Introduction
Statins are medications that lower peripheral cholesterol by inhibiting the liver enzymes 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase (Endo, Kuroda, & Tanzawa, Reference Endo, Kuroda and Tanzawa1976), thus decreasing cardiometabolic morbidity and mortality (Sizar, Khare, Patel, & Talati, Reference Sizar, Khare, Patel and Talati2024). These drugs also express a broader pharmacological activity on neurobiological and immune-metabolic processes likely involved in depression pathophysiology (De Giorgi et al., Reference De Giorgi, Rizzo Pesci, Rosso, Maina, Cowen and Harmer2023; Jones et al., Reference Jones, Farooqui, Kloiber, Husain, Mulsant and Husain2021; Walker et al., Reference Walker, Kim, Borissiouk, Rehder, Dodd, Morris and Berk2021). However, the direction of such composite mechanisms might lead to antidepressant and pro-cognitive (e.g. anti-inflammatory) (Wang & Li, Reference Wang and Li2024; Yin et al., Reference Yin, Ju, Zeng, Duan, Zhu, Liu and Lu2024) or depressogenic and cognitive-impairing (e.g. lipid-lowering) (Rosoff et al., Reference Rosoff, Bell, Jung, Wagner, Mavromatis and Lohoff2022; Taylor & Adhikari, Reference Taylor and Adhikari2025; van der Heijden & Houben, Reference van der Heijden and Houben2023) effects. There have also been historical (Zureik, Courbon, & Ducimetière, Reference Zureik, Courbon and Ducimetière1996) and enduring (Ye et al., Reference Ye, Blais, Ng, Castle, Hayes, Wei and Chan2023) concerns around suicidality and other adverse neuropsychiatric events (Pop et al., Reference Pop, Farcaș, Butucă, Morgovan, Arseniu, Pumnea and Gligor2022) of statin treatment. There is therefore considerable interest in assessing their neuropsychiatric safety within at-risk populations and potential to repurpose statins for use in patients with depressive disorder (Chen et al., Reference Chen, Wang, Teng, Huang, Mo, Qu and Xia2024; De Giorgi, Cowen, & Harmer, Reference De Giorgi, Cowen and Harmer2022; Jiang, Hu, McIntosh, & Shah, Reference Jiang, Hu, McIntosh and Shah2023).
Developing safe antidepressant treatments with innovative mechanisms of action or, conversely, identifying alternative physiopathological pathways leading to depression that can be targeted with new therapeutic agents is a research priority in mental health (Harmer, Duman, & Cowen, Reference Harmer, Duman and Cowen2017). The potential for novel therapeutics, including repurposed compounds, can be assessed in early phase experimental medicine studies using validated measures based on the cognitive neuropsychological model of depression (Godlewska & Harmer, Reference Godlewska and Harmer2021). Depressive disorders are associated with negative biases in emotional processing (Roiser, Elliott, & Sahakian, Reference Roiser, Elliott and Sahakian2012), and administration of conventional antidepressants can reduce these biases in healthy controls and depressed populations (Harmer et al., Reference Harmer, O’Sullivan, Favaron, Massey-Chase, Ayres, Reinecke and Cowen2009). Reward learning appears similarly affected, as the presence of depressive symptoms can be associated with both reduced sensitivity to reward and heightened sensitivity to loss (Halahakoon et al., Reference Halahakoon, Kieslich, O’Driscoll, Nair, Lewis and Roiser2020), which normalize following antidepressant use (Herzallah et al., Reference Herzallah, Moustafa, Natsheh, Abdellatif, Taha, Tayem and Myers2013; Scholl et al., Reference Scholl, Kolling, Nelissen, Browning, Rushworth and Harmer2017). Importantly, early changes in emotional biases or reward deficits may be associated with later antidepressant response (Harmer & Browning, Reference Harmer and Browning2022). Deficits in non-emotional cognition, such as memory and concentration, are also common in depression (Roiser & Sahakian, Reference Roiser and Sahakian2013); however, conventional antidepressants do not effectively treat these cognitive symptoms (Colwell et al., Reference Colwell, Tagomori, Chapman, Gillespie, Cowen, Harmer and Murphy2022; Prado, Watt, & Crowe, Reference Prado, Watt and Crowe2018).
People who are at-risk for developing depression due to the presence of predisposing factors, such as temperament patterns or adverse life events (Malhi & Mann, Reference Malhi and Mann2018), display affective biases that resemble those seen in patients with depressive disorders, and such biases also improve following treatment with traditional antidepressants (Di Simplicio, Norbury, & Harmer, Reference Di Simplicio, Norbury and Harmer2012). In this context, social isolation and ensuing loneliness, which were exacerbated by the COVID-19 pandemic, have been associated with vulnerability to depression (Blanco, Wall, & Olfson, Reference Blanco, Wall and Olfson2020), possibly via neurobiological mechanisms involving immune-metabolic functions (Pourriyahi, Yazdanpanah, Saghazadeh, & Rezaei, Reference Pourriyahi, Yazdanpanah, Saghazadeh and Rezaei2021). Anomalies in waking salivary cortisol (WSC), a stress hormone that suppresses immunological function, which can be used as a measure of hypothalamic–pituitary–adrenal (HPA) axis functionality, have been consistently linked to depression (Nandam, Brazel, Zhou, & Jhaveri, Reference Nandam, Brazel, Zhou and Jhaveri2019) and correlate with emotional information biases in at-risk states (Le Masurier, Cowen, & Harmer, Reference Le Masurier, Cowen and Harmer2007). However, the combined influence of loneliness and HPA function on cognitive processes has not been formally examined.
Taken together, these observations offer an intriguing perspective to further define the neuropsychological profile associated with statin use. One of the mechanisms by which statins could express an antidepressant effect would be mediated by their activity on the HPA axis, whose correct functioning is tightly related to lipid metabolism, stress, inflammation, and mood regulation in animals and humans (Pellosmaa et al., Reference Pellosmaa, Orsak, Tebbe, Jenney, Das, Thompson and Dougall2015; Pellosmaa & Dougall, Reference Pellosmaa and Dougall2016; Pellosmaa & Dougall, Reference Pellosmaa and Dougall2018). These and other immune-metabolic processes, which may underlie the affective costs of social isolation and loneliness (Pourriyahi, Yazdanpanah, Saghazadeh, & Rezaei, Reference Pourriyahi, Yazdanpanah, Saghazadeh and Rezaei2021), appear influenced by statin administration. An observational study examining the neuropsychological effects of statins compared to several other drugs suggested that the former may be associated with less negative affective bias and reduced learning about reward loss (Gillespie et al., Reference Gillespie, Wigg, van Assche, Murphy and Harmer2022). Findings of two further experimental medicine trials differed, though: seven days of the highly potent, moderately lipophilic atorvastatin worsened negative affective bias (De Giorgi et al., Reference De Giorgi, Martens, Rizzo Pesci, Cowen and Harmer2021), while seven days of the highly lipophilic, moderately potent simvastatin only mildly improved it (De Giorgi et al., Reference De Giorgi, Waters, Pesci, Rosso, Cowen and Harmer2022). Equally, a negative effect on these neuropsychological processes would constitute an important neuropsychiatric safety signal for statins; for example, previous studies of the medication rimonabant (Horder et al., Reference Horder, Cowen, Di Simplicio, Browning and Harmer2009; Horder et al., Reference Horder, Browning, Di Simplicio, Cowen and Harmer2012; Horder, Harmer, Cowen, & McCabe, Reference Horder, Harmer, Cowen and McCabe2010) led to withdrawal from the market after numerous reports of suicidal behavior (Sam, Salem, & Ghatei, Reference Sam, Salem and Ghatei2011). Such discrepancies may be disentangled by investigating the neuropsychological effects of longer periods of statin treatment in individuals at risk for depression – such as those who have been exposed to high levels of social isolation and loneliness during the COVID-19 pandemic.
To test this hypothesis, we designed the current experimental medicine trial: the Oxford simvaSTatin and Emotional Processing (OxSTEP) study, which assesses the effects of 28-day simvastatin administration versus placebo on emotional processing, reward learning, working memory, and WSC in people who are at-risk for depression due to high levels of loneliness (Waters et al., Reference Waters, De Giorgi, Quinton, Gillespie, Murphy, Cowen and Harmer2023). In view of the evidence presented above, we hypothesized that longer-term simvastatin use in a group of predisposed participants may lead to positive changes on emotional processing and, secondarily, on reward learning and working memory, while also lowering WSC concentrations.
Methods
Ethics and study design
OxSTEP is an online, double-blind, parallel groups, randomized, gender-stratified, placebo-controlled, experimental medicine study approved by the University of Oxford Central University Research Ethics Committee (MS-IDREC R73946/RE001). The study protocol, which describes the methods in full, was prospectively registered on ClinicalTrials.gov (NCT04973800) and previously published (Waters et al., Reference Waters, De Giorgi, Quinton, Gillespie, Murphy, Cowen and Harmer2023). Members of the Oxford Health NIHR Biomedical Research Centre patient and public contributor pool with lived experience of depression were recruited during development of the study to consult remotely on the study population, design, and protocol. Further details of such discussions can be found in the study protocol.
This trial leveraged a novel approach to experimental medicine that involved a wholly online, remotely conducted design to resolve the recruitment and retention challenges due to the COVID-19 pandemic. This approach was also motivated by the lived experience consultation, which highlighted the increased accessibility and inclusivity that enrolling participants at a UK-nationwide level enables and the likely increase in sample diversity.
Participants
Complete inclusion and exclusion criteria are in Supplementary Material, S1. Briefly, we aimed to recruit an overall healthy adult population (i.e. 21–65 year old, no medical or psychiatric illness, no regular medications) for whom it was safe to take the study medication and who were considered at-risk for developing depression due to a score ≥ 6 on the UCLA 3-item Loneliness Scale (Hughes, Waite, Hawkley, & Cacioppo, Reference Hughes, Waite, Hawkley and Cacioppo2004), which indicates moderate to severe loneliness (i.e. a conservative score for the risk of depression (Matthews et al., Reference Matthews, Bryan, Danese, Meehan, Poulton and Arseneault2022). The decision to select an at-risk group was guided by the lived experience consultation, which indicated this would (a) be more acceptable to participants in the context of long-term pharmacological intervention and (b) provide results of greater value and impact for patients.
The sample size calculation was based on the primary outcome of accuracy on a facial expression recognition task (FERT), a validated tool to assess emotional processing (Harmer et al., Reference Harmer, O’Sullivan, Favaron, Massey-Chase, Ayres, Reinecke and Cowen2009) – see details in the Outcomes section. Using an online power calculator tool (https://www.sealedenvelope.com/power/continuous-superiority/), we computed that a sample size of 25 participants per study arm would give 0.9 power at a 0.05 significance level to detect changes of the magnitude of those seen in a previous key study using the emotional test battery (EMT) with antidepressant treatment [drug mean 10.64 versus placebo mean 5.96, standard deviation 5.1) (Harmer, Shelley, Cowen, & Goodwin, Reference Harmer, Shelley, Cowen and Goodwin2004)]. To account for potentially reduced sensitivity of online testing compared to face-to-face in a laboratory setting as well as lower predicted effect sizes of statins compared to conventional antidepressants, the calculated sample size was doubled to 50 participants per study arm. Withdrawn participants were replaced, and their data were not included in the analysis.
Intervention/comparator
A researcher uninvolved in the study used an online randomization tool (https://www.sealedenvelope.com/simple-randomiser/v1/lists) to produce a randomization code in blocks of four, which was stored in a sealed envelope in a lockable cabinet. Enrolled participants were randomized to either simvastatin 20 mg, a safe dose used in previous clinical trials of simvastatin in depression (Abbasi et al., Reference Abbasi, Mohammadinejad, Shahmansouri, Salehiomran, Beglar, Zeinoddini and Akhondzadeh2015; Gougol et al., Reference Gougol, Zareh-Mohammadi, Raheb, Farokhnia, Salimi, Iranpour and Akhondzadeh2015), or sucrose placebo, in identical capsules so that both participant and study researcher were masked to the study intervention/comparator. Randomized participants were posted 30 days of simvastatin/placebo to their address, including full written instructions of how and when to take them, and secure pre-paid postal boxes to return to the researchers with their saliva samples. Participants received daily automated text messages, reminding them to take the study capsule, and were required to have weekly contact with the study investigators to confirm adherence.
Outcomes
All measures (i.e. WSC, questionnaires, and neuropsychological tasks) were taken both at baseline (pre-intervention) and after 28–30 days of study treatment (post-intervention). Waking saliva samples were collected by the participant and sent by post, then processed and stored as per standard operating procedure, and finally analyzed at the end of the study for WSC – methods are fully reported in Supplementary Material, S2. Questionnaires included: a bespoke COVID-19-related anxiety questionnaire (C19AQ), the Centre for Epidemiologic Studies-Depression scale (CES-D) (Radloff, Reference Radloff1977), an adapted social isolation scale (ELSA) (Bu, Abell, Zaninotto, & Fancourt, Reference Bu, Abell, Zaninotto and Fancourt2020), the Perceived Deficit Questionnaire (PDQ) (Strober et al., Reference Strober, Binder, Nikelshpur, Chiaravalloti and DeLuca2016), the Positive and Negative Affect Scale (PANAS) (Watson, Clark, & Tellegen, Reference Watson, Clark and Tellegen1988), a bespoke side effects questionnaire (SEQ), the Snaith-Hamilton Pleasure Scale (SHAPS) (Snaith et al., Reference Snaith, Hamilton, Morley, Humayan, Hargreaves and Trigwell1995), and the State–Trait Anxiety Inventory (STAI) (Spielberger, Gorsuch, & Lushene, Reference Spielberger, Gorsuch and Lushene1970). Feasibility outcomes for the remote design included participant dropout rate and adherence, successful completion of study tasks, and overall data quality and demonstration of expected main effect of condition across the tasks. Neuropsychological tasks involved the Oxford ETB (O-ETB) for emotional processing, including the FERT (with accuracy as the study primary outcome), the emotional categorization task (ECAT), and the emotional recall task (EREC) (Harmer et al., Reference Harmer, O’Sullivan, Favaron, Massey-Chase, Ayres, Reinecke and Cowen2009), the probabilistic instrumental learning task (PILT) for reward learning (Pessiglione et al., Reference Pessiglione, Seymour, Flandin, Dolan and Frith2006), the N-back task (NBT) for working memory (Redick & Lindsey, Reference Redick and Lindsey2013). These tasks had been made suitable for remote online testing on a data protection-compliant platform and had also been previously validated in a similar study with observational design (Gillespie et al., Reference Gillespie, Wigg, van Assche, Murphy and Harmer2022). A full description of the neuropsychological tasks is in Supplementary Material, S3.
Study procedures
The study procedures are depicted in Supplementary Material, S4. Recruitment involved online adverts, whereby interested volunteers could access the Participant Information Sheet for the study. They were asked to complete a short pre-screening form, namely an online questionnaire referring to the inclusion/exclusion criteria (Supplementary Material, S1). Those who preliminarily met such criteria were offered a pre-screening call with a researcher, where key criteria were re-checked and informed consent was obtained via an online form.
Potential participants then had a video-call screening session with a study medic who collected information about physical and mental health history, including use of medications, using the Structured Clinical Interview for DSM-5 (SCID-5) (First, Reference First, Williams, Karg and Spitzer2016) to confirm eligibility.
Enrolled participants were sent a secure parcel to their address, which included the study medications, the kits for collecting saliva samples, and instructions for all the above. They were also asked to contact the study researchers once their parcel was received. At the point of contact, a researcher called them to confirm the understanding of the study procedures and to schedule the research testing dates. Specifically, participants had to complete a remote baseline session, followed by 28–30 days of study medication, and then by a remote final day session. This structure was expressly designed to enable participants to arrange both sessions around their own commitments.
On an agreed initial day of research testing (pre-intervention), enrolled participants collected baseline waking cortisol saliva: one sample every 15 minutes to a total of four (Supplementary Material, S2). They then completed online questionnaires and neuropsychological tasks over about 1.5 hours, with a study researcher readily available over the phone to provide support as needed. On that evening, participants started taking the study medication, which they continued for 28–30 days (see Intervention/Comparator).
A second research testing session was scheduled after 28–30 days of study medications (post-intervention). On that morning, participants took their waking cortisol saliva samples again, then they repeated the questionnaires and a second version of the neuropsychological tasks. Although most studies previously employing these tests have used an entirely between-subject design (i.e. no pre-intervention versus post-intervention testing), these neuropsychological tasks have been shown to be robust to the effects of test–retest reliability (Thomas, Higgs, & Dourish, Reference Thomas, Higgs and Dourish2016). Therefore, outcomes were measured before and after study treatment to account for a more heterogenous cohort.
To measure the success of intervention/comparator masking, participants were asked to guess whether they were taking simvastatin or placebo.
Statistical analysis
All data processing, analyses, and visualization were conducted using R (version 4.3.2, 2023), specifically using packages lmer, rstatix, report, and brms.
Participants’ socio-demographic characteristics and baseline loneliness scores (UCLA) were reported descriptively. WSC and repeated questionnaires (C19-AQ, CES-D, PANAS, SEQ, SHAPS, and STAI-state subscale) were analyzed via repeated measures analysis of variance (ANOVA) with group (simvastatin versus placebo) as the between-subject factor and time (pre-intervention versus post-intervention) as the within-subject factor. Feasibility outcomes for the remote design (participant dropout rate and adherence, successful completion of study tasks, and overall data quality and demonstration of expected main effect of condition across the tasks) were reported descriptively. For all neuropsychological tasks, data distributions were visually checked using boxplots, and data were excluded when variables were three standard deviations above or below the mean or when total accuracy was below 20%, as per previous studies (Gillespie et al., Reference Gillespie, Wigg, van Assche, Murphy and Harmer2022). The resulting data were analyzed using repeated measures ANOVA with group as the between-subject factor and emotion/valence (O-ETB), win or loss (PILT), and trial condition (N-back) as the within-subject factors, and baseline values included as a covariate. Significant interactions were followed up using simple main effect analyses. When assumptions of equality of variances were not fulfilled, the Greenhouse–Geisser correction was used. Partial eta squared (η2) was reported for the main significant comparisons as a measure of effect size (η2 = 0.01, small effect; η2 = 0.06, medium effect; η2 = 0.14, large effect). Additionally, we conducted post-hoc Bayesian mixed-effects modelling for the FERT accuracy data (i.e. primary outcome) and computational reinforcement learning modelling for the PILT. Full details of the pre-specified and post-hoc statistical analyses are in Supplementary Material, S5.
Results
Participants enrolment started in July 2021 and was completed in February 2023 – study flow chart in Figure 1. Of the 152 people who were eligible at pre-screening, 121 proceeded to the screening visit. Of these, 14 were excluded as they did not meet eligibility criteria, and 107 participants were randomized. Participant dropout following randomization was 5.6% (N = 2 for simvastatin, N = 4 for placebo).
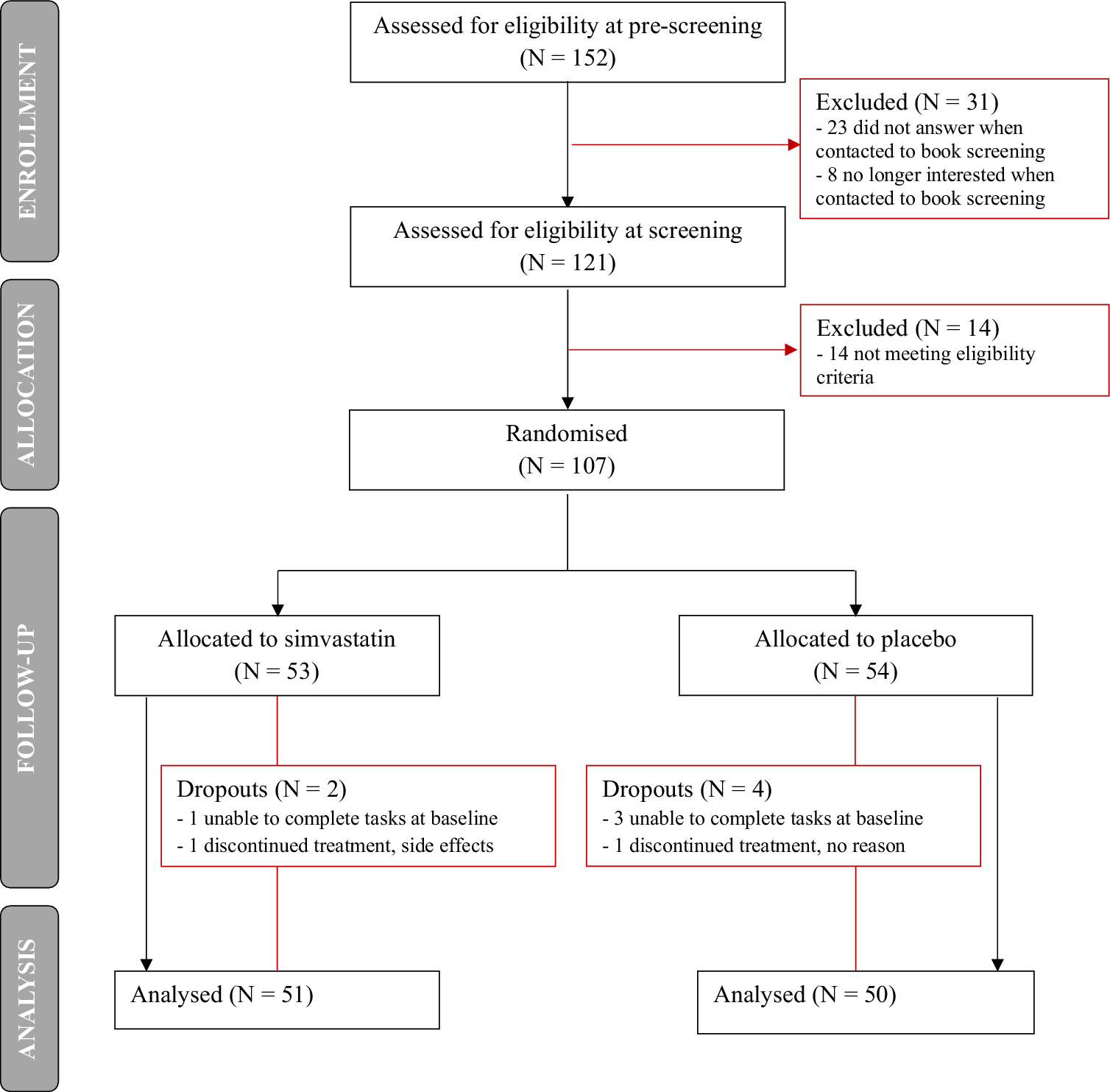
Figure 1. Study flow chart.
Participants’ socio-demographic characteristics and baseline loneliness scores are reported in Table 1: of the final study sample analyzed, 51 participants were randomized to simvastatin (33 females, mean age = 42.5 ± 13.6 years, mean BMI 24.8 ± 2.7) and 50 (33 females, mean age = 43.9 ± 12.5 years, mean BMI 24.4 ± 2.8) to placebo. More people randomly assigned to placebo had undergraduate or postgraduate education (84%) compared to those assigned to simvastatin (65%), and English was the first language for the majority (88%) of the study sample. Average loneliness scores at baseline were comparable between the two groups (mean UCLA 7.2 ± 1.2 for simvastatin, mean UCLA 7.4 ± 1.2 for placebo).
Table 1. Participants’ socio-demographic characteristics and baseline loneliness scores
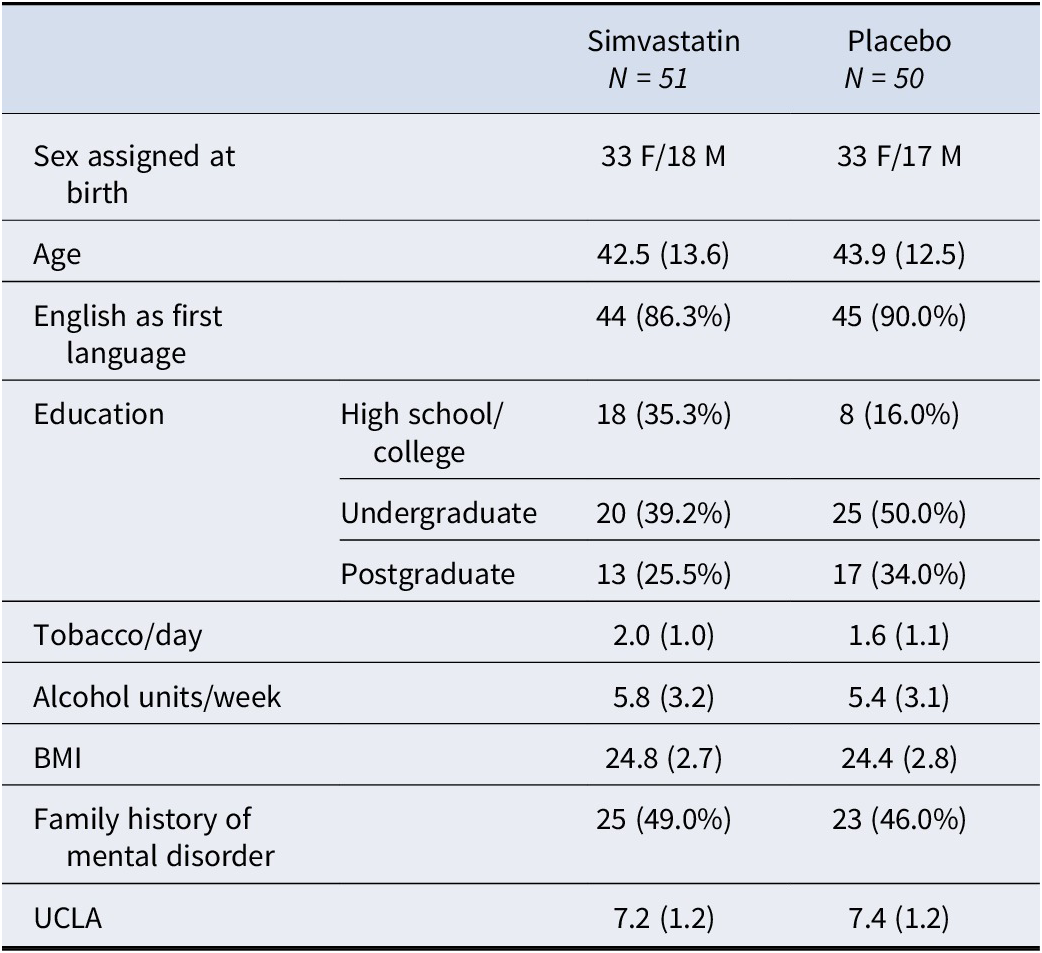
Note: Values are means (standard deviations) but for categorical variables.
Abbreviations: BMI, body mass index; UCLA, loneliness score.
Participants’ baseline (pre-intervention) and final (post-intervention) measures for WSC (averaged across the four samples) and all questionnaires are reported in Table 2: notably, average scores on the CES-D at baseline were above the threshold of 16 for ‘at-risk for clinical depression’ at baseline (17.84 ± 9.57 for simvastatin, 19.12 ± 10.72 for placebo) but not on the final visit (14.33 ± 9.13 for simvastatin, 15.88 ± 11.63 for placebo); however, there was no significant group-time interaction for any of the questionnaires administered or for cortisol (F’s < 1.82, p’s > 0.20). Adequate intervention/comparator masking was achieved (χ2 = 0, df = 1, p = 1.000). Missing data rates (including data excluded for being incomplete, invalid, or an outlier) were minimal (<5% for all but one task - an early version of the N-back task instructions provided to participants included a mistake, sensitivity analysis was used on these participants, N = 10) and comparable to typical in-person experimental studies – details in Supplementary Material, S6. Supplementary figures for main outcomes (i.e. WSC, CES-D, and neuropsychological tasks) are in Supplementary Material, S7.
Table 2. Participants’ baseline (pre-intervention) and final (post-intervention) measures
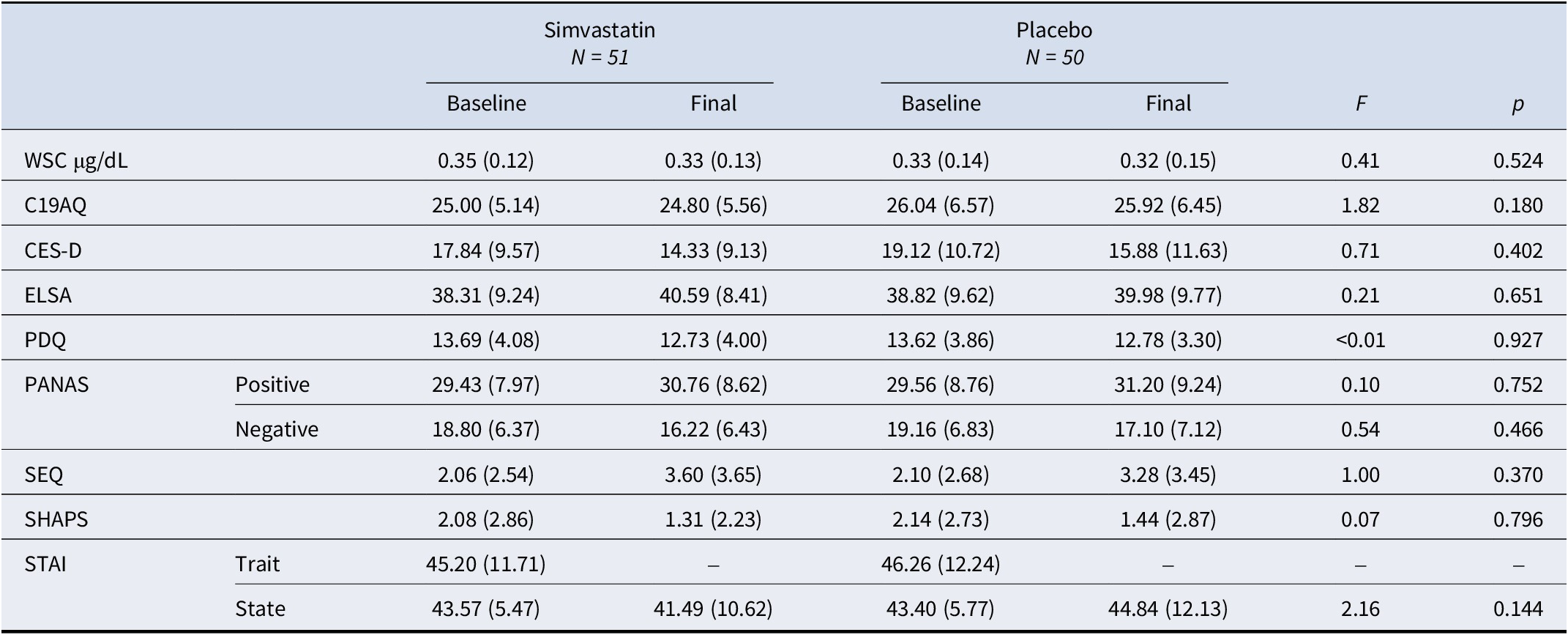
Note: Values are means (standard deviations); F’s indicate between-group interactions on the final day (post-intervention), where baseline scores were used as within-subject factors.
Abbreviations: WSC, waking salivary cortisol; C19AQ, COVID-19-related anxiety questionnaire; CES-D, Centre for Epidemiologic Studies-Depression scale; ELSA, adapted social isolation scale; F, F-statistics; p, p-value; PDQ, perceived deficit questionnaire; PANAS, positive and negative affect scale; SEQ, side effects questionnaire; SHAPS, Snaith-Hamilton Pleasure Scale; STAI, State–Trait anxiety inventory.
A summary of findings for the neuropsychological tasks’ outcomes is in Table 3.
Table 3. Summary of findings for neuropsychological tasks
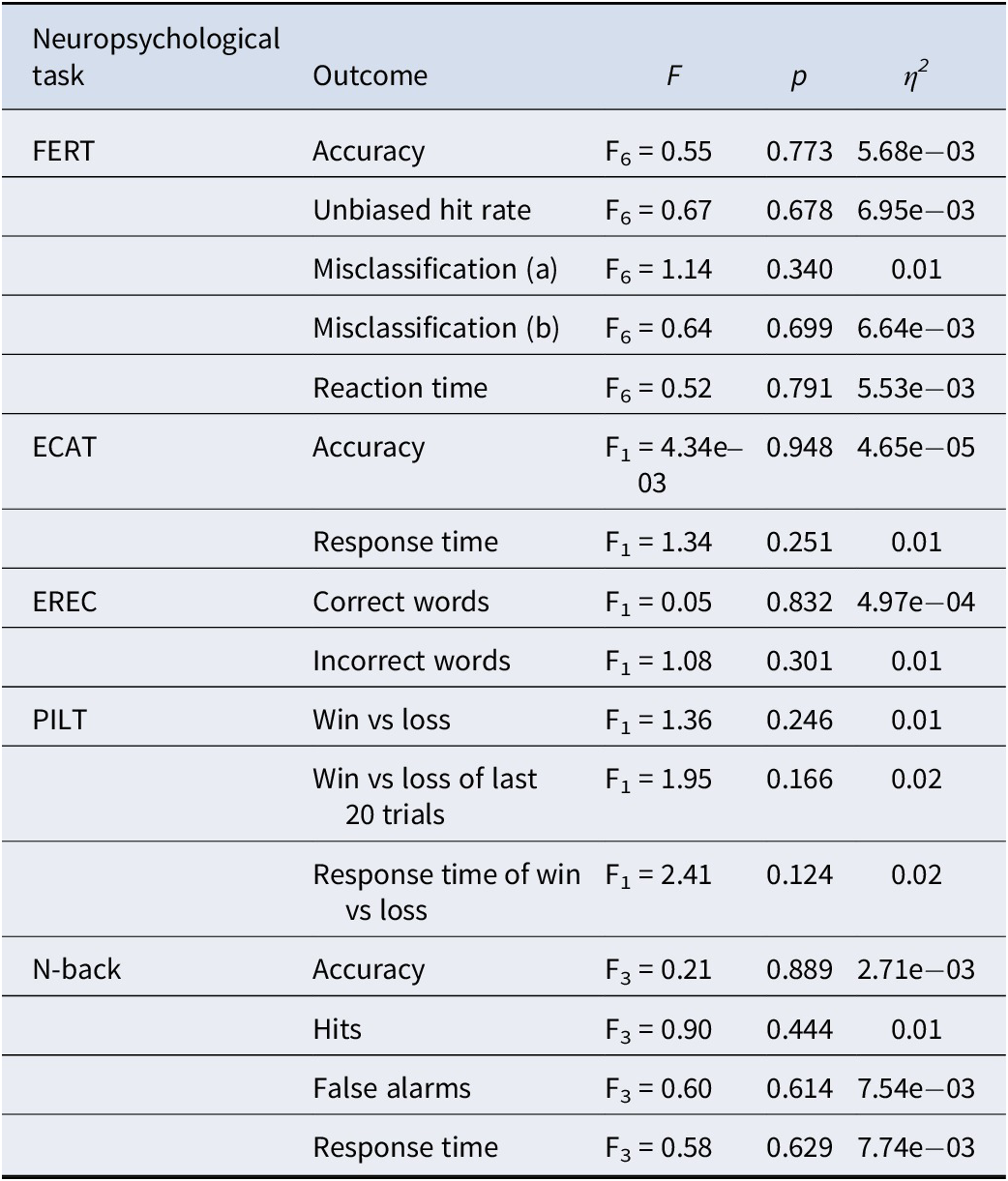
Note: F’s indicate between-group interactions on the final day (post-intervention) with condition as a within-subject factor and baseline scores included as a covariate.
Abbreviations: ECAT, emotional categorization task; EREC, emotional recall task; η2, partial Eta squared; F, F-statistics; FERT, facial expression recognition task; Misclassification (a), misclassify this emotion; Misclassification (b), misclassify this emotion as a different emotion; p, p-value; PILT, probabilistic instrumental learning task.
Oxford emotional test battery (O-ETB, emotional processing)
FERT: As expected, for the primary outcome of percentage accuracy of emotion recognition, the task produced a significant main effect of emotion (F6 = 16.61, p < 0.001; η2 < 0.14). Accuracy was highest for happy faces and lowest for fearful faces, comparable to other similar studies completed in the laboratory (De Giorgi et al., Reference De Giorgi, De Crescenzo, Rizzo Pesci, Martens, Howard, Cowen and Harmer2021; De Giorgi et al., Reference De Giorgi, Waters, Pesci, Rosso, Cowen and Harmer2022).
The interaction between emotion and group was not significant and very small (F6 = 0.55, p = 0.77; η2 < 0.01) – see Figure 2. Moreover, there was no significant group-emotion interaction for any of the other outcome measures (i.e. unbiased hit rate, misclassifications, and reaction time) for this task (F6’s < 1.15, p’s > 0.20, η2’s < 0.01). This remained the same whether controlling for baseline values as a covariate or not.
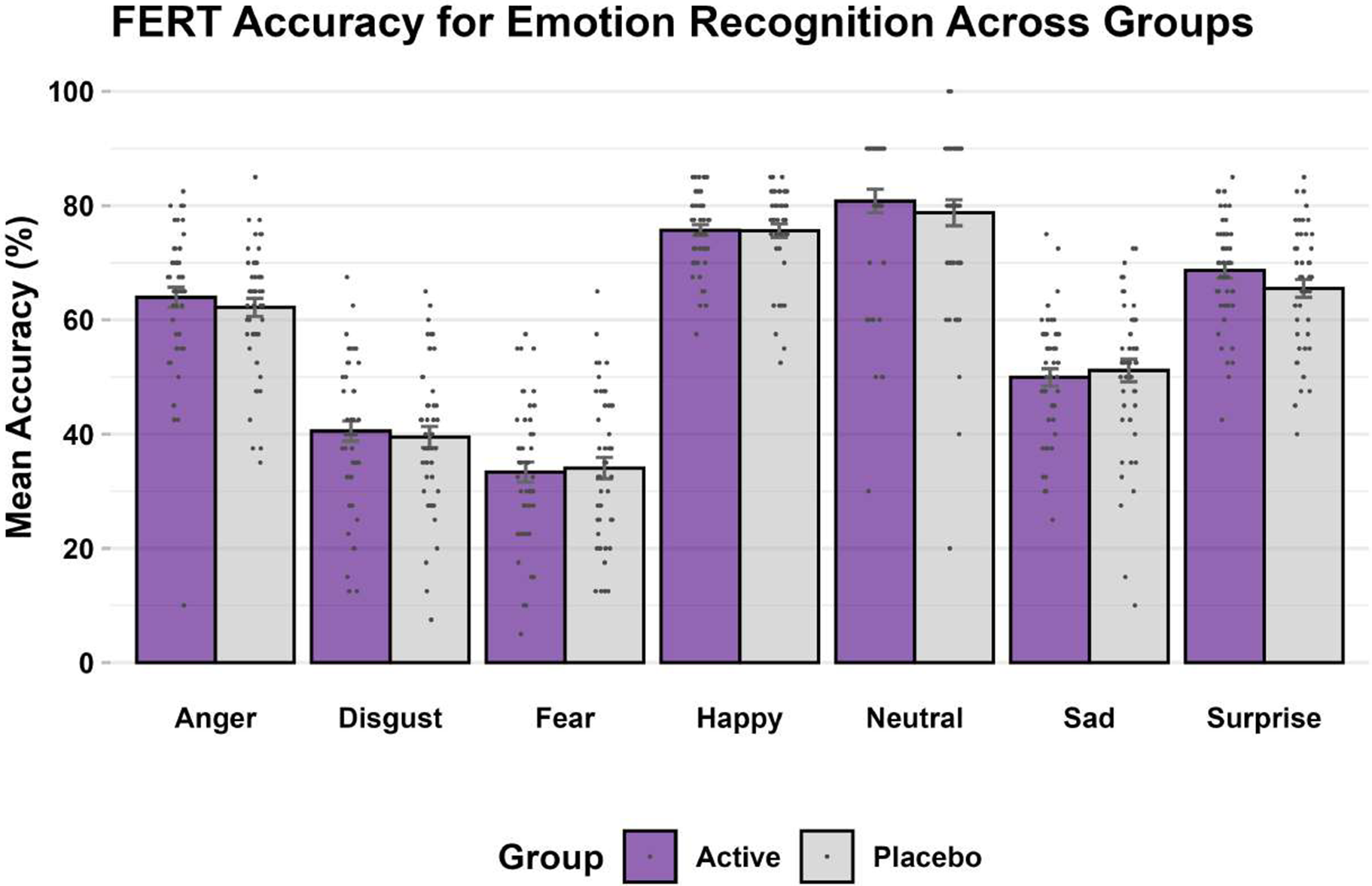
Figure 2. FERT, accuracy (primary outcome) at endpoint.
Note: Bars are mean accuracy scores expressed as percentages (purple: simvastatin, gray: placebo), error bars correspond to standard errors, and dots correspond to individual subject values.
Briefly, findings for the post-hoc Bayesian mixed-effects modelling were consistent with those of frequentist-based modelling (i.e. repeated measure ANOVA) for the FERT accuracy data. Bayes Factor (BF) analyses showed strong evidence for a lack of interaction between group and emotion on unbiased hit rate (BF10 = 0.003). Full details are in Supplementary Material, S8.
ECAT, EREC: As expected, the task produced a significant main effect of valence on EREC intrusions, with participants recalling more positive intrusions than negative intrusions (F1 = 35.33, p < 0.001; η2 = 0.25), in keeping with other studies performed in the laboratory (De Giorgi et al., Reference De Giorgi, De Crescenzo, Rizzo Pesci, Martens, Howard, Cowen and Harmer2021; De Giorgi, Quinton et al., Reference De Giorgi, Quinton, Waters, Cowen and Harmer2022).
There were no significant group-valence interactions for any of the ECAT (i.e. accuracy and response time) and EREC (i.e. correct words and incorrect words) outcomes measured (F1’s < 1.35, p’s > 0.20, η2’s < 0.01).
Probabilistic instrumental learning task (PILT, reward learning)
As expected, participants learned the association between the stimuli for reward and loss across the trials, with participants selecting the stimuli with a high probability of reward 74.8% of the time and selecting the stimuli with a high probability of loss 29.54% of the time across the last 20 trials of each block (De Giorgi et al., Reference De Giorgi, De Crescenzo, Rizzo Pesci, Martens, Howard, Cowen and Harmer2021; De Giorgi, Quinton et al., Reference De Giorgi, Quinton, Waters, Cowen and Harmer2022).
There was no significant effect of simvastatin (F2’s < 2.41, p’s > 0.20, η2’s < 0.01) on any of the outcome measures (i.e. win versus loss, win versus loss last 20 trials, win versus loss response time). Post-hoc computational reinforcement learning modeling did not materially change these results, as shown in Supplementary Material, S8.
N-back task (NBT, working memory)
As expected, there was a significant main effect of condition, with working memory performance reducing as task demands increased (F3 = 116.03, p < 0.001; η2 = 0.59) (Gillespie et al., Reference Gillespie, Wigg, van Assche, Murphy and Harmer2022). However, no significant differences between the simvastatin and placebo groups were identified (F3’s < 0.90, p’s > 0.20, η2’s < 0.01) for any of the outcomes measured (i.e. accuracy, hits, false alarms, and response time).
Discussion
In this study of a sample of people at risk for depression due to high levels of loneliness, we investigated the effects of 28-day simvastatin on a battery of neuropsychological tests, questionnaires, and WSC, using an innovative remote design for conducting experimental medicine trials. This design was approved by the University of Oxford Central University Research Ethics Committee (MS-IDREC R73946/RE001) as an experimental medicine study in the United Kingdom; the safety of the participants was ensured via extensive medical and psychiatric screening and availability of a medic on-call as required. The study completion rate, overall task adherence and data quality, and evidence of anticipated main effects of task condition were comparable to those seen for in-person laboratory studies (De Giorgi et al., Reference De Giorgi, De Crescenzo, Rizzo Pesci, Martens, Howard, Cowen and Harmer2021; De Giorgi, Quinton et al., Reference De Giorgi, Quinton, Waters, Cowen and Harmer2022). This suggests the remote design was successful and comparable to in-person administration. In a large sample (101 participants), there was no significant effect of simvastatin administration compared with placebo on measures of emotional processing, reward learning, and working memory, nor on any of the questionnaires and WSC levels. In particular, accuracy in FERT, the study’s primary outcome, was not affected by simvastatin use, and this null result was confirmed by post-hoc Bayesian analysis. Similarly, other cognitive tasks assessing ECAT, EREC, PILT, and working memory capacity (N-back) did not reveal significant differences between the two groups. WSC response and self-reported measures of mood/affect, anxiety, and other psychosocial functions (C19AQ, CES-D, ELSA, PDQ, PANAS, SHAPS, and STAI-s) also did not show significant group-time interactions, suggesting that simvastatin does not alter waking cortisol or meaningfully alter mood. Taken together, these findings indicate that 28-day administration of simvastatin does not have the hypothesized positive effects on emotional and other cognitive processes, nor on cortisol regulation, in individuals likely at risk for depression.
The absence of significant effects observed in OxSTEP contrasts with prior studies that suggested statins might affect negative emotional biases or influence reward learning (De Giorgi et al., Reference De Giorgi, De Crescenzo, Rizzo Pesci, Martens, Howard, Cowen and Harmer2021; De Giorgi, Quinton et al., Reference De Giorgi, Quinton, Waters, Cowen and Harmer2022; Gillespie et al., Reference Gillespie, Wigg, van Assche, Murphy and Harmer2022). Previous experimental medicine trials had shown mixed results regarding the effects of statins on mood and cognition, with shorter statin regimens indicating modest changes in emotional processing (De Giorgi et al., Reference De Giorgi, De Crescenzo, Rizzo Pesci, Martens, Howard, Cowen and Harmer2021; De Giorgi, Quinton et al., Reference De Giorgi, Quinton, Waters, Cowen and Harmer2022). The rationale for this study indeed stemmed from the assumption that a longer administration period of 28 days would be more effective, especially in individuals vulnerable to depression due to loneliness. Our findings, however, align with more recent literature (De Giorgi et al., Reference De Giorgi, De Crescenzo, Cowen, Harmer and Cipriani2023; De Giorgi, Cowen, & Harmer, Reference De Giorgi, Cowen and Harmer2022; De Giorgi et al., Reference De Giorgi, Quinton, Waters, Cowen and Harmer2022; Hang et al., Reference Hang, Zhang, Li, Li, Zhang, Ye and Sun2021; Lee et al., Reference Lee, Peng, Lee, Wang, Lee, Chen and Shiang2021; Zhang et al., Reference Zhang, Bao, Tao, Zhao and Liu2022), and especially with the largest, most robust clinical trial to date (Husain et al., Reference Husain, Chaudhry, Khoso, Kiran, Khan, Ahmad and Young2023), which questions any direct antidepressant potential of statins. We used a relatively low dosage of 20 mg simvastatin, which is in line with previous trials investigating this medication in depression (Abbasi et al., Reference Abbasi, Mohammadinejad, Shahmansouri, Salehiomran, Beglar, Zeinoddini and Akhondzadeh2015; Gougol et al., Reference Gougol, Zareh-Mohammadi, Raheb, Farokhnia, Salimi, Iranpour and Akhondzadeh2015; Husain et al., Reference Husain, Chaudhry, Khoso, Kiran, Khan, Ahmad and Young2023), but it is possible that higher doses are needed to see an effect on behavioral tasks. The immune-metabolic model of depression suggests that factors like inflammation and lipid metabolism may play a significant role in mood regulation, but this trial does not support the notion that statins can independently ameliorate depressive symptoms or cognitive biases in at-risk populations with short-term treatment. Still, it remains possible that any presumed antidepressant activity of statins in clinical practice, as proposed by other studies (De Giorgi et al., Reference De Giorgi, De Crescenzo, Rizzo Pesci, Martens, Howard, Cowen and Harmer2021; Molero et al., Reference Molero, Cipriani, Larsson, Lichtenstein, D’Onofrio and Fazel2020; Xiao et al., Reference Xiao, Deng, Li, Sun and Tian2023; Yang et al., Reference Yang, Yang, Zeng, Jia and Sun2024), might relate to neural pathways that are not accounted for by the neuropsychological tasks employed in OxSTEP or that would require exploration via more sensitive measures on functional magnetic resonance imaging (fMRI) (Godlewska & Harmer, Reference Godlewska and Harmer2021). Further, the benefit of statins may only be evident in older populations (Gillespie et al., Reference Gillespie, Wigg, van Assche, Murphy and Harmer2022; Redlich et al., Reference Redlich, Berk, Williams, Sundquist, Sundquist and Li2014), compared to our relatively young sample, who are the most likely to be prescribed these drugs for cardiovascular and metabolic ailments in an observational setting and who might present with age-related higher baseline inflammation (Ferrucci & Fabbri, Reference Ferrucci and Fabbri2018).
Conversely, the lack of negative effects on any of the assessed neuropsychological domains or questionnaires for mood/anxiety provides reassurance against previously described adverse effects of statins on emotional and cognitive processes and disorders (Leutner et al., Reference Leutner, Matzhold, Kautzky, Kaleta, Thurner, Klimek and Kautzky-Willer2021; Rosoff et al., Reference Rosoff, Bell, Jung, Wagner, Mavromatis and Lohoff2022; Taylor & Adhikari, Reference Taylor and Adhikari2025; van der Heijden & Houben, Reference van der Heijden and Houben2023), including suicidality (Zureik, Courbon, & Ducimetière, Reference Zureik, Courbon and Ducimetière1996) – as it had been the case instead with rimonabant (Horder et al., Reference Horder, Cowen, Di Simplicio, Browning and Harmer2009; Horder et al., Reference Horder, Browning, Di Simplicio, Cowen and Harmer2012; Horder, Harmer, Cowen, & McCabe, Reference Horder, Harmer, Cowen and McCabe2010). Again, these results are in line with more recent evidence advocating the safety of statins in people with mental illness (De Giorgi et al., Reference De Giorgi, De Crescenzo, Ostinelli, Cowen, Harmer, Fazel and Cipriani2024; Ye et al., Reference Ye, Blais, Ng, Castle, Hayes, Wei and Chan2023), thus supporting ongoing prescribing of these medications in this population at high risk for cardiovascular morbidity and mortality (Rajan et al., Reference Rajan, McKee, Rangarajan, Bangdiwala, Rosengren, Gupta and Yusuf2020).
Limitations
Several limitations may have contributed to the null findings of the OxSTEP study.
First, the remote study design may lead to possible issues in ensuring consistent participant engagement and precise measurement of outcomes in neuropsychological tasks. At-home testing introduces a less rigorously controlled environment and may result in different emotional states that introduce potential biases when compared with in-person testing. Nevertheless, this novel approach had been previously validated in an observational study (Gillespie et al., Reference Gillespie, Wigg, van Assche, Murphy and Harmer2022), and the current study had the additional benefit of participants being supported by a study researcher via phone during the testing sessions. All data quality checks indicated anticipated effects of task condition and minimal data loss due to poor performance. Moreover, this setup allowed us to reach a UK-wide, more geographically diverse population, hence potentially producing more generalizable results as suggested for this kind of study (Clark et al., Reference Clark, Watkins, Piña, Elmer, Akinboboye, Gorham and Regnante2019). Other advantages reported by our patient and public involvement and engagement group included avoidance of unnecessary travel by study participants, which saved time and money and further reduced barriers to participation .
Another particular challenge with remote studies, which is partly shared by in-person studies, is ascertaining whether participants have taken the medications as prescribed. There may be advantages in using independent measures of concordance, for example, salivary drug concentration or similar biomarkers. Indeed, lipophilic statins such as simvastatin have been reported to increase plasma cortisol levels (Sahebkar, Rathouska, Simental-Mendía, & Nachtigal, Reference Sahebkar, Rathouska, Simental-Mendía and Nachtigal2016), but no such effect was observed in the WSC measures obtained in this study. Although all participants self-reported a strict compliance with daily medication intake and this was aided by daily text reminders, we did not have any other objective, independent measure of compliance.
Second, as study recruitment occurred between July 2021 and February 2023 across several unpredictable waves of COVID-19 lockdowns and subsequent reopening, we could not account for the effects that these may have had on our findings, while the remote design allowed for this study to be resilient to changes in government policies as well as participants’ infections. We did not serially record participants loneliness scores but rather utilized this as a screening questionnaire, as those meeting the cut-off were presumed ‘at-risk’ due to loneliness. Due to the subjective nature of loneliness, unpredictability of lockdown measures or potential shielding at the time, we cannot presume that this risk remained stable across the 28 days.
Third, the sample size of 101 participants, despite being well-powered for the primary outcome and significantly larger than earlier studies (De Giorgi et al., Reference De Giorgi, De Crescenzo, Rizzo Pesci, Martens, Howard, Cowen and Harmer2021; De Giorgi, Quinton et al., Reference De Giorgi, Quinton, Waters, Cowen and Harmer2022), may have lacked the statistical power to detect more subtle cognitive or emotional effects of simvastatin, especially in a healthy population at risk for depression but without active symptoms. Furthermore, the inclusion criteria targeting loneliness as a risk factor for depression do not fully capture the heterogeneity of depression vulnerability (Malhi & Mann, Reference Malhi and Mann2018). Indeed, loneliness is a complex construct, and its relationship with emotional processing might require longer periods of intervention than the 28-day simvastatin administration.
Lastly, the study measured WSC as an indicator of HPA axis function, but other markers of immune-metabolic response were not assessed due to the constraints of the remote study design. Since statins exert anti-inflammatory effects that might influence mood via pathways beyond cortisol regulation (Pellosmaa et al., Reference Pellosmaa, Orsak, Tebbe, Jenney, Das, Thompson and Dougall2015; Pellosmaa & Dougall, Reference Pellosmaa and Dougall2018), the absence of such measures could have limited the understanding of the broader biological impacts of simvastatin. Additionally, statins would also affect lipid metabolism with possible consequences on brain functioning (Pellosmaa et al., Reference Pellosmaa, Orsak, Tebbe, Jenney, Das, Thompson and Dougall2015; Pellosmaa & Dougall, Reference Pellosmaa and Dougall2018), but this was not assessed in this study.
Conclusions
In conclusion, 28-day administration of simvastatin did not lead to changes in emotional processing, reward learning, working memory, or WSC levels in individuals likely at-risk for depression due to loneliness. These findings contrast with earlier studies that suggested potential mood benefits of statins but are consistent with more recent literature refuting their independent efficacy in the treatment or prevention of depressive episodes. The lack of adverse neuropsychological mechanisms adds to the evidence base supporting the safety, but not efficacy, of statins in people with mental disorders. A definitive answer regarding any potential antidepressant effect of statins may come from further research testing these medications in people with a clinical diagnosis of depression and raised markers of immune-metabolic dysfunction (Otte et al., Reference Otte, Chae, Nowacki, Kaczmarczyk, Piber, Roepke and Gold2020). Meanwhile, suboptimal prescribing of statins in people with depressive disorders due to concerns around adverse neuropsychiatric effects (Ljung, Köster, Björkenstam, & Salmi, Reference Ljung, Köster, Björkenstam and Salmi2021; Magin et al., Reference Magin, Quain, Tapley, van Driel, Davey, Holliday and Hilmer2021; Park, Tickle, & Cutler, Reference Park, Tickle and Cutler2022; Ter Braake et al., Reference Ter Braake, Fleetwood, Vos, Blackbourn, McGurnaghan, Wild and Jackson2024; Vinuesa-Hernando et al., Reference Vinuesa-Hernando, Aguilar-Palacio, Rabanaque, García-Cárdenas, Lallana, Gamba and Malo2024) should be avoided.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0033291725001187.
Data availability statement
The data produced by this study will be available from the corresponding author upon reasonable request.
Acknowledgements
Our sincere thanks to the participants who have volunteered for this study, the patient and public contributors who advised us on the study design (including Roger Ede and Jane Turner), and the department administrators who helped us at short notice with various requests during the COVID-19 pandemic and beyond.
Author contribution
The study concept and design were developed by RDG, AMGQ, ALG, SEM, PJC, and CJH. The online testing sessions were developed by AMGQ and ALG. SW and RDG were responsible for participant recruitment and management, as well as data collection. Analyses were conducted by SW, ALG, and RDG, post-hoc analyses by MJC. MJC, SEM, PJC, and CJH supported the interpretation of the findings. RDG prepared the first draft of the manuscript. All authors critically revised the manuscript and approved the submitted version.
Funding statement
This study was funded by the Wellcome Trust (grant: 216452/Z/19/Z award: 102176/Z/13/Z to RDG) and the Medical Research Council (grant: MR/K022202 to PJC), and it has been delivered through the National Institute for Health and Care Research (NIHR) Oxford Health Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the Wellcome Trust, the Medical Research Council, the NIHR, the NHS, or the Department of Health and Social Care.
Competing interests
CJH has received consultancy fees from P1vital, Janssen, Sage Pharmaceuticals, Zogenix, Pfizer, and Lundbeck outside of the current work. ALG has received consultancy fees from Zogenix. SEM has received consultancy fees from Janssen Pharmaceuticals, Zogenix, and Sumitomo Dainippon Pharma. CJH and SEM hold grant income from UCB Pharma, Janssen Pharmaceuticals, Zogenix, and Pfizer. The other authors declare that they have no conflict of interest.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







