Introduction
Elephant grass [Cenchrus purpureus (Schum.) Morrone syn. Pennisetum purpureum (Schum.)] is a grass of African origin that stands out for its high potential of herbage accumulation (Pereira et al., Reference Pereira, Lira, Machado, Gomide, Martins, Lédo and Daher2021), being one of the most widespread forage grasses in tropical and subtropical regions worldwide, due to its adaptability to thermal stress (Jin et al., Reference Jin, Luo, Yang, Jia, Sun, Wang, Khan, Huang and Huang2023). This forage species has high potential of dry matter accumulation with an average of 38 t/ha (Pereira et al., Reference Pereira, Lédo and Machado2017) and good nutritional value, with crude protein (CP) of 91 g/kg and in vitro digestible dry matter (IVDDM) of 721 g/kg at 60 days of regrowth (Silva et al., Reference Silva, Santos, Mello, Sales-Silva, Simões Neto, Silva, Dubeux, Coelho, Souza and Cunha2023), as well as vigor and persistence when well managed (Pereira et al., Reference Pereira, Lira, Machado, Gomide, Martins, Lédo and Daher2021). It can be used in cut-and-carry systems or under grazing conditions (Maranhão et al., Reference Maranhão, Cândido, Lopes, Pompeu, Carneiro, Furtado, Silva and Alves2018). However, despite being a grass widely used for feeding ruminants, the harvest frequency adopted may contribute to reduction of its nutritional value and forage accumulation. It has been reported that the use of lesser harvest frequency in elephant grasses may decrease the quality leaf component due to longer regrowth, making it a very fibrous and poorly digestible forage (Ferreira et al., Reference Ferreira, Abreu, Martinez, Braz and Ferreira2018).
Elephant grass genotypes are classified into distinct groups, considering the size of the plant. Dwarf elephant grass has shorter internode lengths than tall genotypes (Chaves et al., Reference Chaves, Ribeiro, Gomide, Paciullo, Morenz and Gama2016), which contribute to the differentiation of morphological characteristics, forage accumulation, and nutritional value (Silva et al., Reference Silva, Sales, Lemos, Silva, Ribeiro, Santos, Mello and Cunha2021b). Generally, dwarf genotypes present greater nutritional value than tall genotypes, with average of 91 g/kg of CP, 646 g/kg of neutral detergent fibre (NDF), 308 g/kg of acid detergent fibre (ADF) and 626 g/kg IVDDM compared to 73 g/kg of CP, 690 g/kg of NDF, 338 g/kg of ADF and 552 g/kg of IVDDM for tall genotypes harvested every 60 days (Souza et al., Reference Souza, Santos, Cunha, Gonçalves, Silva, Mello and Dubeux2021). This suggests that morphological characteristics and plant size need to be considered for defining the utilization of elephant grass in animal production systems (Silva et al., Reference Silva, Cunha, Santos, Magalhães, Mello, Silva, Souza, Carvalho and Souza2021a) due to its impact on defining appropriate management (Maldonado-Méndez et al., Reference Maldonado-Méndez, Guerra-Medina, Avendaño-Arrazate, Gálvez-Marroquín and Ariza-Flores2023).
There are few studies evaluating the performance of dwarf elephant grass genotypes in cut-and-carry systems. Dwarf elephant grass may be an option for production of high-nutritive value forage in cut-and-carry systems, including small producers who harvest forage and use it daily (Silva et al., Reference Silva, Cunha, Santos, Magalhães, Mello, Silva, Souza, Carvalho and Souza2021a). Mott elephant grass is a dwarf type released as a cultivar in 1989, with high forage nutritive value (Sollenberger et al., Reference Sollenberger, Prine, Ocumpaugh, Hanna, Jones, Schank and Kalmbacher1989). The dwarf-type Taiwan A-146 2.37 has been considered a promising elephant grass genotype due to important characteristics such as more erect leaves, lower occurrence of foliar diseases, characteristics that contribute to greater productivity (Silva et al., Reference Silva, Santos, Lira, Dubeux, Freitas and Ferreira2009).
Harvesting elephant grass at lesser harvest frequency generally contributes to greater forage yield; however, it contributes to lesser forage nutritive value, with an increase in components of lower digestibility (Carvalho et al., Reference Carvalho, Arruda, Abreu, Souza, Rodrigues, Lima, Cabral and Behling2018; Islam et al., Reference Islam, Garcia, Sarker, Islam and Clark2023). In a study evaluating tall (Elephant B and IRI-381) and dwarf (Mott and Taiwan A-146 2.37) genotypes with or without irrigation at a 60-day harvest interval, grasses showed differences in growth dynamics, with tall genotypes presenting greater canopy light interception while dwarf genotypes showed greater number of tillers (Ribeiro et al., Reference Ribeiro, Mello, Cunha, Costa, Coelho, Souza and Santos2023). When comparing forage nutritive value, the dwarf-type Mott genotype stood out with the greatest nutritional value and leaf/stem ratio, while the tall genotypes presented a greater proportion of lignified tissues (Souza et al., Reference Souza, Santos, Cunha, Gonçalves, Silva, Mello and Dubeux2021). The authors suggested adjustments in the harvest frequency to balance forage accumulation and nutritive value.
Our hypothesis was that (i) tall and dwarf elephant grass genotypes, when managed under different harvesting frequencies, present morphological differences and (ii) the dwarf genotypes maintain greater forage nutritive value and less variation in rumen fermentation kinetics compared to tall genotypes with the advancing maturity (reduction in harvest frequency). Thus, the objective of this study was to evaluate morphological characteristics and kinetics of in vitro gas production of elephant grass genotypes of different sizes under the harvest frequencies of 60 and 90 days.
Materials and methods
Experimental site, treatments and experimental design
The experiment was conducted in Garanhuns, in the Agreste Meridional Region, Pernambuco, Brazil, at the Experimental Farm of the Federal Rural University of Pernambuco (UFRPE). The experimental site is located at 8°53’ S, 36°29’ W, 896 m a.s.l. The climate is classified as tropical Aw’ according to the Köppen-Geiger climate classification (Alvares et al., Reference Alvares, Stape, Sentelhas, Gonçalves and Sparovek2013), with average 21.2°C annual temperature, rainy seasons from autumn to winter, hot and dry summers and mild and humid winters.
Weather data for the experimental period recorded total precipitation of 1305 mm, with an average temperature of 21.5°C, minimum 18.9°C and maximum 25.3°C. The rainy and dry seasons were defined according to the water balance, with total precipitation in the rainy season being 316 mm with an average temperature of 19.9°C and in the dry season 162 mm with an average temperature of 23.6°C (Fig. 1).
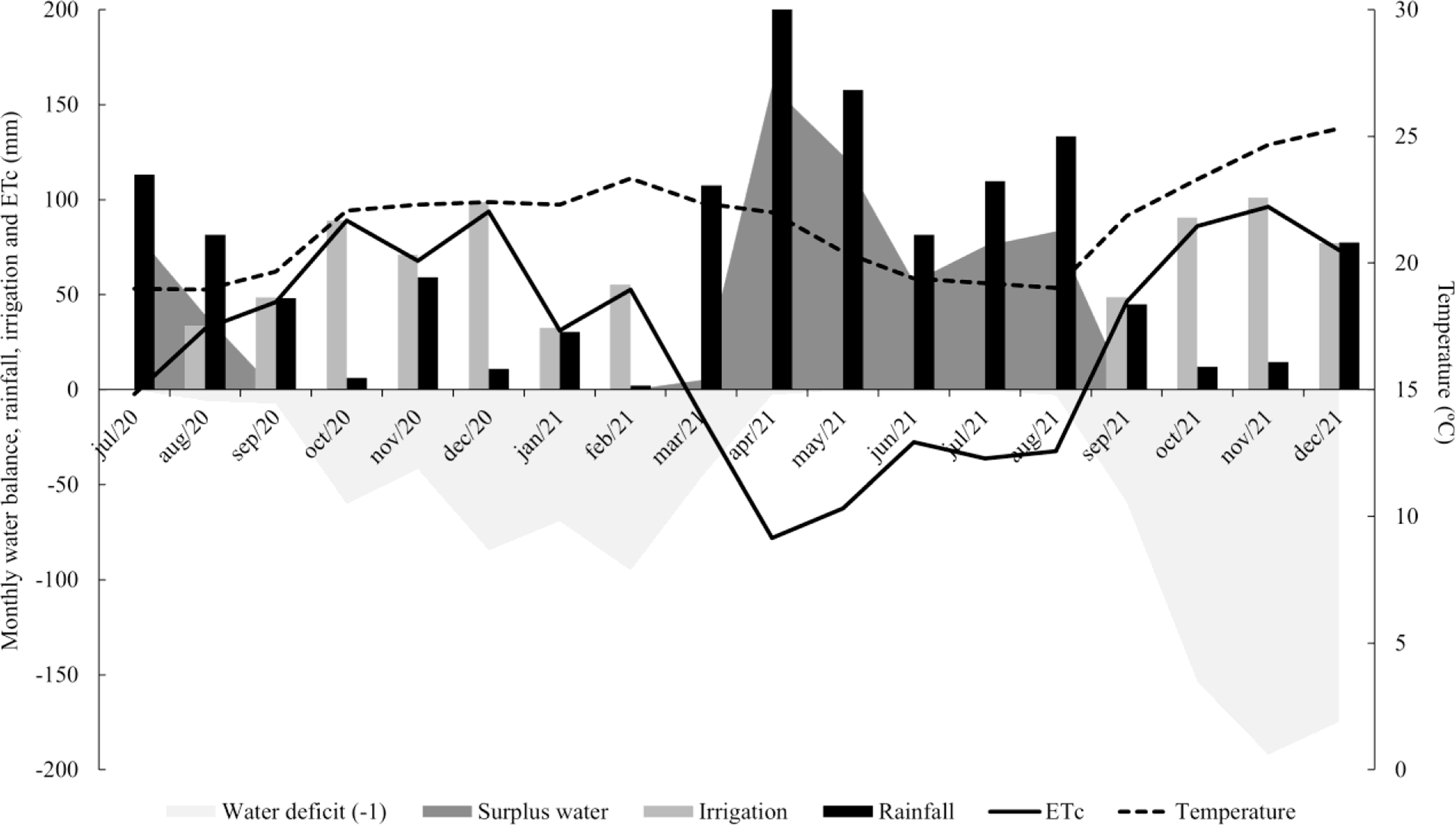
Figure 1. Water balance, rainfall, monthly irrigation, crop evapotranspiration (ETc) and air temperature recorded during the experimental period in Garanhuns, PE, Brazil.
The soil of the experimental area is classified as Ultisol, and the texture was sandy clay loam. The average soil chemical characteristics at the 0–20 cm layer were pH (H2O) = 5.0; phosphorus (P) = 8 mg/dm3 (Mehlich−1 Extractor); potassium (K) = 0.07 cmolc/dm3; sodium (Na) = 0.03 cmolc/dm3; aluminium (Al) = 0.15 cmolc/dm3; calcium (Ca) = 3.25 cmolc/dm3; magnesium (Mg) = 0.95 cmolc/dm3; potential acidity (H+Al) = 1.8 cmolc/dm3; sum of bases (SB) = 4.3 cmolc/dm3; cation exchange capacity (CEC) = 6.1 cmolc/dm3; base saturation (V) = 70.5% and organic matter (OM) = 22.8 g/kg.
The experimental design was a randomized complete block with split-plot arrangement and four replications. The main plots consisted of harvest frequency (60 and 90 days), and the subplots corresponded to four elephant grass genotypes, two tall (Elephant B and IRI-381) and two dwarf-types (Mott and Taiwan A-146 2.37, hereafter referred to as Taiwan).
The Elephant B genotype, also known as Mecker, is one of the first genotypes introduced in Brazil. IRI-381 genotype is a cultivar recommended for use in cut-and-carry systems in the state of Pernambuco (Lira et al., Reference Lira, Cunha, Pereira, Lira, Santos, Dubeux and Mello2010). Mott genotype is a dwarf type selected in 1977 from a progeny of self-fertilized Markeron cultivar in Georgia (USA) (Sollenberger and Jones Júnior, Reference Sollenberger and Jones1989), and Taiwan genotype was generated by the joint elephant grass breeding program of the Pernambuco Agronomic Institute (IPA) and UFRPE.
Plot establishment and management
Elephant grass genotypes were established in 2016 by vegetative propagation with 1 m spacing between rows, using mature stems. The area was 1820 m2 (91 × 20 m). Each plot consisted of 546 m² (91 × 6 m), with spacing of 8 m between main plots. Each subplot had 24 m² (4 × 6 m), and the sampling area was 15 m² (3 × 5 m).
The maintenance soil fertilization was performed 15 days after harvest, following the regional fertilizer recommendation for elephant grass in Pernambuco State (Cavalcanti, Reference Cavalcanti2008), consisting of 100 kg nitrogen/ha and 80 kg potassium/ha in the planting furrow. The fertilization sources consisted of ammonium sulphate [(NH4)2SO4] and potassium chloride (KCl), respectively.
Irrigation management distributed the water needed for restoring 100% crop evapotranspiration (ETc) based on the standardized Penman-Monteith method by FAO/56 (Allen et al., Reference Allen, Pereira, Raes and Smith1998). The weather data used for ETc calculations were obtained from a weather station from located in Garanhuns, Pernambuco, Brazil. In the first year, 82.3 and 170 mm of water were applied in the irrigated plots in the rainy and dry seasons, respectively. In the second year, 48.8 and 178 mm was applied in the plots irrigated in the rainy and dry seasons, respectively (Fig. 1).
Response variables
Morphological variables
Sampling and morphological evaluations were carried out during the rainy season, corresponding to harvests occurred in the months of July and September and the dry season, considering the harvests that occurred in the months of November and December of the years of 2020 and 2021. Rainy and dry seasons were separated according to the pattern of rainfall distribution at the location.
The morphological variables evaluated were: plant height (cm), number of leaves per tiller, leaf length (cm), leaf width (cm), stem diameter (mm), internode length (cm) and leaf angle (°). To estimate the leaf/stem ratio, six tillers from each subplot were collected, manually separated into leaf blade and stem (stem plus sheath), dried in a forced air oven at 55°C (Tecnal®, TE-394/3, Piracicaba, Brazil) for 72 hours and weighed. Leaf/stem ratio was obtained by dividing the dry weight of leaves by stems.
Plant height was obtained by taking measurement from ground level up to the curvature height of the upper leaf using a graduated ruler. Leaf blade length and width were measured in the newly fully expanded leaves with an obvious collar region. Length was measured from collar to blade tip, and width was measured at the midpoint of the leaf blade based on the measurement of blade length. The mean length of the internodes (from the base to the apex) was measured using a caliper. The leaf angle was measured with the aid of a protractor, using the stem as a reference.
In vitro gas production analysis
For in vitro gas production, dry samples of leaves, stems and whole plant were ground in a Willey mill (Tecnal®, R-TE-650/1, Piracicaba, Brazil), to pass 2 mm sieves. From each sample, 1.0 g was added to glass bottles (160 mL) containing 90 mL of nutrient medium according to Theodorou et al. (Reference Theodorou, Williams, Dhanoa, McAllan and France1994). Subsequently, 10 mL of rumen fluid was added to each bottle, sealed with rubber corks and aluminium seals. The ruminal inoculum was obtained immediately before starting the incubation, from samples composed of the solid and liquid fractions of the ruminal contents of a cattle (female) fistulated in the rumen, with a diet composed of elephant grass and concentrate, with unrestricted access to water. The pressure caused by fermentation was measured using a pressure transducer (Datalogger Universal Logger AG100 – Agricer). The readings were made with a higher rate during the initial time and with a lower rate towards the end of the study time (2, 4, 6, 8, 10, 12, 15, 18, 21, 24, 30, 36, 42, 48 and 72 hours of incubation). The pressure in pound-force per square inch (PSI) was converted into volume of gas (V) by the equation:
generated in the Laboratory of Gas Production at the Federal Agreste University of Pernambuco, based on 937 observations (−8°90’ S, −36°49’; W, 844 m a.s.l.), considering 1 PSI = 4.859 mL of gas.
Cumulative gas production data were adjusted by the two-compartment model according to Schofield et al. (Reference Schofield, Pitt and Pell1994):
where V(t) is the maximum total volume of produced gas (mL/g DM); Vf1 (mL/g DM) is the maximum volume of gas for the degradation of non-fibrous carbohydrates (DNFC); VF2 (mL/g DM) is the maximum volume of gas for the degradation of fibrous carbohydrates (DFC); kd1 (h) is the rate of the degradation of non-fibrous carbohydrates (RDNFC); kd2 (h) is the rate of the degradation of fibrous carbohydrates (RDFC); L (h) is the duration of initial digestion events (lag time) common to the two phases; and T (h) is the fermentation time.
Statistical analysis
The data of morphological variables were subjected to the analysis of variance, using the SAS software, with season considered as a repeated measure, using the PROC MIXED. Fixed effects included genotypes, harvest frequencies, seasons and their interactions. The blocks and their interactions were considered random effects. When there was a significant effect of the factors studied and their interactions in the F test in ANOVA, the means were compared using the PDIFF procedure adjusted for Tukey test. Significant differences were considered when P < 0.05.
To determine the parameters of gas production, the bicompartmental logistic model was used with the aid of PROC NLIN of the SAS software. Afterwards, the parameters were analyzed using PROC MIXED of SAS. The treatments (harvest frequencies and genotypes) and their interactions were considered as fixed effects and the blocks as random. Successive harvests were considered as repeated measures and means were compared using the PDIFF procedure adjusted for Tukey test. Significant differences were considered when P < 0.05.
Results
Morphological characteristics
The interaction of harvest frequency and genotype had significant effects on plant height (cm) (P < 0.004) and number of leaves per tiller (NLT) (P < 0.001) (Table 1). Greater plant height was observed for tall genotypes at both harvest frequencies. When comparing in harvest frequencies, the highest plant heights were observed at 90 days of harvest (Table 1). Mott genotype showed a greater NLT at a harvest frequency of 60 days, while there was no significant difference between Mott and IRI-381 at 90 days of harvest, with IRI-381 showing an increase 28% in NLT compared to harvest every 60 days (Table 1).
Table 1. Plant height (cm) and number of leaves per tiller of irrigated elephant grass genotypes affected by harvest frequency × genotype interaction in Garanhuns, PE, Brazil
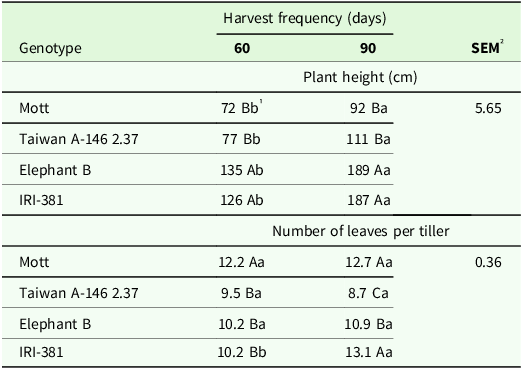
1 Means followed by the same uppercase letter in the column and lowercase in the row do not differ from each other by the Tukey test (P < 0.05).
² SEM = standard error of the means.
The leaf/stem ratio was affected by genotype (P < 0.001) and harvest frequency (P < 0.001) (Fig. 1). There was a greater leaf/stem ratio for the Mott genotype in the harvest frequency of 60 days, followed by the Taiwan and IRI-381 genotypes (Fig. 1a). The harvest frequency at 60 days showed a greater leaf/stem ratio (Fig. 1b), being 54% higher when compared to the harvest frequency at 90 days.
The interaction of season and genotype had significant effects on stem diameter (P < 0.001) and number of leaves per tiller (P < 0.019) (Table 2). In the rainy season, tall genotypes and Mott showed larger stem diameters, while in the dry season, only Mott showed greater stem diameter (Table 2). The Mott and IRI-381 genotypes showed greater stem diameter in the dry season, with an increase of 60% and 12%, respectively (Table 2). IRI-381 genotype showed the greater NLT in the rainy season with a decrease of 19% in the dry season. Mott showed the greater NLT for the Mott genotype in the dry season (Table 2).
Table 2. Stem diameter (mm), number of nodes and number of leaves per tiller of irrigated elephant grass genotypes affected by season year × genotype interaction in Garanhuns, PE, Brazil
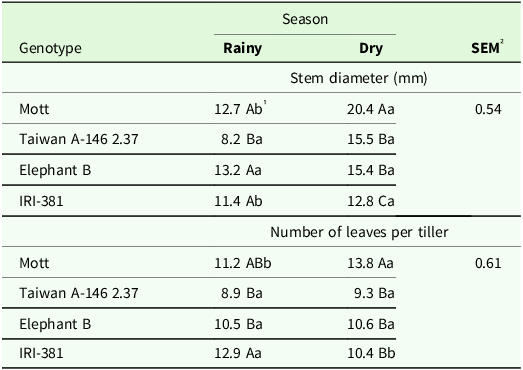
1 Means followed by the same uppercase letter in the column and lowercase in the row do not differ from each other by the Tukey test (P < 0.05).
² SEM = standard error of the means.
The internode length was affected by season (P < 0.001) and genotype (P < 0.001) (Fig. 3). Internode length was 37% greater in the rainy season compared to the dry season (Fig. 3a). As expected, the greater internodes length was observed for the tall genotypes (Fig. 3b).
Plant height (P < 0.002), leaf angle (P < 0.002), leaf length (P < 0.001), stem diameter (P < 0.001), leaf width (P < 0.001) and number of leaves per tiller (P < 0.010) were affected by season × harvest frequency interaction (Table 3).
Table 3. Plant height (cm), leaf angle (°), leaf length (cm), stem diameter (mm), leaf width (cm) and number of leaves per tiller of irrigated elephant grass genotypes affected by the interaction season × harvest frequency in Garanhuns, PE, Brazil

1 Means followed by the same uppercase letter in the column and lowercase in the row do not differ from each other by the Tukey test (P < 0.05).
² SEM = standard error of the means.
A greater plant height was observed when the harvest frequency was 90 days, regardless of season, with values 41% greater than when harvested every 60 days (Table 3). Leaf angle was greatest in the rainy season at a harvest frequency of 90 days. Leaf length, stem diameter and leaf width were generally greater at the harvest frequency of 60 days in the rainy season (Table 3). The number of leaves per tiller, plant height and leaf length and width were generally greater in the dry season (Table 3).
Leaf angle and stem diameter were greater in the harvest frequency of 60 days in the dry season, while the variables leaf length, stem diameter, leaf width and number of leaves per tiller were greater when harvested every 90 days (Table 3).
In vitro gas production
There was a significant effect of the genotype × harvest frequency interaction on the volume of gas produced of DNFC (P < 0.001), RDNFC (P < 0.004), volume of gas produced of DFC (P < 0.01) and RDFC (P < 0.001) (Table 4).
Table 4. Volume of gas produced (mL/g DM) and degradation rate (/h) of non-fibrous and fibrous carbohydrates from the whole plant of the irrigated elephant grass genotypes affected by the genotype × harvest frequency interaction in Garanhuns, PE, Brazil
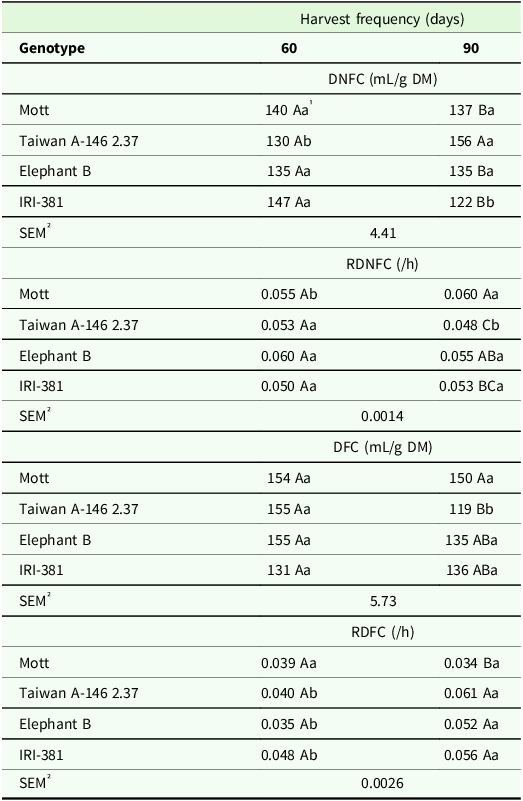
DNFC = degradation of non-fibrous carbohydrates; RDNFC = rate of the degradation of non-fibrous carbohydrates; DFC = degradation of fibrous carbohydrates; RDFC = rate of the degradation of fibrous carbohydrates.
1 Means followed by the same uppercase letter in the column and lowercase in the row do not differ from each other by the Tukey test (P < 0.05).
² SEM = standard error of the means.
The Taiwan genotype presented the greatest volume of gas produced by DNFC at the harvest frequency of 90 days (Table 4). Higher RDNFC and volume of gases by DFC were observed for the Mott genotype at 60 d harvest frequency, while the tall genotypes and Taiwan showed the greatest RDFC when harvested at the 90 days harvest frequency (Table 4).
Taiwan genotype showed a higher volume of gas produced by DNFC, volume of gases by DFC and RDFC at the 90 days harvest frequency, while the highest RDNFC was observed when the forage was harvested every 60 days (Table 4). Opposite response was observed for Mott genotype, which showed a greater RDNFC at the 90 days harvest frequency (Table 4).
IRI-381 genotype forage showed a higher volume of gas produced by DNFC when harvested every 60 days, while the tall genotypes and Taiwan showed a higher RDFC at the 90 days harvest frequency (Table 4).
There was a significant effect of harvest frequency × season interaction on lag time (P < 0.001) and total gas production (P < 0.02) (Table 5). A 43% longer lag time was observed for the forage harvested every 90 days compared to 60 days in the rainy season, while greater total gas production was observed for forage harvested at a 60-day frequency in the dry period (Table 5). Forage harvested in the rainy season provided lag time for ruminal microorganisms, regardless of harvest frequency. In turn, the highest volume of total gas production was observed at a harvest frequency of 60 days in the dry season (Table 5).
Table 5. Lag time (h) and total gas production (mL/g DM) of irrigated elephant grass genotypes affected by the harvest frequency × season interaction in Garanhuns, PE, Brazil
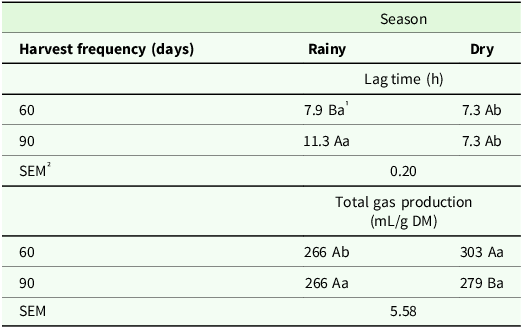
1 Means followed by the same uppercase letter in the column and lowercase in the row do not differ from each other by the Tukey test (P < 0.05).
² SEM = standard error of the means.
There was a significant effect of the genotype × harvest frequency interaction on volume of gas produced by DNFC (P < 0.001) and DFC (P < 0.001), RDFC (P < 0.01), lag time (P < 0.03) and total gas production (P < 0.001) by fermentation of leaf blades (Table 6). It was observed a greater lag time and total gas production for the Elephant B and Taiwan genotypes harvested harvest frequency of 60 days. At the harvest frequency of 90 days, the leaf blades of the Taiwan genotype produced greater volume of gas by DNFC, while the leaf blades of the Mott genotype produced greater volume of gas by DFC (Table 6). Leaves of dwarf types of genotypes generally showed greater (Table 6).
Table 6. Volume of gas produced (mL/g DM) by non-fibrous carbohydrates and fibrous carbohydrates, rate of degradation of fibrous carbohydrates (/h), lag time (h) and total gas production of the leaf blade of the genotypes of Irrigated elephant grass affected by genotype × harvest frequency interaction in Garanhuns, PE, Brazil
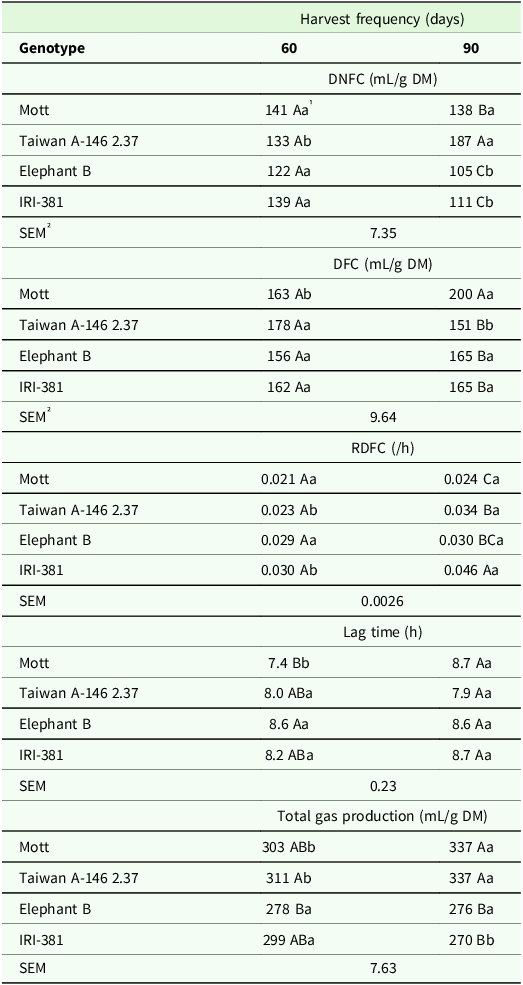
DNFC = degradation of non-fibrous carbohydrates; DFC = degradation of fibrous carbohydrates; RDFC = rate of the degradation of fibrous carbohydrates.
1 Means followed by the same uppercase letter in the column and lowercase in the row do not differ from each other by the Tukey test (P < 0.05).
² SEM = standard error of the means.
Taiwan genotype produced a greater volume of gas during DNFC, a greater RDFC and total gas production when harvested every 90 days, while the tall genotypes showed a greater volume of gas during DNFC at a harvest frequency of 60 days (Table 6). IRI-381 genotype presented a greater RDFC at a harvest frequency of 90 days.
A greater lag time was observed for the Mott genotype at a harvest frequency of 60 days, as well as greater total gas production by the leaf blades of the IRI-381 genotype (Table 6). Both dwarf genotypes showed higher total gas production during fermentation of leaf blades at a harvest frequency of 90 days.
The interaction of season and genotype had significant effects on volume of gas produced (P < 0.003) and RDFC (P < 0.01) of the leaf blades (Table 7). The leaf blades of the dwarf genotypes produced a greater volume of gas during DFC in the dry season, with an average of 195 mL of gases, while the greater RDFC was observed in the fermentation of leaf blades for the IRI-381 genotype (Table 7). Taiwan genotype showed a greater volume of gas produced by DFC of leaf blades in the dry season, 43% higher than the volume produced in the rainy season. Tall genotypes had a higher rate of leaf blades degradation in the dry season (Table 7).
Table 7. Volume of gas produced (mL/g DM) and rate of degradation of fibrous carbohydrates (/h) in the leaf blade of irrigated elephant grass genotypes affected by the genotype × season of the year interaction in Garanhuns, PE, Brazil
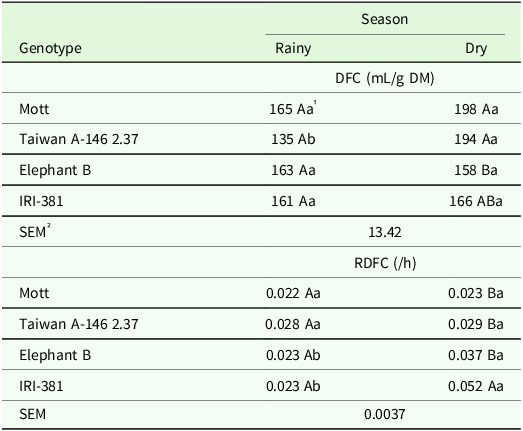
DFC = degradation of fibrous carbohydrates; RDFC = rate of the degradation of fibrous carbohydrates.
1 Means followed by the same uppercase letter in the column and lowercase in the row do not differ from each other by the Tukey test (P < 0.05).
² SEM = standard error of the means.
There was a significant effect of genotype for volume of gas produced by DNFC (P < 0.001), lag time (P < 0.001), and total gas production (P < 0.01) by stem fermentation (Table 8). Mott genotype showed a greater gas volume and total gas production during stems DNFC and presented shorter lag time, approximately 11% lesser than the value observed for the Taiwan, Elephant B and IRI-381 genotypes (Table 8). The tall genotype Elephant B showed similar total gas production compared to dwarf Mott genotype.
Table 8. Volume of gas produced by non-fibrous carbohydrates (mL/g DM), lag time (h) and total gas production (mL/g DM) of the stem of irrigated elephant grass genotypes in Garanhuns, PE, Brazil
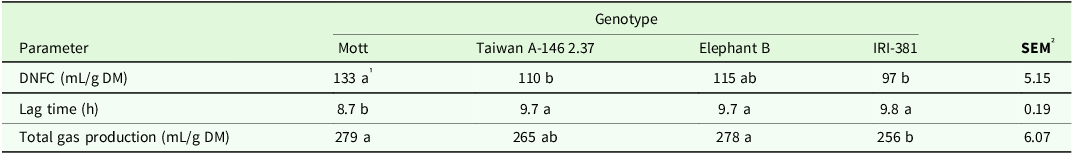
DNFC = degradation of non-fibrous carbohydrates.
1 Means followed by the same lowercase in the row do not differ from each other by the Tukey test (P < 0.05).
² SEM = standard error of the means.
There was a significant effect of genotype on volume of gas produced (P < 0.001) and RDNFC (P < 0.001) and total gas production (P < 0.001) during stem fermentation (Table 9). Greater total gas production and volume and RDNFC were observed for stems at the 60 days harvest frequency.
Table 9. Volume of gas produced (mL/g DM) and rate of degradation of non-fibrous carbohydrates (/h) and total gas production (mL/g DM) of the stem of irrigated elephant grass genotypes affected by harvest frequency in Garanhuns, PE, Brazil
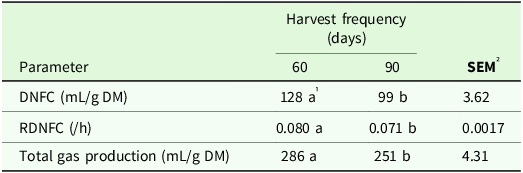
DNFC = degradation of non-fibrous carbohydrates; RDNFC = rate of the degradation of non-fibrous carbohydrates.
1 Means followed by the same lowercase in the row do not differ from each other by the Tukey test (P < 0.05).
² SEM = standard error of the means.
Volume of gas produced (P < 0.001), RDNFC (P < 0.001), lag time (P < 0.001) during stem fermentation were affected by season (Table 10). The largest volume of gas produced during of DNFC of stems were observed in the dry season, with a difference of approximately 31 mL compared to rainy season. During the rainy season occurred greater stems RDNFC and lag time (Table 10).
Table 10. Volume of gas produced (mL/g DM) and rate of degradation of non-fibrous carbohydrates (/h) and lag time (h) of the stem of irrigated elephant grass genotypes affected by the season of the year in Garanhuns, PE, Brazil
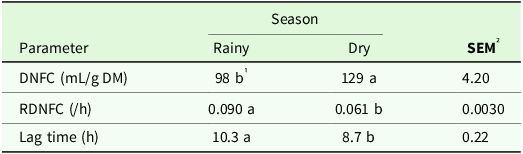
DNFC = degradation of non-fibrous carbohydrates; RDNFC = rate of the degradation of non-fibrous carbohydrates.
1 Means followed by the same lowercase in the row do not differ from each other by the Tukey test (P < 0.05).
² SEM = standard error of the means.
The interaction of season and genotype had significant on RDFC (P < 0.02) of stems (Table 11). In the dry season, a higher RDFC was observed for the tall genotypes and the dwarf Taiwan.
Table 11. Degradation rate of fibrous carbohydrates (/h) of the stem of irrigated elephant grass genotypes affected by the genotype × season of the year interaction in Garanhuns, PE, Brazil
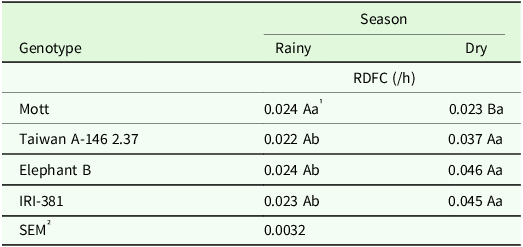
RDFC = rate of the degradation of fibrous carbohydrates.
1 Means followed by the same uppercase letter in the column and lowercase in the row do not differ from each other by the Tukey test (P < 0.05).
² SEM = standard error of the means.
Discussion
Dwarf elephant grass genotypes usually present lesser stem elongation, and consequently plant height, compared to tall genotypes (Souza et al., Reference Souza, Santos, Cunha, Gonçalves, Silva, Mello and Dubeux2021; Ribeiro et al., Reference Ribeiro, Mello, Cunha, Costa, Coelho, Souza and Santos2022; Silva et al., Reference Silva, Santos, Mello, Sales-Silva, Simões Neto, Silva, Dubeux, Coelho, Souza and Cunha2023), which may contribute to greater investment in leaf emission likely contributing to greater leaf/stem ratio. However, reducing harvest frequency contributes to an increase plant height (Table 1) and the participation of stems in the harvested forage mass (Fig. 2a), reducing leaf/stem ratio (Fig. 2a), and potential of fermentation by microorganisms in the rumen. As expected, tall-size genotypes Elephant B and IRI-381 showed greater plant height independently of harvest frequency (60 and 90 days), which likely contributed to reduce leaf/stem ratio, despite maintaining (Elephant B) or even increasing (IRI-381) the number of leaves per tiller (NLT) as the harvest frequency was reduced from 60 to 90 days (Table 1).

Figure 2. Leaf/Stem Ratio of irrigated elephant grass genotypes affected by (a) genotype and (b) harvest frequency in Garanhuns, PE, Brazil. Means followed by the same capital letter do not differ from each other by F test (P < 0.05). Bars indicate the standard error of the mean (SEM = 0.04, 0.02).
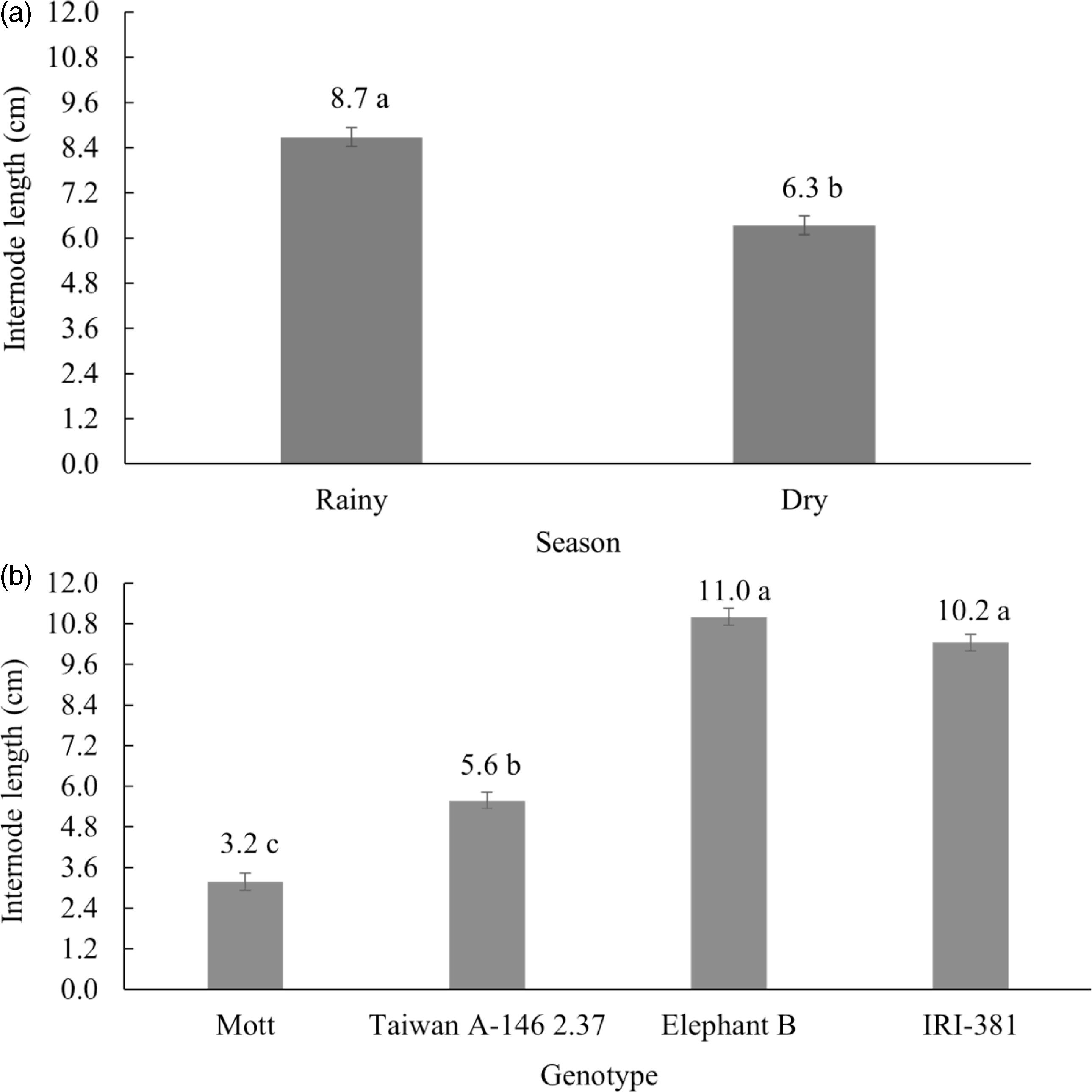
Figure 3. Internode length (cm) of irrigated elephant grass genotypes affected by (a) season and (b) genotype in Garanhuns, PE, Brazil. Means followed by the same capital letter do not differ from each other by F test (P < 0.05). Bars indicate the standard error of the mean (SEM = 0.25, 0.25).
The leaf/stem ratio was reduced with lesser harvest frequency (90 days) (Fig. 2b), and the dwarf Mott genotype stood out with the greatest leaf/stem ratio (Fig. 2a). According to Viana et al. (Reference Viana, Mello, Guim, Lira, Dubeux, Santos and Cunha2018), grasses with a greater leaf/stem ratio may present improved forage nutritive value, and the presence of more leaves may facilitate the animal’s reach and grasp during grazing, while the use of dwarf types may facilitate small ruminants grazing. Reducing the harvest frequency of elephant grass increased more the stem fraction relative to the leaf fraction, resulting in a greater proportion of cell wall structural components in the plant, which may contribute to decrease the nutritive value of the harvested forage (Tessema et al., Reference Tessema, Mihret and Solomon2010).
The number of leaves per tiller is a genetic-specific characteristic (Table 1); however, it can be affected by harvest frequency and season (Table 2). Dwarf Mott genotype presented the greatest NLT and showed greater NLT in the dry season compared to other genotypes, while the IRI-381 genotype showed greater NLT at the 90-day harvest frequency. Ribeiro et al. (Reference Ribeiro, Mello, Cunha, Costa, Coelho, Souza and Santos2022) evaluating the same genotypes also reported that the Mott genotype had the greatest NLT, followed by the IRI-381. This suggests that the potential effects of stem in fermentation may be more affected by the increase of stem participation in the forage mass, which can be combined with variation in leaves nutritional value due maturity.
The stem diameter may affect the tolerance of grasses to the dry season and increased harvest frequency due to storage of reserve compounds (Mello et al., Reference Mello, Lira, Dubeux, Santos and Freitas2002). In the present study, the largest stem diameter (Table 2) was observed for the Elephant B, Mott and IRI-381 genotypes in the rainy season, and for the Mott genotype, followed by the Taiwan and Elephant B genotypes in the dry season. Despite the importance stem in the storage or organic reserves for plant recovery after cutting or grazing (Silva et al., Reference Silva, Santos, Lira, Dubeux, Freitas and Ferreira2009), the presence of stems with large diameter may contribute to an increase its participation in the forage mass, contributing to a reduction in the leaf/stem ratio and forage nutritive value.
The longer internode length observed in tall genotypes generally contributes to a reduced forage nutritive value. This effect seems to be amplified during the rainy season, likely due to a greater increase in cell wall elongation as a result of water availability (Fig. 3a and 3b). In contrast, the shorter internode length found in dwarf genotypes, such as Mott and Taiwan, may favour their nutritional value, as reduced stem elongation limits the conversion of assimilates and nutrients towards lignified tissue accumulation, likely favouring the presence of more digestible components (Silva et al., Reference Silva, Santos, Lira, Dubeux, Freitas and Ferreira2009; Viana et al., Reference Viana, Mello, Guim, Lira, Dubeux, Santos and Cunha2018).
The harvest frequency of 90 days resulted in taller plants during the rainy season, while the plant height was similar between rainy and dry season in the harvest frequency of 60 days (Table 3). The greater height observed in the rainy season may be related to the use of irrigation, which reduced the effect of variability of the distribution of rainfall. In the dry season, the use of irrigation probably was not the main limiting factor, but the availability of solar radiation and temperature, which may reduce plant photosynthesis and accumulation of new tissues. According by Habermann et al. (Reference Habermann, Oliveira, Contin, Delvecchio, Viciedo, Moraes, Prado, Costa, Braga and Martinez2019), the reduced availability of light and temperature affect plant growth, considering that plants present an optimal temperature range suitable for their optimal development. In the experimental site, there are several periods with cloudiness, which also affects both quantity and quality of available solar radiation (Andrade et al., Reference Andrade, Fonseca, Lopes, Nascimento, Cecon, Queiroz, Pereira and Reis2005).
The availability of resources for plant growth may affect leaf angle (Shimoya et al., Reference Shimoya, Cruz, Ferreira, Pereira and Carneiro2002). In the present study, the leaf angle was higher in the 90 days harvest frequency in the rainy season, while in the 60 days harvest it was higher in the dry season (Table 3). Leaf angle may affect light interception by the canopy, which can also impact plant growth and forage production (Alencar et al., Reference Alencar, Santos, Cunha, Mello, Lucena, Leopoldino Neto, Peixôto and Silva2023). According by Shimoya et al. (Reference Shimoya, Cruz, Ferreira, Pereira and Carneiro2002), the leaf angle influences the photosynthetic activity and the density of plants per area, which can be altered due to the leaf insertion angle and its impacts in capturing photosynthetically active radiation. Additionally, more erect leaves with greater leaf angles may also require more deposition of lignified tissues, potentially affecting its potential of fermentation and nutritive value.
The length and width of the leaf blade are directly related to the leaf area index, which may affect canopy photosynthesis and growth. In the present study, leaf length and width of elephant grasses were higher at harvest frequency at 60 days in the rainy season, while at the harvest frequency of 90 days was higher in the dry season (Table 3). The greater leaf length and width likely contributed to reduce the leaf angle (Table 3). According to Silva et al. (Reference Silva, Santos, Dubeux, Lira, Ferreira, Freitas, Cunha and Silva2010), stem diameter, leaf length and width, and leaf/stem ratio present high heritability, indicating that much of the variation between genotypes has genetic causes, with lesser influence of the environment. The leaf component generally presents less proportion of lignified tissues compared to stems (Souza et al., Reference Souza, Santos, Cunha, Gonçalves, Silva, Mello and Dubeux2021), favoring the fermentation process.
At the 60 days harvest frequency, the genotypes showed no differences in the parameters of in vitro gas production for the plant (Table 4), and for the volume of gas produced by DNFC and DFC, and RDFC of leaf blades (Tables 6 and 7), probably due to the lesser plant age, not differentiating the deposition of structural compounds, suggesting that at this point all genotypes may present high levels of soluble carbohydrates available to microorganisms. Souza et al. (Reference Souza, Santos, Cunha, Gonçalves, Silva, Mello and Dubeux2021) evaluating the anatomical characteristics of the leaf blades of the same genotypes, the leaf blades of the elephant grass genotypes show a predominance of mesophyll, regardless of plants size (dwarf and tall). Generally, young plant cells presents greater cellular, which is fermented more quickly in the rumen (Jung, Reference Jung1989).
The volume of gas produced by DNFC, which corresponds to the volume of gases (mL) from the fermentation of the fast digestion fraction, was greater for the Taiwan genotype harvested every 90 days (Table 4). As it is a dwarf genotype, it has lesser stem elongation (Fig. 3) and greater leaf/stem ratio (Fig. 2), which probably contributed to increase protein content, and decrease lignin in NDF. The greater volume of gas can be a result of the high solubility fraction, as the most digestible fractions of the plant are present in the leaf blades (Paciullo et al., Reference Paciullo, Gomide, Silva, Queiroz and Gomide2002). Similarly, Maranhão et al. (Reference Maranhão, Cândido, Lopes, Pompeu, Carneiro, Furtado, Silva and Alves2018) reported that greater leaf/stem ratio may indicate greater forage nutritional value. According by Ramin and Huhtanen (Reference Ramin and Huhtanen2013), dwarf cultivars have lower lignin and NDF content, and greater CP content.
The higher RDNFC in the Mott genotype (Table 4) may indicate greater energy availability for rumen microorganisms, which may enhance microbial activity and overall fermentation efficiency compared to the other dwarf type Taiwan genotype. This increase in degradation occurs, possibly, because Gram-negative bacteria, such as non-fibrous carbohydrates fermenting bacteria, are able to use a greater variety of available substrates, such as different sources of nitrogen (ammonia, amino acids and peptides) for the synthesis of their proteins, whereas Gram-positive bacteria use only ammonia, such as structural carbohydrate-fermenting bacteria (Santos et al., Reference Santos, Pereira, Pereira, Silva, Santos, Carvalho, Almeida, Silva and Ribas2021), corroborating the greater volume of gas produced by DNFC (156 mL, Table 4). Furthermore, soluble nutrients are the substrates used for initial growth, multiplication and microbial activity at the beginning of fermentation and how much greater the availability of proteins and soluble carbohydrates at the beginning of fermentation, greater the rate of feed degradation (Eger et al., Reference Eger, Graz, Riede and Breves2018).
The dwarf genotype Mott exhibited the highest volume of gas produced by DFC (Tables 4 and 6), indicating the degradation of slowly fermenting carbohydrates at a harvest frequency of 90 days,. The greater gas volume may be associated with the high levels of slowly degrading carbohydrates available for ruminal microorganisms. In the case of the Mott genotype, the harvesting every 90 days may have to the decrease in rapidly fermentable carbohydrates and an increase in slowly degrading carbohydrates. The long regrowth period with reduction of harvest frequency may increase forage accumulation, but decrease the participation of the leaf component and, consequently, the quality of the forage harvested (Ferreira et al., Reference Ferreira, Abreu, Martinez, Braz and Ferreira2018).
The rate of degradation of the fibrous fraction represents the fraction of slowly fermenting carbohydrates available for degradation by ruminal microorganisms. The RDFC was greater for Taiwan, Elefante B and IRI-381 genotypes (Table 4). When comparing RDFC of leaf blades, the greater value were observerd for IRI-381 genotype showed greater RDFC, followed by Elefante B and Taiwan genotypes in harvest frequency at 90 days (Table 6), which may indicate a longer time for degradation of the fibrous fraction in the frequency of harvest at 90 days. Souza et al. (Reference Souza, Santos, Cunha, Gonçalves, Silva, Mello and Dubeux2021) reported that the Elefante B and IRI-381 genotypes harvested every 60 days showed a smaller concentration of potential degradable fractions, which may contribute to the reduction of energy available to the rumen microorganisms responsible by fermentation of fiber carbohydrates, potentially contributing to reduce animal performance.
Lag time is the time required for rumen microorganisms to adhere to food particles. A greater lag time was observed in the rainy season in the harvest frequency at 90 days (Table 5), which was probable a result of the presence of morefibrous fraction, since microorganisms generally adhere more slowly to the particle to start the degradation process. Additionally, the reduced concentration of nonfibrous carbohydrates available to the microorganisms at the beginning of fermentation of the substrate increases the lag time.
The greatest total gas production adjusted by the bicompartmental model was observed for forage harvested at 60 days in the dry season of the evaluation season (303 mL) (Table 5) and was probably a result of greater availability of nonfibrous carbohydrates for ruminal fermentation, resulting in lesser lag time at the same harvest frequency. When evaluating elephant grass genotypes harvested at two levels of light interception (90 and 95%) and two stubble heights (30 and 50 cm), Chaves et al. (Reference Chaves, Ribeiro, Gomide, Paciullo, Morenz and Gama2016) reported a total volume of gas produced of approximately 165 mL for the dwarf elephant grass cv. BRS Kurumi and approximately 168 mL for the intermidiate dize CNPGL 00-1-3 genotype, with lag time ranging from 2.19 to 4.59 h−1, much lesser than the observed in the current study.
The lesser lag time and greater total gas production were observed in forage harvested at 60 days and in the dry season (Table 5), which may indicate a greater concentration of readily fermentable substrates for microorganisms and physical and chemical characteristics of the cell wall (Guimarães Jr et al., Reference Guimarães, Gonçalves, Maurício, Pereira, Tomich, Pires, Jayme and Sousa2008). It is important to mention that during the dry season the genotypes presented generally shorter heights, which probably contributed to reduce the presence of structural tissues (Sanchês et al., Reference Sanchês, Rodrigues, Araújo, Santos, Silva, Figueredo, Cabral, Araújo and Costa2019).
The gas volume produced during DNFC and DFC and RDFC of leaf blades were similar for the genotypes at the 60-day harvest frequency (Table 6) probable due to young deposited soluble carbohydrate contents (Santana et al., Reference Santana, Ítavo, Ítavo, Dias, Niwa, Moraes, Arcanjo, Gurgel, Borges, Formigoni and Difante2022). At the harvest frequency of 90 days, the leaf blades of the Taiwan genotype had a higher volume of gas by DNFC, followed by the Mott genotype, both dwarf size (Table 6). It is important to mention that dwarf genotypes presented greater leaf/stem ratio (Fig. 2), which may contribute to greater fermentation of soluble carbohydrates, considering that the leaf blade represents the fraction with the greatest solubility (Paciullo et al., Reference Paciullo, Gomide, Silva, Queiroz and Gomide2002).
Dwarf size genotypes (Mott and Taiwan) showed greater volume of gas produced by DFC in the dry season (Table 6), and the 60-day harvest frequency showed greater volume of gas from DNFC of stems, greater RDNFC, as well as the greatest total gas production (286 mL) (Table 9). According to Souza et al. (Reference Souza, Santos, Cunha, Gonçalves, Silva, Mello and Dubeux2021), dwarf genotypes (Mott and Taiwan) present a greater concentration of soluble and potential degradable fractions of carbohydrates in their leaf blades, compared to the stem fraction. Fast ruminal fermentation carbohydrates are considered a primary source of energy for rumen microorganisms for cell multiplication (Singh et al., Reference Singh, Kushwaha, Nag, Mishra, Singh and Anele2012). The availability of the energy/nitrogen ratio for microorganisms is determined by degradation rates affects the production and microbial protein synthesis. Silva et al. (Reference Silva, Cunha, Santos, Magalhães, Mello, Silva, Souza, Carvalho and Souza2021a) reported that tall-sized elephant grasses Elephant B and IRI-381 presents a reduced energy/nitrogen ratio than dwarf cultivars, negatively affecting degradability, microbial protein synthesis, negatively impacting metabolic status and animal weight gain.
Tall-sized elephant grass Elephant B genotype had the longest lag time (Table 6), comparable to that reported by Malafaia et al. (Reference Malafaia, Valadares Filho, Vieira, Silva and Pereira1998) evaluating the tall-size cv. Napier at 60 days of regrowth time (8.45 h). According to Campos et al. (Reference Campos, Lanna, Bose, Boin and Sarmento2002), the longer lag time for in vitro gas production with elephant grass may be linked to the dilution effect of nonstructural carbohydrates, which, depending on its concentration, may be more available for microbial activity. The reduced concentration of nonstructural carbohydrates may increase the competition for this substrate, initially affecting the time for cell wall colonization by cellulolytic bacteria.
The total in vitro gas production during the fermentation of leaf blades of the dwarf genotypes (Taiwan and Mott) harvested every 90 days (Table 6) suggest that these genotypes may retain a higher content of readily fermentable carbohydrates, indicating their potential for effective fermentation in the rumen, which supports a significant increase in gas production at this harvest frequency (Souza et al., Reference Souza, Santos, Cunha, Gonçalves, Silva, Mello and Dubeux2021).
The greater gas production from the DNFC of stems and the shorter lag time in the Mott genotype (Table 8) indicate a higher nutritional value of this morphological component, which may facilitate the fermentation process and reduce its potential negative impact on animal consumption and performance (Brandstetter et al., Reference Brandstetter, Costa, Silva, Araújo Neto, Silva, Neves, Souza and Oliveira2018). Furthermore, both Mott and Elephant B presented greater total gas production during the fermentation of the stem fraction (Table 2), likely related to the stored soluble reserves.
The volume of gas produced by DNFC of stem was greater in dry season (Table 10), moment in which the lag time was reduced. This probably occurred due to less stem elongation (Table 1). Taiwan, Elephant B and IRI-381 genotypes showed RDFC in the dry season (Table 11). According to Peixôto et al. (Reference Peixôto, Mello, Santos, Cunha, Pimentel, Silva, Simões Filho and Coelho2022), evaluating dwarf and tall size elephant grass, climatic variations throughout the year results in variations in the nutritive value, regardless of the size of the plant, which may affect the potential of fermentation of elephant grasses.
Tomich et al. (Reference Tomich, Gonçalves, Maurício, Pereira and Rodrigues2003) indicated that more fermentable or digestible forages generally produce more volume of gas and presents greater rates of gas production, resulting in a greater fermentation of the material in a shorter incubation time. In the present study, a greater volume of gas was observed in the harvest frequency at 60 days, and when harvest frequency was reduced to 90 days, dwarf genotypes produced a greater volume of gas during the fermentation of leaf blades, while the tall size genotypes showed lesser degradation rates (Tables 6 and 7). This indicates that the presence of cellular content with greater concentration of soluble carbohydrates and its availability for ruminal microorganisms decline more quickly in tall genotypes compared to dwarf types.
Conclusion
The morphological characteristics of tall and dwarf elephant grass genotypes are affected by harvest frequency and season and contributes to differences in the fermentation parameters. Mott has a greater leaf/stem ratio and number of leaves compared to tall genotypes and the dwarf Taiwan. The harvest frequency of 60 days during the rainy season favours the production of high-nutritive value forage of dwarf elephant grass genotypes, and it can be reduced to 90 days during the dry season without large negative impacts in fermentation parameters. A reduced harvest frequency (90 days) contributes to significant increase in fibrous content in tall genotypes, decreasing the potential of fermentation.
Author contributions
Conceptualisation: MVFS; methodology: MVFS, RTAS and ALRM; software: RTAS and MVC; validation: MVFS, VJS, ALRM and RTAS; formal analysis: RTAS, NVS, MVC and RLCF; investigation: NVS, RTAS and EJSA; resources: MVFS and ALRM; data curation: NVS and RTAS; writing – original draft: NVS and MVFS; writing – review and editing: NVS, VJS, MVC and EJOS; visualization: NVS and MVFS; supervision: MVFS, ALRM, RTAS and VPS; project administration: MVFS; funding acquisition: MVFS and MVC.
Funding statement
This research was funded by the National Council for Scientific and Technological Development (CNPq) through research grants. Additionally, this study was partially financed by the Coordination for the Improvement of Higher Education Personnel (CAPES) – Brazil, Finance Code 001.
Competing interests
The authors declare there are no conflicts of interest.
Ethical standards
Not applicable.

















