers, Shaffer, Villamarin and Tan112 The DMN, which is involved in self-referential thinking and emotional processing, is particularly significant in PPD as disruptions in this network may contribute to the negative self-appraisal and rumination often observed in the disorder. Other fMRI studies observed reduced amygdala response to negative stimuli and decreased striatal activation after reward in PPD.Reference Moses-Kolko, Perlman, Wisner, James, Saul and Phillips113,Reference Moses-Kolko, Fraser, Wisner, James, Saul and Fiez114 The reward network is also critical in PPD, as altered responses to reward may be associated with the anhedonia often seen in depression. Additional studies noted alterations within the DMN, limbic system (LIN) and interhemispheric connectivity.Reference Zhang, Li, Liu, Hou and Zhang115 By contrast, Schnakenberg et alReference Schnakenberg, Hahn, Stickel, Stickeler, Habel and Eickhoff116 did not find any structural or functional changes in PPD.
Structural and functional imaging can identify brain regions associated with symptoms, while magnetic resonance spectroscopy (MRS) and positron emission tomography (PET) imaging can examine differences of biochemical systems at the molecular level in the brain. An initial MRS study of PPD found no significant differences in occipital cortex GABA levels compared with healthy controls.Reference Epperson, Gueorguieva, Czarkowski, Stiklus, Sellers and Krystal31 Another study reported higher glutamate concentrations in the medial prefrontal cortex (MPFC).Reference McEwen, Burgess, Hanstock, Seres, Khalili and Newman117 Conversely, lower combined glutamate plus glutamine and combined N-acetylaspartate (NAA) plus N-acetylaspartylglutamate were reported in the left DLPFC, with no differences in the anterior cingulate gyrus (ACG), in PPD versus healthy controls.Reference Rosa, Soares, Figueiredo, Cavalli, Barbieri and Schaufelberger118 In addition, blunted HPA axis responses were found to be linked with low levels of glutamate and glutamine in the ACG in PPD.Reference de Rezende, Rosa, Garcia-Leal, de Figueiredo, Cavalli and Bettiol119 A combined fMRI and MRS study noted a link between cortical GABA concentration and RSFC in the DMPFC in postpartum women, with no GABA differences, in PPD and healthy controls.Reference Deligiannidis, Fales, Kroll-Desrosiers, Shaffer, Villamarin and Tan112 PET studies in PPD revealed elevated brain monoamine oxidase-A (MAO-A) binding in the ACC and prefrontal cortex,Reference Sacher, Wilson, Houle, Rusjan, Hassan and Bloomfield120,Reference Sacher, Rekkas, Wilson, Houle, Romano and Hamidi121 and decreased postsynaptic serotonin 1 A receptor binding in regions of the ACC, the mesiotemporal and the lateral orbitofrontal cortices,Reference Moses-Kolko, Wisner, Price, Berga, Drevets and Hanusa122 while no differences were found in dopaminergic D2/3 receptor binding in the striatum.Reference Moses-Kolko, Price, Wisner, Hanusa, Meltzer and Berga123
Perinatal anxiety
Very few papers have examined imaging features in women with perinatal anxiety without mood symptoms. Those that exist are confined to the postpartum period. Two studies reported that greater neural responses to negative infant cues were correlated with higher postpartum anxiety symptoms.Reference Finnegan, Kane, Heller and Laurent124,Reference Malak, Crowley, Mayes and Rutherford125 Increased maternal anxiety was associated with stronger connections between caregiving behaviours and amygdala and right posterior superior temporal sulcus connectivity.Reference Guo, Moses-Kolko, Phillips, Swain and Hipwell126 In addition, several studies have explored perinatal anxiety in relation to PPD. Women with PPD with anxiety showed changes in the subgenual anterior cingulate cortex (sgACC) and dynamic functional connectivity (dFC) between the sgACC and superior temporal sulcus compared to women with PPD and without anxiety.Reference Cheng, Wang, Roberts, Zhou, Wang and Deng127 Functional connectivity density linked both anxiety and depression to common and specific circuit disruptions.Reference Cheng, Zhou, Kwok, Li, Wang and Zhao128 PPD with anxiety disrupted the lateral prefrontal-striatal circuit, while PPD without anxiety affected the occipito-parieto-frontal circuit.Reference Cheng, Zhou, Kwok, Li, Wang and Zhao128 Irregularities in the DMN distinguished PPD from PPD with anxiety.Reference Chen, Yang, Li, Chen, Chen and Shao129
pOCD
One study that compared postpartum women with OCD to those without (as well as to a comparison group of mothers past the postpartum period) found that, compared to healthy postpartum women, those with OCD had heightened stress responses (both self-report and endocrine) in response to psychosocial stress, and that this response was associated with a distinct pattern of activation in the orbitofrontal and temporal cortices.Reference Lord, Steiner, Soares, Carew and Hall130
PPP
Research on neuroimaging in PPP is limited, but some evidence of structural and functional differences has been noted. One study found decreased volume in the parahippocampal gyrus, anterior cingulate cortex (ACC), postcentral gyrus and superior temporal gyrus (STG) in postpartum women with recent psychotic episodes compared to at-risk women without such episodes.Reference Fusté, Pauls, Worker, Reinders, Simmons and Williams131 Two other studies reported increased connectivity between the DLPFC and other areas along with functional differences in working memory and emotional recognition in women at risk of PPP.Reference Kowalczyk, Pauls, Fusté, Williams, Hazelgrove and Vecchio132 In addition, one study found that, while connectivity in areas related to executive functioning increased overall, it decreased during emotional tasks.Reference Sambataro, Cattarinussi, Lawrence, Biaggi, Fusté and Hazelgrove133
PMDD
A major recent advancement in the structural neuroimaging literature on PMDD comes from the Comasco lab, which conducted studies based on an unprecedentedly large sample. The findings highlight the value of large-scale, phase-specific imaging studies to better characterise the neurobiological basis of PMDD. These studies investigated structural changes in grey matter in women with PMDD during the luteal phase. One study found higher depressive symptoms in PMDD correlated with lower bilateral amygdala volume.Reference Dubol, Wikström, Lanzenberger, Epperson, Sundström-Poromaa and Comasco134 Another study reported that PMDD is associated with greater white matter volume in the right uncinate fasciculus and increased fractional anisotropy in several brain regions compared to controls.Reference Gu, Dubol, Stiernman, Wikström, Hahn and Lanzenberger135 The same group also investigated structural differences in PMDD in both mid-follicular and late luteal phases and found that women with PMDD consistently showed thinner cortices and reduced gyrification compared to controls, regardless of menstrual cycle phase – suggesting trait-like brain differences.Reference Dubol, Stiernman, Sundström-Poromaa, Bixo and Comasco136 In addition, menstrual cycle-related changes in cortical architecture were observed across all participants.Reference Dubol, Stiernman, Sundström-Poromaa, Bixo and Comasco136 While Dubol et alReference Dubol, Stiernman, Wikström, Lanzenberger, Epperson and Sundström-Poromaa137 reported reduced volume in the cerebellum, right amygdala and ventral putamen, earlier studies noted increased volume in posterior cerebellar volume and in the left hippocampal gyrusReference Berman, London, Morgan and Rapkin138 and decreased parahippocampal gyrus in PMDD compared to healthy controls.Reference Jeong, Ham, Yeo, Jung and Joe139
fMRI studies reveal significant changes in brain reactivity to emotional stimuli during the luteal phase in PMDD. These include increased amygdala response decreased prefrontal cortex activity to negative and social stimuliReference Protopopescu, Tuescher, Pan, Epstein, Root and Chang140,Reference Petersen, Ghahremani, Rapkin, Berman, Liang and London141 and reduced ACC activity to social and emotion regulation tasks.Reference Gingnell, Ahlstedt, Bannbers, Wikström, Sundström-Poromaa and Fredrikson142,Reference Comasco, Hahn, Ganger, Gingnell, Bannbers and Oreland143 In addition, increased insula activation occurs during emotional tasks and social tasks,Reference Gingnell, Ahlstedt, Bannbers, Wikström, Sundström-Poromaa and Fredrikson142,Reference Bannbers, Gingnell, Engman, Morell, Comasco and Kask144 alongside hyperconnectivity in the basal ganglia and thalamus.Reference Dan, Reuveni, Canetti, Weinstock, Segman and Goelman145
PET and MRS imaging studies have examined biochemical changes in PMDD. Increased glucose metabolism in the cerebellum and abnormal activation during working memory tasks in the DLPFC were observed from the follicular to the late luteal phase in PMDD compared to healthy controls.Reference Baller, Wei, Kohn, Rubinow, Alarcón and Schmidt146,Reference Rapkin, Berman, Mandelkern, Silverman, Morgan and London147 Alterations in the serotonergic system included a smaller increase in serotonin 1A receptor (5-HT1A) binding in dorsal raphe nuclei from the follicular to the late luteal phase in PMDD,Reference Jovanovic, Cerin, Karlsson, Lundberg, Halldin and Nordström148 altered levels of serotonin precursor 5-hydroxytryptophan (5-HTP) in prefrontal regions and the striatum correlated with symptom severity in PMDD.Reference Eriksson, Wall, Marteinsdottir, Agren, Hartvig and Blomqvist149 Recently, increased availability of serotonin transporter in the midbrain during the luteal phase was described.Reference Sacher, Zsido, Barth, Zientek, Rullmann and Luthardt150 MRS studies found higher GABA levels in the occipital cortex from the follicular to the mid- and late luteal phasesReference Epperson, Haga, Mason, Sellers, Gueorguieva and Zhang151 and lower GABA levels in the ACC/MPFC and left basal ganglia.Reference Liu, Wang, Gao, Gao, Zhao and Qiao152 However, some studies reported no differences in glutamate or other metabolite levels in the MPFC and cingulate gyrus between PMDD and healthy controls.Reference Batra, Seres-Mailo, Hanstock, Seres, Khudabux and Bellavance153,Reference Rasgon, Thomas, Guze, Fairbanks, Yue and Curran154
Perimenopausal depression
To date, brain imaging research has not focused on perimenopausal depression. (While there are reports of brain changes in response to the hormone shifts that are characteristic of perimenopause – with possible implications that could be important for future studies on depression – those studies are outside the scope of this paper. Similarly, there are imaging studies of cognitive changes in perimenopause as well.Reference Metcalf, Duffy, Page and Novick155) Wang et alReference Wang, Tao, Zhong, Liu, Han and Zhang156 is the only study to have used DTI to investigate this subgroup and found alterations in white matter microstructure in the insula and subcortical areas that correlated with depressive symptoms in perimenopausal women.
Summary
In summary, most neuroimaging studies to date on psychiatric illness at times of reproductive hormonal transition has focused on PPD and PMDD. Structural imaging studies have shown changes in grey matter volume, cortical thickness and white matter integrity, with significant findings in the DMN and reward networks linked to emotion regulation. Functional imaging, most notably fMRI, has highlighted abnormal connectivity in regions such as the amygdala, prefrontal cortex and insula during emotional stimuli. MRS and PET imaging have detected biochemical changes in neurotransmitter systems, such as glutamate, GABA, serotonin and MAO-A. However, heterogeneity in imaging modalities, methodologies and study designs, as well as small sample sizes, present challenges for validating and replicating findings. Longitudinal, multi-modal imaging studies are required to confirm previous findings, identify reliable biomarkers and explore their potential to predict future illness. Although MRI can be conducted during pregnancy and postpartum period, logistical challenges such as participant availability, motion artefacts and minimising stress or discomfort complicate study designs and data collection. The use of PET imaging is further restricted during pregnancy because of radiation exposure, thereby underscoring the need for alternative approaches. Nonetheless, PMDD and perimenopause offer the unique window of opportunity to study neurobiological changes across reproductive transitions. These studies can develop our understanding of psychiatric illness at other stages of reproductive hormonal transition, such as pregnancy and postpartum; improve diagnostic and treatment strategies; and address ethical and practical challenges in vulnerable populations.
Synthesis and future directions
Biomarker research in reproductive psychiatric disorders, including exploration of epigenetics, immunological markers, NASs and neuroimaging, holds immense promise for enhancing our understanding of these conditions (Table 1 and Fig. 1). While PPD has been the primary focus of research, a comprehensive understanding of reproductive psychiatric disorders requires integrating findings across all reproductive transitions (including AND and PND, perinatal anxiety, pOCD, PPP, PMDD and perimenopausal depression) and biomarker types (epigenetics, immunology, NASs, neuroimaging). The state of the literature thus far is primarily one of association, not causation – so few of the markers covered here are close to being studied as clinically useful tests. There are nevertheless some commonalities that can point us toward future research.
Table 1 Summary of potential biomarkers
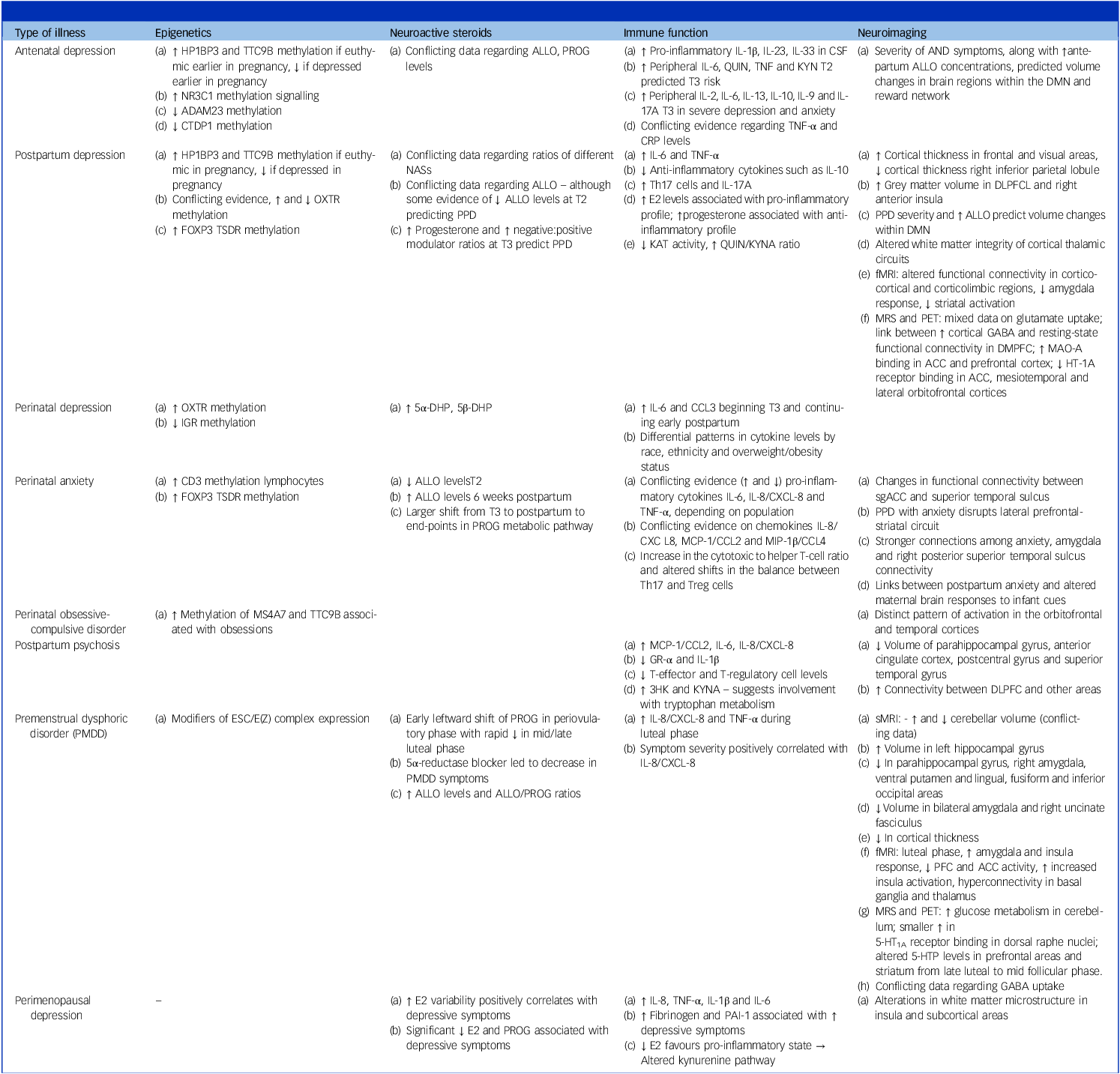
3HK, 3-hydroxykynurenine; 5α-DHP, 5-alpha-dihydroprogesterone, intermediary molecule between PROG and ALLO; 5β-DHP, 5-beta-dihydroprogesterone, intermediary molecule between PROG and pregnanolone; 5-HT1A, serotonin 1A receptor; ACC, anterior cingulate cortex; ADAM23, a disintegrin and metalloproteinase domain-containing protein 23; ALLO, allopregnanolone, metabolite of PROG; AND, antenatal depression; CCL2, chemokine (C-C motif) ligand 2; CCL-3, chemokine (C-C motif) ligand 3; CCL4, chemokine (C-C motif) ligand 4; CRP, C-reactive protein; CSF, cerebrospinal fluid; CTDP1, CTD phosphatase subunit 1; CXCL-8, C-X-C motif chemokine ligand 8; DLPFCL, Dorsolateral prefrontal cortex; DMN, default mode network; E2, oestradiol; FOXP3, Forkhead box P3; GR-α, glucocorticoid receptor alpha; HP1BP3, heterochromatin protein 1, binding protein 3; HT-1A, serotonin 1A; IL-1β, interleukin 1-Beta; IL-6, interleukin 6; IL-10, interleukin 10; IL-17A, interleukin 17A; IL-23, interleukin 23; IL-33, interleukin 33; KAT, kynurenic aminotransferase; KynA, kynurenic acid; KYN, kynurenine, tryptophan metabolite; MAO-A, monoamine oxidase-A; MCP-1, monocyte chemoattractant protein 1; MIP-1β, macrophage inflammatory protein 1 beta; MPFC, medial prefrontal cortex; MRS, magnetic resonance spectroscopy; MS4A7, membrane-spanning 4A subfamily member 7; NAS, neuroactive steroids; NR3C1, nuclear receptor subfamily 3 group C member 1; OXTR, oxytocin receptor gene; PAI-1, plasminogen activator type 1; PET, positron emission tomography; PFC, prefrontal cortex; PND, perinatal depression; PPD, postpartum depression; PROG, progesterone; QUIN, quinolinic acid; sgACC, subgenual anterior cingulate cortex; T2, second trimester; T3, third trimester; Th17, T-helper 17; TNF, tumour necrosisf factor; TSDR, treg-cell-specific demethylated region; TTC9B, tetratricopeptide repeat domain 9B.

Fig. 1 Potential biomarkers of reproductive psychiatric disorders (created with BioRender.com). HP1BP3, heterochromatin protein 1, binding protein 3; TTC9B, tetratricopeptide repeat domain 9B; OXTR, oxytocin receptor gene; NR3C1, nuclear receptor subfamily 3 group C member 1; FOXP3, Forkhead box P3; ESC/E(Z), extra sex combs/enhancer of zeste; ADAM23, a disintegrin and metalloproteinase domain-containing protein 23; CTDP1, CTD phosphatase subunit 1; IL-6, interleukin-6; TNF-α, tumour necrosis factor-alpha; IL-1β, interleukin-1β; KYN, kynurenine; Trp, tryptophan; QUIN, quinolinic acid; Th17, T-helper 17; GABA, gamma-aminobutyric acid; HPA, hypothalamic–pituitary–adrenal; MRI, magnetic resonance imaging; PFC, prefrontal cortex; fMRI, functional magnetic resonance imaging; DMN, default mode network; MRS, magnetic resonance spectroscopy; PET, positron emission tomography; MAO-A, monoamine oxidase-A.
Much of the data – no matter which section we fit it into above – points to individual vulnerability to hormone fluctuation. Epigenetic markers are on hormone-responsive genes; immune markers vary as hormones change; and genetic polymorphisms that underlie key steps in the metabolism of hormones appear to be related to symptoms. It is thus clear that our next step is to determine not which of the various protein markers examined here is the ’smoking gun’ that can serve as a predictive biomarker of all reproductive psychiatric illness, but rather whether one or a few of them they can be used as markers for the various reasons for individual vulnerability to hormonal change. Some individuals, for example, may be vulnerable because of genetic defects in genes for enzymes; others may be vulnerable because of unique patterns of interaction between the immune system and neurotransmitters in the setting of hormone change; still others because of failures in receptor plasticity in response to hormone change. Useful biomarkers will be those that capture a shared biological signal, and can indicate risk in those who may be vulnerable for different reasons.
To arrive at such biomarkers, future research must prioritise longitudinal studies with standardised methodologies and diverse samples to address inconsistencies and limitations in current findings. Critically, exploring the complex interplay among genetic, epigenetic, hormonal, immunological and neural factors is essential, utilising a multi-omics approach. While some studies examine the interplay between hormones and brain imaging (such as those of the Deligiannidis group),Reference Deligiannidis, Fales, Kroll-Desrosiers, Shaffer, Villamarin and Tan112,Reference Duan, Cosgrove and Deligiannidis157 surprisingly few studies look at interactions among these systems, which is a clear necessity to advance this research further.
It will also be crucial to delineate whether these illnesses are distinct entities or variations of common psychiatric disorders, especially concerning timing of onset, and identify individuals sensitive to hormonal fluctuations. Paying attention to timing and predictive utility is also of high importance. Biomarkers will be most helpful if they can predict who will become ill – and therefore studies that collapse together periods that may have distinct biological risks (such as pregnancy and postpartum) will continue to be of limited use. Ultimately, translating these findings into clinical practice, through improved diagnostics, risk prediction and personalised treatments, is the ultimate goal, requiring collaborative, interdisciplinary efforts.
Data availability
Data availability is not applicable to this article as no new data were created or analysed in this study.
Author contributions
S.E.: conducted literature review, prepared original draft of manuscript, edited, reviewed critically for important intellectual content. K.M.: conducted literature review, prepared original draft of manuscript, edited. S.I.: conducted literature review, prepared original draft of manuscript, prepared figure and table. İ.Ö.: conducted literature review, prepared original draft of manuscript, edited. L.M.O.: conceived and designed the work, edited, reviewed critically for important intellectual content, gave final approval of the version to be published. All authors agree to be accountable for all aspects of this work.
Funding
This study received no specific grant from any funding agency, commercial or not-for-profit sectors. İ.Ö. was funded by K99EB034768.
Declaration of interest
None.





eLetters
No eLetters have been published for this article.