Importation (or classical) biological control aims to reduce the impact of an invasive species by intentionally introducing host-specific natural enemies, or biological control agents, from its native range into an area it has invaded (Van Driesche and Abell Reference Van Driesche, Abell, Jørgensen and Fath2008; Hiempel and Mills Reference Hiempel and Mills2017). However, potential interactions among imported biocontrol agents once introduced in the invaded range can be challenging to test pre-release and may reduce control efficacy if the more competitive agent species directly or indirectly reduces biocontrol efficacy (Milbrath and Nechols Reference Milbrath and Nechols2004, Reference Milbrath and Nechols2014; Smith and Mayer Reference Smith and Mayer2005; Bourchier and Crowe Reference Bourchier and Crowe2011; Van Hezewijk and Bourchier Reference Van Hezewijk and Bourchier2012). Importation biological control has been used for decades to manage invasive knapweeds, Centaurea Lamarck (Asteraceae), which are found throughout North America, particularly in the west (Gayton and Miller Reference Gayton and Miller2012; Marshall et al. Reference Marshall, Bourchier, Miller, Moffat, Ensing, Vankosky and Martel2024). Thirteen biocontrol agents were imported from the Palearctic, and 12 of these species have subsequently become established on invasive knapweed species in the United States of America, and until 2020, 10 of the 13 were known to be established in Canada (Bourchier et al. Reference Bourchier, Mortensen, Crowe, Mason and Huber2002; Winston et al. Reference Winston, Schwarzländer, Randall and Reardon2015; Marshall et al. Reference Marshall, Bourchier, Miller, Moffat, Ensing, Vankosky and Martel2024).
Of the 13 Centaurea agents introduced to North America, larvae of eight feed in the seed heads (see Wilson and Randall Reference Wilson and Randall2005, table 1), including Larinus minutus Gyllenhal and Bangasternus fausti Reitter (both Coleoptera: Curculionidae). Diffuse knapweed, C. diffusa Lamarck, is a monocarpic herb that reproduces only by sexually produced seed, and seed head agents were therefore likely to be its most effective biocontrol agents because they directly eliminate plant offspring before they can develop. However, the four species of seed head–feeding tephritid flies and the gelechiid moth biocontrol agent appear to have limited influence on C. diffusa populations (Smith and Mayer Reference Smith and Mayer2005; Myers et al. Reference Myers, Jackson, Quinn, White and Cory2009). In contrast, L. minutus has been credited with the substantial declines of diffuse knapweed populations in British Columbia, Canada (Myers et al. Reference Myers, Jackson, Quinn, White and Cory2009; Gayton and Miller Reference Gayton and Miller2012) and likely other areas where it has been released. However, its interactions with other seed-feeding beetle agents have not been evaluated.
Before their release in North America, host-specificity testing indicated that L. minutus and B. fausti can develop only in the seed heads of introduced Centaurea spp. (Maddox and Sobhian Reference Maddox and Sobhian1987; Sobhian et al. Reference Sobhian, Campobasso and Dunn1992; Jordan Reference Jordan1995; Kashefi and Sobhian Reference Kashefi and Sobhian1998; Smith and Mayer Reference Smith and Mayer2005). Larinus minutus was released in Canada and the United States of America in the early 1990s, whereas B. fausti was released only in the United States of America due to concerns that it would affect the performance of other agents in Canada (Müller-Schärer and Schroeder Reference Müller-Schärer and Schroeder1993; Winston et al. Reference Winston, Schwarzländer, Randall and Reardon2015). However, B. fausti has recently been detected in the southern interior of British Columbia (Nelson et al. Reference Nelson, Earley, Marshall, Mancera Barreto, Cock and Williams2023). As such, Canadian populations of L. minutus are now interacting with B. fausti throughout British Columbia’s Okanagan Valley. Apart from apparently using only C. diffusa as a host (Nelson et al. Reference Nelson, Earley, Marshall, Mancera Barreto, Cock and Williams2023), little is known about how Canadian B. fausti interacts with sympatric L. minutus.
As a first step in characterising the interactions of the two species, we recorded the phenology of L. minutus and B. fausti across their sympatric Canadian range. We hypothesised that the relative phenology of Canadian populations would be similar to populations in their native range, where adult B. fausti are most abundant early in the C. diffusa growing season (Sobhian et al. Reference Sobhian, Campobasso and Dunn1992) and adult L. minutus are abundant throughout the season (Kashefi and Sobhian Reference Kashefi and Sobhian1998). Based on this, we predicted that adult B. fausti would be active earlier in the year than adult L. minutus are in Canada. If B. fausti is reducing C. diffusa control by interfering with L. minutus, it could lead to C. diffusa population resurge and increased management costs in British Columbia.
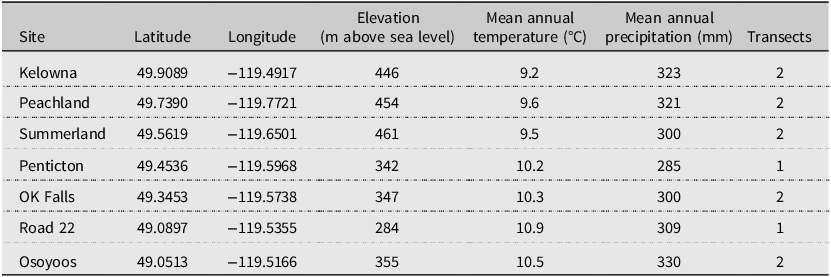
In April 2022, we established seven study sites across the Canadian range of B. fausti (Table 1; Nelson et al. Reference Nelson, Earley, Marshall, Mancera Barreto, Cock and Williams2023). At five sites, we established two 20-m transects within patches of C. diffusa and selected focal study plants that were closest to each 5-m mark, for a total of 10 focal study plants at each site. At the remaining two sites, we were limited to only a single transect, for a total of five study plants, due to vegetation management or small patch size. We visited sites weekly between April and November 2022.
Table 1. Details of Larinus minutus and Bangasternus fausti study sites across the Okanagan Valley, British Columbia, Canada. Environmental data were retrieved using the ClimateNAr package (Wang et al. Reference Wang, Hamann, Spittlehouse and Carroll2016) and represent the annual average condition in 1991–2020
We collected adult weevils in the field by carefully shaking the focal plants over a tray. If focal plants were shorter than 20 cm, we instead collected all weevils by hand to avoid damaging the smaller stem. We spent 10 minutes collecting additional weevils from plants near to the transects to limit false zeroes from focal plants, and we recorded mating events when observed. We collected a total of 30 seed heads from three haphazardly selected, nonfocal plants at each site between mid-June and October 2022 for laboratory assessment.
In the laboratory, we counted B. fausti eggs on the bracts of 10 seed heads per collection event from 15 June to 18 August 2022. We did not count L. minutus eggs because they are laid inside the seed heads (Kashefi and Sobhian Reference Kashefi and Sobhian1998) and are not visible. We stored the remaining 20 seed heads from each collection in plastic containers in a 24 °C climate-controlled rearing chamber with ambient humidity and a 16:8 hour day:night cycle at the Summerland Research and Development Centre (Agriculture and Agri-Food Canada, Summerland, British Columbia, Canada) and collected emergent adult weevils weekly until the end of September and monthly until November 2022. We immediately removed any containers with fungal growth, if found. We identified emerging adult weevils using published literature (B. fausti: Colonnelli and Whitehead Reference Colonnelli and Whitehead1990; Nelson et al. Reference Nelson, Earley, Marshall, Mancera Barreto, Cock and Williams2023; L. minutus: Gültekin and Anderson Reference Gültekin and Anderson2017). We deposited adult specimens of each species in the Summerland Research and Development Centre Collection (ACBC00158–00177; Agriculture and Agri-Food Canada, Summerland, British Columbia), the Entomology Collection of the Royal British Columbia Museum (Victoria, British Columbia, Canada), and the Canadian National Collection of Insects, Arachnids and Nematodes (Agriculture and Agri-Food Canada, Ottawa, Ontario, Canada).
We performed all statistical analyses in R, version 4.3.1 (R Core Team 2023), using the MASS (Venables and Ripley Reference Venables and Ripley2002), MuMIn (Bartoń Reference Bartoń2024), DHARMa, version 0.4.7 (Hartig Reference Hartig2024), ape, version 5.0 (Paradis and Schliep Reference Paradis and Schliep2019), and ClimateNAr. version 1.2.0 (Wang et al. Reference Wang, Hamann, Spittlehouse and Carroll2016) packages.
We used negative binomial generalised linear models (to account for zero inflation of count data) with the glm.nb function (MASS) to compare the phenology of adult L. minutus and B. fausti in the field. Specifically, we predicted adult abundance with date, species, and their interaction. We used the same model structure to predict the number of emerged adults from collected seed heads.
We characterised the relationship between environmental conditions and mean adult field abundance for B. fausti and L. minutus separately. For each species, we used linear models (lm function in base R) to predict mean weevil abundance, with latitude, degree-day accumulation (> 5 °C), and mean annual precipitation including snowfall (mm) as covariates (environmental data from ClimateNAr). We predicted abundance using: (1) latitude to represent the spatial distribution of B. fausti in Canada, expecting fewer beetles at more northerly sites due to ongoing range expansion, (2) degree days (> 5 °C) because these are relevant time currency for ectotherms like plants and insects (e.g., Schantz et al. Reference Schantz, Hardegree, James, Sheley and Becchetti2023; Pantzke et al. Reference Pantzke, Ferguson, Rajamohan, Rinehart, Prischmann-Voldseth and Prasifka2024), (3) mean annual precipitation because moisture availability influences earlier and more abundant Centaurea spp. growth (Mráz et al. Reference Mráz, Tarbush and Müller-Schärer2014; Kožić et al. Reference Kožić, Hartmann, Callaway, Hensen, Nagy and Mráz2024), and (4) average winter temperature because dormant season conditions can influence vital rates of plants (Evers et al. Reference Evers, Knight, Inouye, Miller, Salguero-Gómez, Iler and Compagnoni2021) and insects (Gill et al. Reference Gill, Goyal and Chahil2017; e.g., Guo et al. Reference Guo, Qin and Wen2021).
For all models, we weighted our field observations by the number of visits and transects per site to account for unequal effort, and for phenology models, we used the dredge function (MuMIn) to compare all possible models using the small-sample corrected Akaike information criterion (AICc), selecting the simplest model if the difference (ΔAICc) was smaller than two among competing models. For all phenology models, we checked for over dispersion of residuals using the testDispersion function and tested for normality of residuals using the parametric simulateResdiuals function (DHARMa). We tested for spatial autocorrelation using Moran’s I with the Moran.I function (ape).
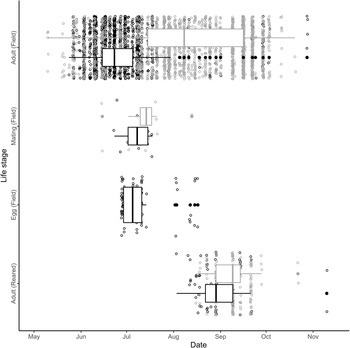
For field-observed adult phenology, date (P < 0.001, Z = –7.428, standard error = 0.004), species (P < 0.001, Z = –5.346, standard error = 99.4), and their interaction (P < 0.001, Z = 5.354, standard error = 0.005) were all significant predictors of adult abundance (AICc = 1216.3, weight: 0.998). The mean date of adult abundance found in the field for adult B. fausti (26 June 2022; Fig. 1) occurred 41.9 days earlier than the mean adult abundance of L. minutus (7 August 2022; Fig. 1).
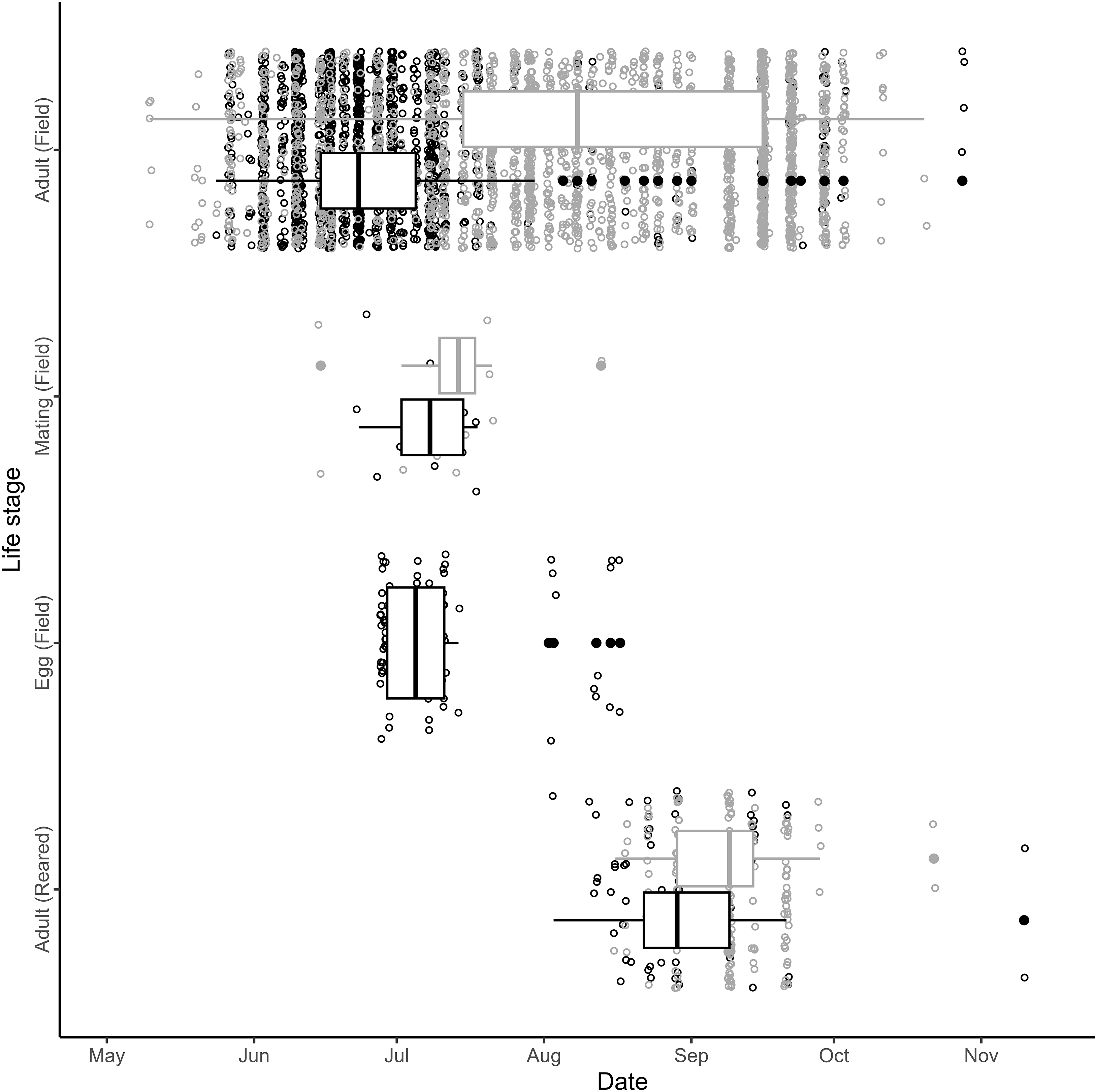
Figure 1. Phenology of Bangasternus fausti (black) and Larinus minutus (grey) in the Okanagan Valley, British Columbia, Canada, in 2022. “Field” indicates beetles collected or observed on Centaurea diffusa in the field, and “reared” indicates beetles that emerged from C. diffusa seed heads in the laboratory. Points jittered for visibility; filled circles represent observation dates outside of the third quartile; tick marks indicate the first day of each month. Larinus minutus eggs were not observed because they are laid within the involucral bracts.
We reared 268 weevils from 543 C. diffusa seed heads and found that only species was a significant predictor for the number of emerged adults (P = 0.003, Z = 3.009, standard error = 0.436; AICc = 173.0, weight = 0.689). Excluding perceived outliers that emerged late in the fall (n = 4), B. fausti adults (n = 70) emerged between 3 August and 21 September 2022, and L. minutus (n = 194) emerged between 16 August and 28 September 2022 (Fig. 1). We observed B. fausti mating between 23 June and 18 July (n = 13) and L. minutus mating between 15 June and 13 August 2022 (n = 15). We found 82 B. fausti eggs on C. diffusa seed heads collected between 28 June and 17 August 2022.
We found that B. fausti abundance (n = 1174,
![]() $\bar x$
= 7.03) was positively associated with mean annual precipitation (df = 1, F = 35.42, P = 0.027) but was not associated with latitude, average winter temperature, nor degree days > 5 °C (all df = 1, F < 5.1, P > 0.15). Mean L. minutus abundance (n = 1759,
$\bar x$
= 7.03) was positively associated with mean annual precipitation (df = 1, F = 35.42, P = 0.027) but was not associated with latitude, average winter temperature, nor degree days > 5 °C (all df = 1, F < 5.1, P > 0.15). Mean L. minutus abundance (n = 1759,
![]() $\bar x$
= 10.53) was not related to any environmental predictors in our study area (all P > 0.23). For both species, results were similar when using the total number of beetles observed instead of the mean per visit.
$\bar x$
= 10.53) was not related to any environmental predictors in our study area (all P > 0.23). For both species, results were similar when using the total number of beetles observed instead of the mean per visit.
We found that adults of both L. minutus and B. fausti are active throughout the C. diffusa growing season in their sympatric Canadian range but that B. fausti tends to be active earlier in the season than L. minutus is, as is observed in the species’ native range in southern Europe and the Mediterranean (Sobhian et al. Reference Sobhian, Campobasso and Dunn1992; Kashefi and Sobhian Reference Kashefi and Sobhian1998). In both British Columbia and Greece, L. minutus adults were first recorded in early May, whereas the new generation of adults emerged in midsummer (Fig. 1; Kashefi and Sobhian Reference Kashefi and Sobhian1998). We suggest that the persistence of adult L. minutus throughout the season in both its native and introduced ranges may be caused by the emergence of the new generation occurring while the first generation is still present. Such overlap of L. minutus generations would explain the abundance of adults observed between late July and early August in both the present and previous native range studies (Kashefi and Sobhian Reference Kashefi and Sobhian1998).
Although we found that B. fausti activity occurred earlier than that of L. minutus, its phenology in British Columbia was delayed in the year of study compared to that which had been recorded in Greece previously. Adult abundance of B. fausti showed a right temporal skew in both the native and introduced ranges: the first adult B. fausti were recorded in Greece in early May (Sobhian et al. Reference Sobhian, Campobasso and Dunn1992), whereas we recorded the first adult B. fausti in late May. Peak adult B. fausti field abundance also occurred about a month later (Greece: late May; British Columbia: late June), as did mating (Greece: late May; British Columbia: late June to mid-July; Sobhian et al. Reference Sobhian, Campobasso and Dunn1992). We observed B. fausti eggs at approximately the same time as mating (Fig. 1), consistent with the results of Sobhian et al. (Reference Sobhian, Campobasso and Dunn1992). We suspect that the later phenology of B. fausti in British Columbia may be related to its distribution, because British Columbia is the northern extent of its introduced range (Nelson et al. Reference Nelson, Earley, Marshall, Mancera Barreto, Cock and Williams2023), and we note that Greece has an appreciably earlier spring than British Columbia does (De Frenne et al. Reference De Frenne, Graae, Rodríguez-Sánchez, Kolb, Chabrerie and Decocq2013).
Environmental covariates seem unlikely to be driving phenological differentiation in adult abundance for either species because there were no significant patterns for L. minutus, whereas mean annual precipitation was the only significant covariate for B. fausti. It is possible that elevated precipitation and moisture increases germination and above-ground biomass in C. diffusa as observed in other Centaurea species (e.g., Mráz et al. Reference Mráz, Tarbush and Müller-Schärer2014; Kožić et al. Reference Kožić, Hartmann, Callaway, Hensen, Nagy and Mráz2024) and that adult B. fausti respond by feeding in greater abundance in locations where (1) more C. diffusa have germinated and (2) C. diffusa plants have more foliage, but we would expect this response to also increase L. minutus. The divergence in median timing of adult weevil activity on C. diffusa (Fig. 1) may suggest resource partitioning between the two weevil species as a result of historical competitive selective pressure in their native range, with B. fausti activity associated with the wetter conditions of late spring and early summer and with the majority of L. minutus adults found later in the season.
Although adult B. fausti are present on C. diffusa significantly earlier in the season than L. minutus is (Fig. 1), it is unclear whether they are competing for host resources. The larvae appear to have separate niches within the seed head: B. fausti larvae feed on the flower receptacle, florets, or ovules before achenes develop (Harris Reference Harris and Delfosse1990; Sobhian et al. Reference Sobhian, Campobasso and Dunn1992), whereas L. minutus larvae feed on the pappus hairs and developing or mature achenes (Harris Reference Harris and Delfosse1990; Jordan Reference Jordan1995; Lang et al. Reference Lang, Hansen, Richard, Ziolkowski and Spencer2000). Because L. minutus can consume larvae of other agent species feeding in knapweed seed heads (Smith and Mayer Reference Smith and Mayer2005; Crowe and Bourchier Reference Crowe and Bourchier2006; Seastedt et al. Reference Seastedt, Knochel, Garmoe and Shosky2007; Bourchier and Crowe Reference Bourchier and Crowe2011), we hypothesise that B. fausti larvae were subjected to allochronic selection pressure to avoid being eaten by L. minutus larvae and, therefore, develop earlier. Considering B. fausti feeds on developing flowers and has been found to have a low survival rate in the field, B. fausti could impact food availability for L. minutus, die before mating, or both, thereby reducing the population growth rate of both agents (Sobhian et al. Reference Sobhian, Campobasso and Dunn1992). As such, the next steps in exploring the relationship between Canadian B. fausti and L. minutus lie within the host’s seed heads. Does L. minutus outcompete B. fausti, or vice versa? If they are competing, how might their re-acquaintance in Canada influence the abundance of C. diffusa? The answers to such eco-evolutionary questions are yet unknown, but it is clear that further study of these biocontrol agents is warranted.
Acknowledgements
The authors thank Nicholas Hivon and Loughlin McQueen for assistance with specimen collection and rearing. N.G.E. was funded in part by a NSERC CGS-D. Funding for this study was granted to C.E.M. and D.J.E. by the British Columbia Ministry of Forests (#1818 and #2569), as well as to C.E.M. and D.J.E. by Agriculture and Agri-Food Canada (AAFC ABASE #2201 and APMS #2839).