Introduction
In 2023, the World Health Organization determined that while the COVID-19 pandemic no longer fit the definition for a public health emergency of international concern, the virus remains well established globally and the pandemic continues [1]. In 2024, between 31500 and 265000 infections and 500 to 4300 deaths due to COVID-19 were reported from across the globe to the World Health Organization every week [2]. Given the ongoing nature of this pandemic, understanding the factors associated with infection, including vaccinations, continues to be pertinent.
A healthy workforce is essential to maintaining our health care systems. As healthcare workers (HCWs) are at higher risk for acquiring or transmitting infectious diseases due to the nature of their jobs, they are a priority for research and prevention efforts. In one study, the odds of being diagnosed with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were 1.62 to 1 for Canadian HCWs compared with the general public [Reference Galarneau3]. HCWs were not only more likely to be tested and diagnosed with SARS-CoV-2 [Reference Waldner4], studies have determined that household members living with HCWs were at higher risk of contracting SARS-CoV-2 than the general public, likely because they were exposed to an infected HCW [Reference Shah5–Reference Carazo8]. On the other hand, 10–18% of SARS-CoV-2 infections among HCWs were likely acquired within their household, making them a potential source of infection for their patients and coworkers [Reference Elgersma7, Reference Carazo8].
Vaccination has proven to be an effective means of protecting people from being infected with SARS-CoV-2 and of reducing the severity of symptoms in those who did become ill [Reference Fischer9]. However, the effectiveness of vaccines has varied depending on the match between the vaccine and the circulating variant as well as the time since vaccination [Reference Wu10, Reference Zhou11]. Authors of a series of papers from the SARS-CoV-2 Immunity and Reinfection Evaluation (SIREN) study of HCWs in the United Kingdom reported vaccine effectiveness (VE) estimates for receipt of two doses of mRNA vaccines that ranged from 50–89% for December 2020 to September 2021 [Reference Hall12] while the receipt of a third dose of vaccine was associated with relative VE estimates of 63% for September to November 2021 and 21–35% for December 2021 to February 2022 [Reference Hall13]. Between September 2022 and March 2023, the relative VE of a fourth, compared with a third, dose of vaccine was 13% [Reference Kirwan14], while the relative VE of a booster dose in October 2023 was 27% [Reference Kirwan15].
Correlates of protection that are easily obtained and can be efficiently analyzed are necessary to estimate the effectiveness of vaccines in a short period [16] (e.g., for new vaccine evaluation) and to evaluate populations or subgroups within populations for their immune status, either vaccine- or naturally-acquired. Antibodies against SARS-CoV-2, including immunoglobulin G (IgG) anti-spike and anti-receptor binding domain (RBD) of the spike protein, are induced by vaccination while infection induces a general response against several viral proteins, including the nucleocapsid protein. The RBD is a target for antibody-mediated neutralization, making it a good choice for serological assays for SARS-CoV-2 [Reference Martínez-Barnetche17]. Some studies have shown that people with high levels of SARS-CoV-2 IgG anti-spike and anti-RBD antibodies had significantly lower risks of infection compared with people with lower levels [Reference Spiteri18, Reference Seekircher19], while other studies reported no association [Reference Baratto20, Reference Erice, Prieto and Caballero21].
In this analysis, we investigate VE and other factors associated with SARS-CoV-2 infection among Canadian HCWs who participated in a cohort study between June 2020 and December 2023. We also investigate the association between antibodies to SARS-CoV-2 using IgG antibodies to the RBD of the spike protein and rate of SARS-CoV-2 infection in the participants who provided blood samples.
Methods
The Canadian COVID-19 Cohort Study was a 41.5-month prospective cohort study conducted with HCWs recruited from acute care hospitals and private practices/clinics in Canada. Funding availability resulted in rolling enrolment that occurred first in Toronto acute care hospitals in June 2020 (Sinai Health, Sunnybrook Health Sciences Centre, Michael Garron, and North York General hospitals) followed by University Health Network, Unity Health Toronto, Markham-Stouffville hospital, and HCWs from private practices/clinics. In 2021, participants were enrolled from William Osler Health, Hamilton Health Sciences Centre and St. Joseph’s Healthcare Hamilton, and the Ottawa Hospitals (Ontario); Grey Nuns, Royal Alexandra, the University of Alberta, and Alberta Children’s hospitals (Alberta); IWK Health Centre and QEII hospitals (Halifax); and the Centre Hospitalier Universitaire de Sherbrooke (Québec). Participants were enrolled until 1 June 2023 and followed until they withdrew or 1 December 2023, whichever occurred first. Local research ethics review boards approved the study prior to enrolment of participants at each site. Supplementary Table S1 provides more information about vaccine availability and requirements for HCWs in Canada.
HCWs were eligible for the study if they were 18–75 years old at enrolment, worked at a participating site for >20 h per week for employees or for ≥8 h per week for physicians or nurse practitioners caring for ill patients, and had convenient access to internet-based web pages. For these analyses, participants must have completed a baseline questionnaire and participated for at least 30 days.
Recruitment processes occurred virtually using email invitations, internal hospital communications, and social media platforms (e.g., Facebook, Twitter). Following consent, participants were asked to complete a baseline questionnaire on a secure, study-generated, and -managed electronic data collection site. Subsequently, annual baseline questionnaires were to be completed to capture changes in exposure profiles including personal, household, and workplace factors. Emails were sent to participants every second week (randomized at enrolment, so half of the participants received an email each week) to provide a direct link to a biweekly questionnaire about symptoms in the previous 14 days. If they reported a cough, fever, or shortness of breath, they were automatically directed to complete an illness report, and it was suggested they test for SARS-CoV-2. Every 10th week (randomly assigned at enrolment), participants who had not submitted an illness report in the previous 14 days were linked to a monitoring questionnaire (instead of the biweekly one) to collect information on symptoms and close contact/exposure to anyone with COVID-19. The email also provided a link and asked participants to provide information on COVID-19 vaccines received and illnesses experienced. Reports could be initiated or updated at any time using a previously-provided link to their study web page.
Participants were asked to provide blood samples, either venous blood or dried blood spots (DBS), at enrolment, 30 days after every positive swab result or COVID-19 vaccination, and every 180 days after the most recent of either (swab or vaccination). All samples were tested in the Gingras laboratory using enzyme-linked immunosorbent assays for detecting IgG antibodies against the spike trimer (S1), its RBD, and nucleocapsid proteins. Luminescence values were normalized to a synthetic standard (relative ratio) and converted to binding antibody units (BAU)/mL using the World Health Organization’s international standard [Reference Abe22, Reference Abubakar23]. See Supplementary Table S2 for sample collection and processing details.
The primary outcome was SARS-CoV-2 infection (respiratory swab that tested positive by polymerase chain reaction (PCR) or rapid antigen test (RAT)) occurring between enrolment and end of study/end of participation. Subsequent infections were defined as positive SARS-CoV-2 tests collected ≥60 days after the previous one [Reference Hadley24]. If the report of a positive test was followed within 1 day by a report of a negative test, the positive test was not eligible. Illness reports collected information on dates and results of tests as well as symptoms experienced and known exposures to people with SARS-CoV-2 in the previous 14 days (with or without masks/other protective equipment). Comparator data were selected from (a) illness reports with a negative test result and no symptoms that were >50% complete or (b) monitoring questionnaires in which no symptoms were reported and were >50% complete. The reports or questionnaires that occurred closest to the midpoint between start and stop dates for the observation period were used. Monitoring questionnaires captured the same exposure information as the illness reports (less test date/results).
The stsplit command in Stata SE v18.0 [25] was used to split participants’ records 14 days after each vaccination and positive test that occurred during study participation. To account for days at risk, start time was either the participant’s date of enrolment, 14 days after receipt of a dose of COVID-19 vaccine, or 60 days after a report of a positive test. Stop time was the date of a positive test, 13 days after receipt of a vaccine, or the last day of active study participation. Following the split, demographic and exposure data were matched to each observation period.
Potential exposures included the total number of COVID-19 vaccines received prior to the outcome as recorded in the online vaccination report that collected the name of the vaccine and date received. Receipt of the primary series of vaccines was defined as the receipt of both doses of a two-dose vaccine schedule or one dose of a single dose vaccine. A single dose vaccine was coded as two doses that were received on the same date for consistency in coding the number of doses received. Baseline questionnaires completed prior and most proximal to the outcome provided data on other potential exposures including age, sex, race, chronic disease, immunosuppressive medication/disease, use of eye glasses, use of refillable drink containers, hands-to-face habits, health status, household size, smoking status, level of patient contact, work unit, province of work, and occupation (see Table 1 for details of categories). To assess previous infection, SARS-CoV-2 infections occurring prior to enrolment were captured on the baseline questionnaires, while those occurring during participation (but previous to the outcome under consideration) were captured on illness reports. If a blood sample indicated an infection (i.e., IgG nucleocapsid and spike and/or RBD antibody levels above threshold) that was not reported, the infection was assumed to have occurred midway between blood samples (or midway between enrolment and sampling, if no earlier sample was available). Close contact (within 2 metres for greater than 2 min) to a person with SARS-CoV-2 in the 14 days before the event was reported on illness reports and monitoring questionnaires.
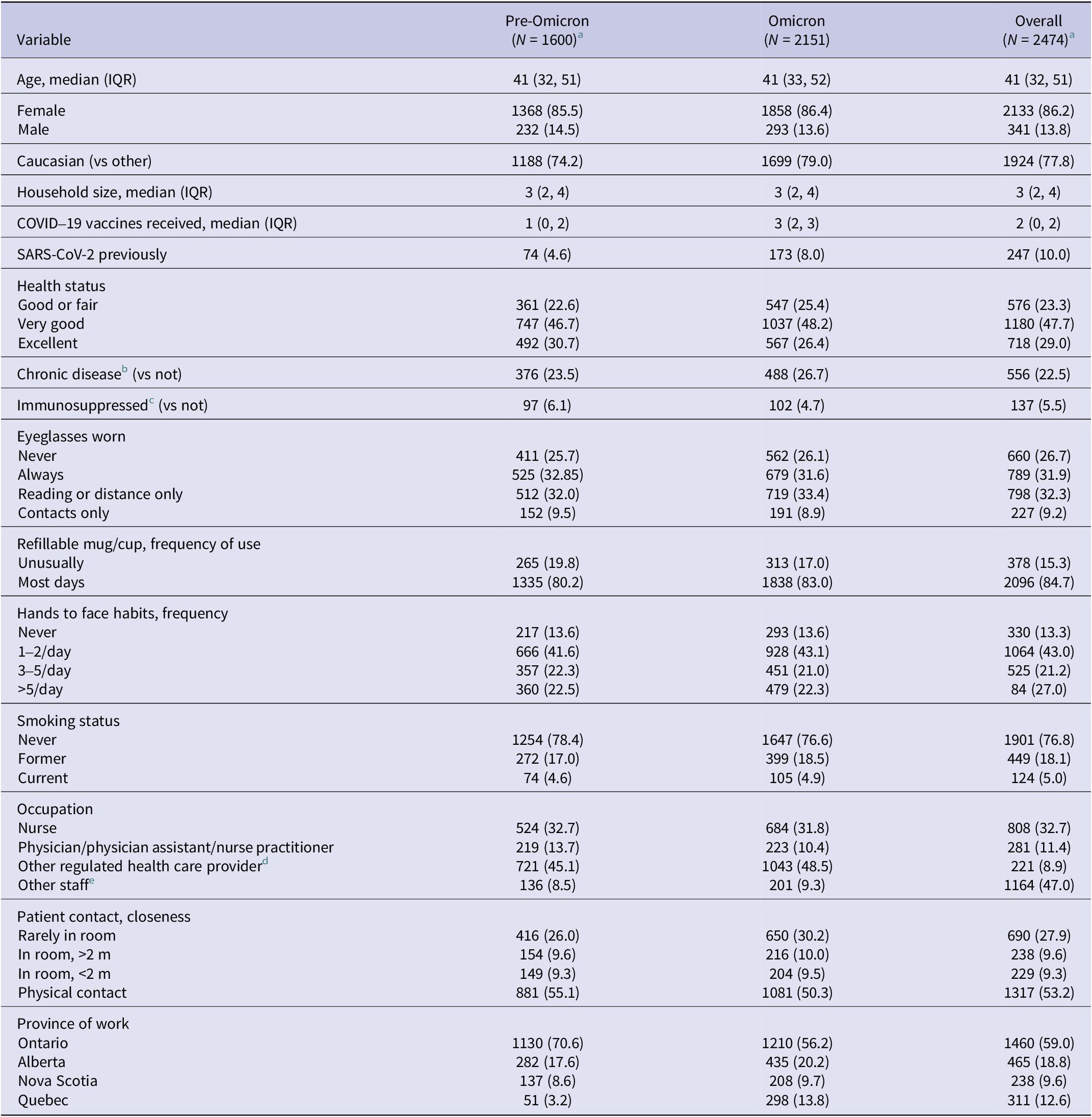
Table 1. Demographic variables of Canadian healthcare workers, 15 June 2020 to 1 December 2023: characteristics at enrolment (pre-Omicron period) or at enrolment/first annual questionnaire completed after 12 December 2021 (Omicron period). Number (per cent) unless otherwise stated
IQR, interquartile range.
a At the time of completion of their first baseline (i.e., at enrolment; enrolment was rolling).
b Asthma, chronic obstructive pulmonary disease or other chronic lung conditions, diabetes, heart disease, cancer treated in the past 5 years, liver or kidney disease, chronic neurological disorder, or other long-term chronic conditions.
c Immunosuppressive medication/treatment or HIV/AIDS.
d Respiratory therapist, laboratory technician, physical therapist, occupational therapist, imaging technician/technologist, pharmacist, pharmacy technician, psychologist, or social worker.
e Food service, ward clerk, administration, healthcare aids, housekeeper, porter, research, or other clinical support.
The weekly estimates of the variants causing illness across Canada, as reported by provinces and territories, were used to determine the periods of variant dominance used in these analyses. In Canada, the Alpha-dominated period started on 2 February 2021, Delta on 27 June 2021, Omicron BA.1 on 12 December 2021, BA.2 on 27 March 2022, BA.4/BA.5 on 26 June 2022, and recombinant variants (XBB, EG.5, others) on 19 February 2023. The rates of hospital admissions for COVID-19, averaged for the period 8 to 14 days prior to the event to account for time between infection and hospital admission, were used as a proxy for the force of infection (local incidence and rates of immunization) [26].
The exposure for the secondary research question was the level of IgG anti-RBD antibodies using binding antibody units (BAU/mL) in blood samples provided most closely preceding the outcome. Due to differences in laboratory procedures, data were provided separately for serum and DBS samples; results of 1235 tests conducted prior to 25 June 2021 were not used in longitudinal analyses as the aggregated BAU/mL values were not comparable with those tested on or after that date due to the addition of extra dilutions.
Analysis
Unadjusted SARS-CoV-2 incidence rates, per 1000 person-days, were summarized by variant-dominant periods and for first versus subsequent infections. Cox regression was used to estimate the association between exposures (vaccination, previous SARS-CoV-2 infection, and other factors) and positive tests for SARS-CoV-2 (either symptomatic or asymptomatic). Due to the likely differences in risk factors over the study period, separate models were conducted for the pre-Omicron (Wuhan, Alpha, and Delta) and Omicron (BA.1, BA.2, BA.4. BA.5, and XBB/EG.5) dominant periods. For the pre-Omicron period, the period of observation started on 24 December 2020, 14 days after the availability of the first COVID-19 vaccines and continued until the beginning of the Omicron period. The Prentice, Williams, and Peterson gap time model analyzes recurrent events through stratification by the number of prior events. Robust standard errors were used to adjust for dependence within participants. This analysis was employed to allow the use of all infections, not just the first one reported [Reference Prentice, Williams and Peterson27]. However, only the first infection was used in the pre-Omicron period because estimates are unstable with small numbers (two re-infections were excluded).
Backward elimination was used to build models using the Bayesian information criteria to assess the best fit. Covariates were assessed for plausible biological interactions (e.g., age and gender). Time-varying covariates (rates of hospitalization and months since vaccination) were assessed and included, as needed, to maintain proportionality. Models were adjusted for variables that were significantly associated with infection at a p-value of <0.10 on either model (pre-Omicron or Omicron). Assessments of the proportional hazards assumption were conducted using the proportional hazards test and graphs of Schoenfeld residuals.
VE estimates were calculated using 1–adjusted hazard ratio (aHR); only relative VE estimates could be calculated during the Omicron period because >99% of HCWs had been vaccinated. All analyses were conducted using Stata SE v18.0 [25].
The secondary research question estimated the association between the levels of IgG anti-RBD antibodies and the hazard of infection using the blood samples collected prior to and most proximal to each event. To allow the use of both types of samples (serum and DBS) in one model, the BAU/mL were categorized into quartiles by sample type [Reference Spiteri18] resulting in one variable. The Prentice, Williams, and Peterson gap time model was used with robust estimates of variance to adjust for repeated infections in the same participant. The models were further stratified by the number of vaccinations received ≥14 days prior to each event and adjusted for: days between blood sample and event, type of sample, previous infection (yes/no), age, gender, race, household size, self-reported health status, province, Canadian rate of hospitalization for SARS-CoV-2 at time of event, occupation, and known exposure to someone with SARS-CoV-2 in the past 14 days. Due to small numbers, participants with four or more vaccines were collapsed together as were participants with no or only one (of a two dose) vaccine. One model was created for the full study period and a separate one for data during the Omicron period using the same methods described for the primary objective. Data were too sparse to create a stable model for the pre-Omicron period.
IgG anti-RBD levels (BAU/mL) for serum and DBS samples tested after 24 June 2021 were depicted separately using box plots by a) the number of doses of vaccine received, limited to samples collected within 14 to 119 days of vaccination, with/without previous infection and b) the number of days between vaccination and blood collection, limited to samples collected ≥14 days after the second or subsequent dose of vaccine, with/without previous infection. Statistical comparisons across categories (vaccine doses or days since vaccination) were made using quantile regression to assess the equality of medians [Reference Conroy28] without adjustment for other variables.
Results
Of the 2474 participants eligible for these analyses, 2133 (86.2%) were female, 32.7% were nurses, 53.2% had direct physical contact with patients, 76.7% were in very good or excellent health at the time of enrolment, and the median age was 41 years (interquartile range [IQR] 32–51). As shown in Table 1, rolling enrolment impacted the number of participants by province and period. For example, 70.6% were from Ontario hospitals in the pre-Omicron period compared with 56.2% in the Omicron period. The median number of days in the study was 731 days (IQR 379–931), 238 days (IQR 110–311) in the pre-Omicron, and 637 days (IQR 274–718) in the Omicron periods. Supplementary Table S3 provides the number of participants, drop-out rates, SARS-CoV-2 infections, and blood samples collected by month.
The uptake of COVID-19 vaccines in this cohort of HCW was high; by the end of the pre-Omicron period (11 December 2021), 99.3% of participants had received either a primary series (hereafter referred to as two doses) or three doses of vaccine. Of those who were participating at the end of the study (1 December 2023), only 14.7% had received two doses of vaccine; 38.2% had received three, 23.6% had received four, and 23.4% had received five or more doses of vaccine against SARS-CoV-2. Most HCWs (95.1%) were vaccinated with BNT162b2 for their first dose (99.4% of them received it for their second dose as well), 4.4% with mRNA-1273 (67.5% of them received it for their second dose as well), and the remainder with a variety of other vaccines approved for use in Canada. Supplementary Figure S1 shows the number of doses of each type of vaccine by month (i.e., original mRNA, bivalent with original and BA.1 or BA.4/5, and monovalent Omicron XBB 1.5).
There were 1470 positive SARS-CoV-2 tests reported by study participants. Overall, 1302 (52.6%) participants had a single infection, while 158 (6.4%) reported two, and 10 reported three infections during their participation in the study. There was a median of 377 days (IQR 260, 515) between infections. The rate of primary infection and subsequent infections was 1.16 (95% CI 1.10, 1.23) and 0.38 (95% CI 0.33, 0.44) per 1000 participant days, respectively. Of the 1470 infections, 42 (2.9%) were asymptomatic: 7 of 59 (11.9%) in the pre-Omicron period compared with 35 of 1411 (2.5%) during the Omicron period. This is likely related to changes in testing requirements over time [Reference Robertson29].
Fifty-nine (57 first and 2 second) infections were reported during the pre-Omicron period (0.16 per 1000 participant-days; 95% CI 0.12, 0.20). During the Omicron period, 1411 infections were reported (1.14 per 1000 participant-days; 95% CI 1.09, 1.20), including 144 second and 8 third infections. As shown in Table 2, incidence rates ranged from 0.12 (95% CI 0.06, 0.23) to 3.10 (95% CI 2.78, 3.44) per 1000 person-days of study participation, with the highest rates reported during the Omicron BA.1 and BA.2 dominated periods. Figure 1 depicts the rate of infections reported by study participants (per 1000 participant days) and the rates of hospital admission for COVID-19 (per 100000 Canadians) used as a proxy for force of infection.
Table 2. Reported SARS-CoV-2 infections, rates of infection per 1000 participant-days of follow-up; Canadian healthcare workers; 15 June 2020 to 1 December 2023
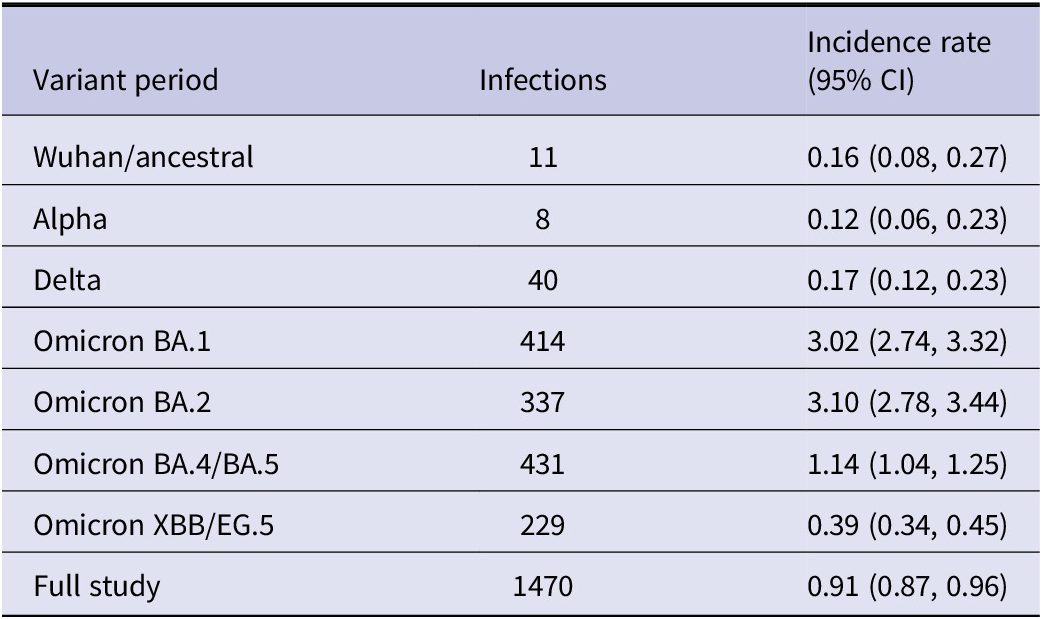
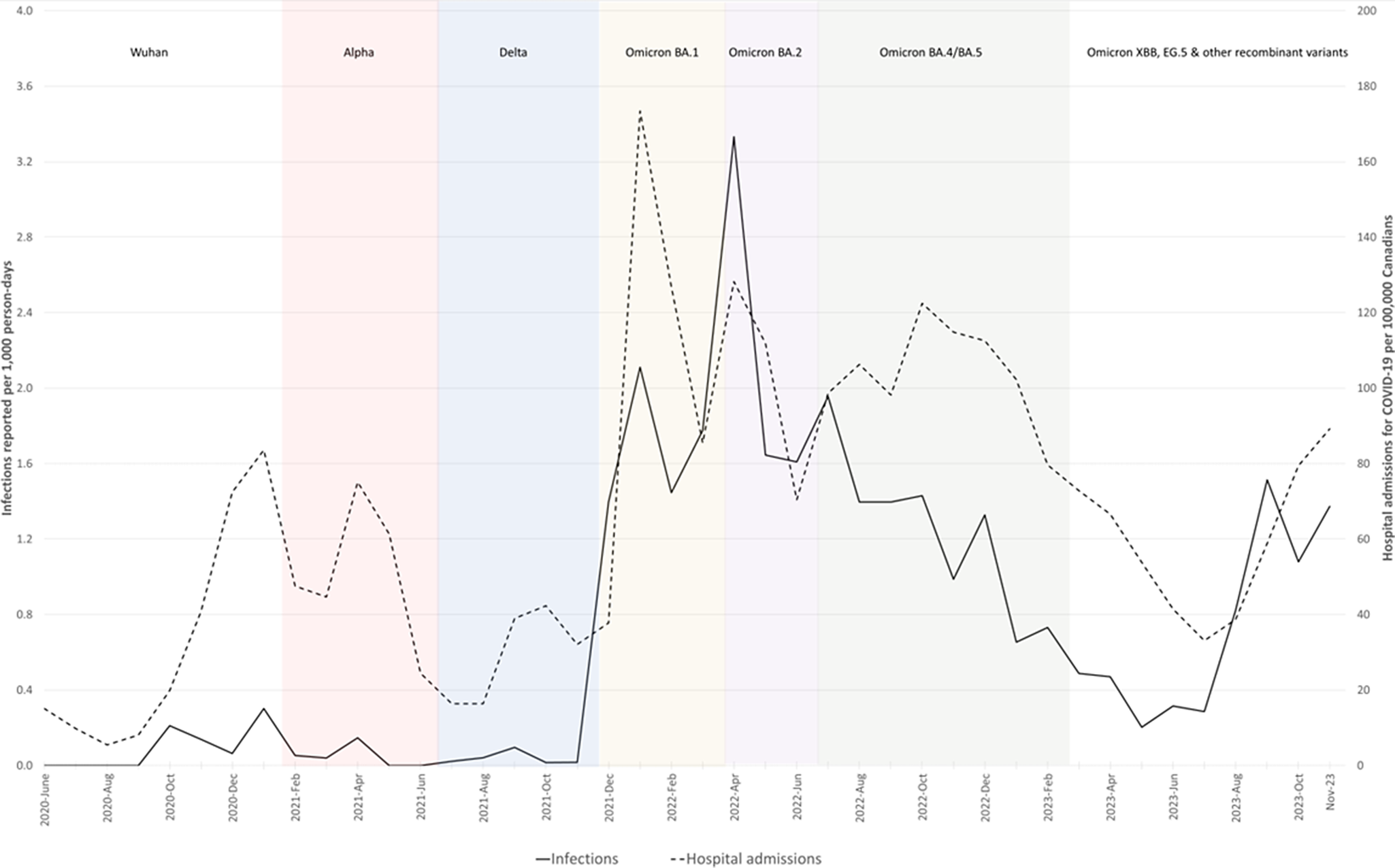
Figure 1. Infections reported per 1000 participant-days per month 15 June 2020 to 1 December 2023, COVID-19 Cohort Study, and rates of hospital admission for COVID-19 per 100000 Canadians.
Vaccine effectiveness and risk factors for infection
During the pre-Omicron (but post-vaccine availability) period (24 December 2020 to 11 December 2021), adjusted VE estimates (1-aHR) were 85% (95% CI 1, 98) and 93% (95% CI 46, 99) for HCWs who received two or three doses of vaccine, respectively. As shown in Table 3, HCWs in excellent health had lower rates of infection than people who rated their general health as fair or good. Older HCWs and males had significantly higher rates of infection than younger and female participants. Participants who reported a known exposure to infected extended family members (e.g., parents/children living outside the household) or friends had significantly higher rates of infection (aHR 7.99 (95% CI 2.58, 24.8)) than those with no known exposure to a person with SARS-CoV-2.
Table 3. Adjusted hazard ratios for infection with SARS-CoV-2, Canadian healthcare workers, by variant period: pre-Omicron (15 June 2020 to 11 December 2021) and Omicron (12 December 2021 to 1 December 2023); COVID-19 Cohort Study

* p ≤ 0.05; †p ≤ 0.01; ‡p ≤ 0.001.
aHR, adjusted hazard ratio; NP, nurse practitioner; PA, physician assistant.
a Adjusted for repeated infections reported by the same participant using the Prentice-Williams and Peterson gap time model.
b Restricted to first infections and events occurring after 24 December 2020; adjusted for variables in table column and province of residence (no one had >3 doses).
c Restricted to participants with ≥2 vaccine doses; adjusted for repeated infections for the same participant within period and variables in table column and province of residence; hospitalization rate included as a time-varying covariate.
d Both doses of two-dose vaccines or one dose of a single dose vaccine ≥14 days before event (one dose of a single-dose vaccine was coded as two so booster doses were counted as third or subsequent, as appropriate).
e Respiratory therapist, laboratory technician, physical therapist, occupational therapist, imaging technician/technologist, pharmacist, pharmacy technician, psychologist, or social worker
f Food service, ward clerk, administration, healthcare aide, housekeeper, porter, research, or other clinical support.
g ≤14 days prior to the report; includes any exposure (i.e., with or without protective equipment).
Compared with participants who received the primary series of vaccine(s), the adjusted relative VE estimates during the Omicron period (12 December 2021 to 1 December 2023) were 43% (95% CI 29, 54), 56% (42, 67), and 46% (24, 62) for third, fourth, and fifth/sixth doses of vaccine (see Table 3); absolute VE could not be estimated since 99.3% of participants had been vaccinated by the start of the period. During this period, having had any previous SARS-CoV-2 infection (i.e., at any time; either prior to or during the study) was protective; the effectiveness (1-aHR) was 38% (95% CI 23, 49) indicating they were less likely to have a subsequent infection than a person without a previous infection. Relative VE estimates for HCWs without a previous infection were similar to those for all participants (see Supplementary Table S4). Participants who reported recent close contact with/exposure to someone in the household (aHR 1.41, 95% CI 1.24, 1.61), a coworker (aHR 1.42, 95% CI 1.09, 1.86), or an extended family member/friend (aHR 1.20, 95% CI 1.01, 1.43) who had SARS-CoV-2 had significantly higher rates of infection than those with no known exposure.
Serological protection
Eligible serological data were available for 1947 (78.7%) participants who had 1298 infections over 1240521 days of participation. As shown in Table 4, participants with IgG anti-RBD antibody levels in the highest quartile were significantly less likely to report a subsequent SARS-CoV-2 infection than those in the lowest quartile in a model stratified by the number of vaccines received and adjusted for the covariates listed in the table footer. Restricting the analysis to HCWs active during the Omicron period made little difference to the estimates, while the sample size was too small to produce stable estimates for the pre-Omicron period.
Table 4. Adjusted hazard ratio for infection with SARS-CoV-2 based on IgG anti-RBD antibody levels (BAU/mL), Canadian healthcare workers, 15 June 2020 to 1 December 2023; COVID-19 Cohort Study
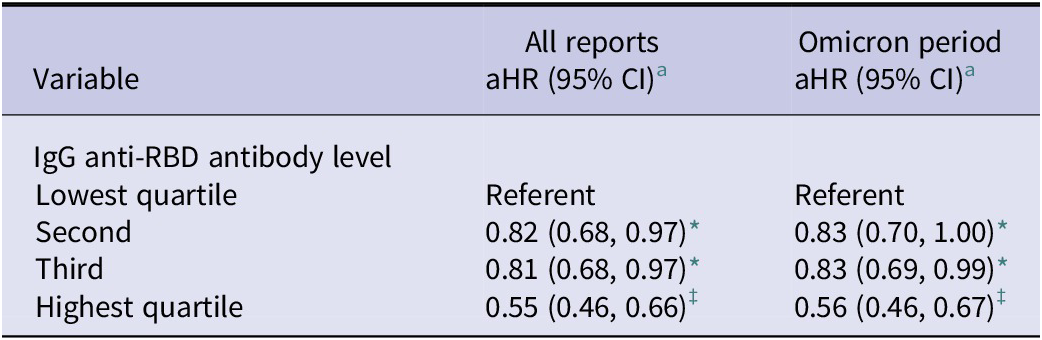
* p < 0.05; †p < 0.01; ‡p < 0.001.
aHR, adjusted hazard ratio; IgG, immunoglobulin G; RBD, receptor binding domain.
a Prentice, Williams, and Peterson proportional hazards models stratified by number of vaccines received ≥14 days prior to event and adjusted for days between blood sample and event, type of sample (serum or DBS), age, gender, race (Caucasian vs. other), household size, self-reported health status, province, Canadian rate of hospitalization for SARS-CoV-2 at time of event, occupation, and known exposure to someone with SARS-COV-2 in the past 14 days.
As shown in Figure 2a, in DBS samples drawn 14–119 days after vaccination from participants without previous SARS-CoV-2 infection, the median antibody level was significantly higher following each subsequent dose of vaccine (p ≤ 0.001). The median anti-RBD level, based on serum samples, was significantly higher following the second dose of vaccine (p = 0.04) than the first. However, there was no difference in the median values for the second through sixth doses (p = 0.20). This is different than samples collected by DBS and for samples collected from participants with a previous SARS-CoV-2 infection (as noted below). This was due, at least in part, to the limited sample sizes in serum categories, so should be interpreted with caution (see Supplementary Table S5).

Figure 2. IgG anti-RBD levels by (a) number of previous vaccine doses and (b) the number of days since the most recent of second or subsequent COVID-19 vaccine doses received, Canadian healthcare workers with no previous SARS-CoV-2 infections, 15 June 2020 to 1 December 2023, COVID-19 cohort study.
For participants providing either serum or DBS samples, the median level of anti-RBD antibodies declined significantly (p < 0.001) between the first (14–59 days) and fifth periods (240–299 days) following their second or subsequent dose of vaccine (see Figure 2b). There were no significant differences in antibody levels for the samples collected from 300 days onward (note, also, the smaller sample sizes for these periods.
Participants who were infected with SARS-CoV-2 had significantly higher anti-RBD levels following vaccination than those without a previous infection (see Figures 3a and 2a and Supplementary Figure S2). For participants who had been infected with SARS-CoV-2 prior to collecting their blood sample, RBD antibody levels increased from their first through the fourth and fifth doses of vaccine when the median values equalled the maximum dilution for testing for both the DBS and serum samples (see Figure 3a). As shown in Figure 3b, the median level of anti-RBD antibodies declined significantly from 59 to 299 days after vaccination. This occurred for participants who provided either serum or RBD samples (p < 0.001). Similar to the samples from participants without prior SARS-CoV-2 infection, there were no significant differences in median anti-RBD levels from 300 days onward.

Figure 3. IgG anti-RBD levels by (a) number of previous vaccine doses and (b) the number of days since the most recent of second or subsequent COVID-19 vaccine doses received, Canadian healthcare workers with previous SARS-CoV-2 infections, 15 June 2020 to 1 December 2023, COVID-19 Cohort Study.
Discussion
In this analysis of a cohort of Canadian HCWs who were followed from 15 June 2020 to 1 December 2023, vaccination against SARS-CoV-2 and previous SARS-CoV-2 infection were associated with lower rates of subsequent SARS-CoV-2 infection. However, the significance of vaccination and previous infection differed by period. Between June 2020 and December 2021, prior to widespread transmission of the Omicron variant in Canada, vaccination was highly protective, and the incidence of SARS-CoV-2 in HCWs was low. During the Omicron period (December 2021 to December 2023), a substantial increase in infections occurred, and the relative effectiveness of booster doses of vaccine was 43–56% when compared with HCWs who had received only the primary series of vaccines. During the Omicron period, any previous infection (i.e., before or during the Omicron period) was found to be protective against subsequent infection while adjusting for vaccination status; data were too sparse to detect differences in the effect of previous infection during the pre-Omicron period.
Prior to the widespread transmission of the SARS-CoV-2 Omicron variants, the estimated VE in the cohort of HCWs who participated in this study was 85–93%, similar to estimates from other studies of HCWs [Reference Hall13, Reference Galgut30, Reference Thompson31]. Authors of a systematic review reported that mRNA vaccines had a pooled VE estimate of 92% against any SARS-CoV-2 infection (symptomatic or asymptomatic) in studies of HCWs, most of which were conducted prior to the circulation of the Omicron variants [Reference Galgut30]. Similarly, authors of an American study reported a VE estimate of 90% in fully vaccinated healthcare and other essential workers between December 2020 and March 2021; protection remained high during a Delta-dominated period [Reference Thompson31]. Between September and November 2021, relative VE estimates for HCWs who participated in the UK SIREN study and who received a third dose of vaccine was 63% among previously uninfected and 88–90% among previously infected HCWs when compared with HCWs who had received only two doses of vaccine [Reference Hall13].
However, the effectiveness of the original vaccines waned over time as new variants emerged. Compared with participants in this study who had received only their primary series of vaccines, the relative VE estimates were 43–56% for HCWs who received booster doses between December 2021 and December 2023 (estimates were adjusted for previous SARS-CoV-2 and other factors). Due to the cumulative number of infections, it is inaccurate to estimate VE without taking previous infections into account because previous infection with SARS-CoV-2 has been shown to be protective in other studies. However, protection from infection, like protection from vaccination alone, wanes over time [Reference Hall13–Reference Kirwan15] which may be, at least in part, due to the degree of [dis]similarity between the circulating variants [Reference Hou32]. Although the relative VE for participants in our study who had not had SARS-CoV-2 were similar to those for the overall cohort (46–63%), this is not unexpected given that 88% of all infections were the first for the participant. These data suggest that vaccination remains an important tool to reduce illness caused by SARS-CoV-2 [33].
In our study, higher IgG anti-RBD antibody levels were associated with reduced rates of infection. Unlike our findings, some smaller studies were not able to detect a relationship between IgG antibody titres and the probability of infection [Reference Baratto20, Reference Erice, Prieto and Caballero21]. Similar to our results, Spiteri et al. [Reference Spiteri18] reported that of HCWs who had received a booster dose of COVID-19 vaccine, those with IgG anti-spike levels in the top quartile were significantly less likely than those with levels in the second or third quartiles to become infected. Seekircher et al. [Reference Seekircher19] also reported that participants with IgG anti-spike antibody levels twice that of others were at significantly lower risk of infection. Of note, both of these studies [Reference Spiteri18, Reference Seekircher19] reported reduced, but still significant, effect sizes during the Omicron, compared with the pre-Omicron periods.
We found that IgG anti-RBD levels generally continued to increase following the first five doses of COVID-19 vaccine, but that levels waned significantly within 60 days after receipt of a vaccine. The anti-RBD levels from vaccinated HCWs who had a breakthrough infection also started to wane by day 60 following a positive test, but the antibody levels started higher and waned more slowly than among those who had not previously had SARS-CoV-2. These results are similar to those reported by other researchers [Reference Baratto20, Reference Hou32, Reference Matsumoto34]. The waning of antibody levels has been reported to start as early as 50 days after receipt of a second [Reference Terpos35–Reference Bonnet37] or third [Reference Hönning38] dose of vaccine. Waning was also reported within 90 days of a second dose of vaccine for people who had previously tested positive for SARS-CoV-2 [Reference Keeshan39]. In our study, HCWs with hybrid (vaccination and infection) related anti-RBD antibodies had levels that were consistently higher (when comparing by the number of vaccine doses received) than those with vaccine-induced antibodies alone. Similar results were reported by other researchers [Reference Baratto20, Reference Hönning38, Reference Keeshan39]. However, antibody levels alone do not provide the full picture. Kirwan et al. [Reference Kirwan15] noted that participants who were infected during the Delta or Omicron BA.1 periods were less likely to be infected during the Omicron BA.4/5 period than participants infected in the Alpha period or earlier, even with the same IgG anti-spike antibody levels. Given the antigenic drift of the SARS-CoV-2 virus, it is important to consider the specificity of the antibodies created by vaccination and/or previous infection. [Reference Dépéry40] With the use of updated antigens, RBD and/or spike antibody levels to determine rates of seroconversion and seroprotection may be useful for the licensure of COVID-19 vaccines as is done with influenza vaccine candidates. They will also be useful for seroepidemiologic studies assessing the level of protection in various populations.
In this study, other factors that affected the rate of infection included exposure to an infected household member, coworker, or friend/a member of their extended family (e.g., children/parents not living in the household). Other studies reported similar findings. Being exposed to an infected person outside of work [Reference Belan41–Reference Plumb43], including members of the household [Reference Elgersma7, Reference Alghader, Valvi and de la Hoz44], or being exposed to an infected colleague [Reference Belan41, Reference Alghader, Valvi and de la Hoz44, Reference Emecen45] were reported as risks for SARS-CoV-2 among HCWs in other settings. These findings suggest that transmission was associated with longer-lasting and [very likely] unprotected exposures that would be more common in social, rather than patient-HCW, interactions. In a study done in 2021, Passaretti et al. [Reference Passaretti46] reported that the odds that an unvaccinated HCW who was exposed to a household member with SARS-CoV-2 would subsequently test positive themselves were eight times higher than for a fully vaccinated HCW. These data indicate a strong need to increase the awareness of HCWs about the risk of contracting SARS-CoV-2 and other diseases, such as influenza [Reference Buckrell47–Reference Kuster49], in the community as well as the healthcare setting.
Authors of some studies reported that occupational risk factors included having a patient-facing role [Reference Belan41], being exposed to a patient with SARS-CoV-2 [Reference Alghader, Valvi and de la Hoz44], being a nurse [Reference Bahrs50, Reference Porru51], a service worker or a physician trainee [Reference Alghader, Valvi and de la Hoz44], working in an emergency department or inpatient medical unit (vs the intensive care unit) [Reference Alghader, Valvi and de la Hoz44], or, in another study, working in an emergency department, intensive care unit, or COVID-19 ward [Reference Chivu52]. These factors were not associated with an increased the rate of infection in our cohort nor in some other studies of HCWs [Reference Baker42, Reference Emecen45]. One thing that appears to differentiate the studies that reported occupational factors as being significantly associated with the risk of infection from those that do not is whether they considered the role of community exposures, a vital component in the determination of risk factors for infection since HCWs do not live in isolation.
Study strengths and limitations
This study followed HCWs for up to 41.5 months during the COVID-19 pandemic, with a rich collection of exposure and outcome data collected prospectively using a secure online platform. However, as with all research, our study has limitations. Over the course of the study, there were many withdrawals due to study fatigue/conflicting priorities or job change/retirement. However, by continuing to enroll new participants until June 2023, we were able to maintain a sample size large enough to answer our research questions with good representation across occupations, ages, and geographic regions. Although prospective studies are prone to selection bias, our study included participants from four provinces across Canada who represented people working in acute care hospitals and private practice alike, providing a breadth of exposures to SARS-CoV-2. There was also the potential for depletion of susceptible bias whereby VE estimates are attenuated when unvaccinated/minimally vaccinated participants are no longer susceptible to the disease due to immunity following infection. However, we accounted for previous infections in VE estimates using both self-report and, as available, blood test results to reduce the impact of the bias. There was also the possibility that some participants delayed booster vaccinations following an infection. A post-hoc analysis determined that there were no differences in the number of days between subsequent (e.g., fourth and fifth) vaccines for participants who did and did not have previous infections.
Given that the study started in 2020, there were several methodological changes. For example, an additional dilution was added to measure antibody levels in 2021, resulting in 1235 early results being excluded due to differences in maximal values that, in turn, made it impossible to compare values over time and reduced the sample sizes for first and second vaccines. Also, we collected only serum samples until DBS sampling and analysis was approved (and continued it thereafter); this required the separation of test results by test type. Both issues somewhat reduced the ability to detect significant differences in antibody level analyses. In addition, self-collection of DBS samples may have resulted in lower sensitivity and specificity compared with serum samples. However, we were able to collect samples from a wide geographic area using the DBS samples. Another limitation was the change in respiratory sampling used to detect SARS-CoV-2: HCWs were tested using PCR until early 2022 when RAT was widely available in Canada [53]. The RAT is not as sensitive as PCR testing; this may have resulted in some false negative results and thus have an impact on effect sizes.
Although one advantage of a prospective cohort design is the ability to gather data as it occurs, rather than depending on the recollection of participants, our participants were required to reliably report data (including all infections and vaccinations) and to test themselves/be tested for SARS-CoV-2 whenever they had any symptoms or known exposures (an early requirement for all HCWs). For this study, participants were emailed every second week to complete a short online questionnaire that also took them to the secure website used to report illnesses and vaccinations and to complete monitoring and baseline questionnaires. The purpose of this was to maximize the reporting of events. Despite this, rates of testing varied significantly during the study, suggesting that some infections were likely missed because participants were not tested for SARS-CoV-2. However, the rates of testing did not vary by the recency of vaccination but, rather, generally mirrored the rate of hospital admissions for COVID-19 [Reference Robertson29].
Conclusions
Relative COVID-19 VE estimates during the follow-up of this cohort of highly vaccinated HCWs indicated that vaccination remained an effective way to reduce the probability of infection. IgG anti-RBD antibody levels may be useful as correlates of protection for vaccine licensure and for population-level studies. The strongest associations with infection, outside of vaccination, were exposures to infected household members, extended family members, friends, and coworkers, indicating a need to increase the awareness among HCWs about the risk of contracting SARS-CoV-2 during non-patient contacts.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0950268825100101.
Data availability statement
The data that support the findings of this study are available upon request from the corresponding author due to privacy restrictions.
Acknowledgements
The investigators thank their staff, who worked tirelessly throughout the study, and the participants, who gave freely of their time amidst the stress of working during the pandemic.
We also thank the non-author members of the CCS Working Group for their contributions to the study including: Kevin Katz, Infection Prevention & Control, North York General Hospital, 4001 Leslie St, Toronto, ON, M2K 1E1, Canada; Shelly A. McNeil, Nova Scotia Health, 5820 University Ave, Halifax, NS, B3H 2Y9, Canada; Mark Loeb, Division of Infectious Disease, Hamilton Health Sciences Centre, 1200 Main St. W., Hamilton, ON L8N 3Z5, Canada; Samira Mubareka, Sunnybrook Health Sciences Centre, 2075 Bayview Ave, Toronto, ON, M4N 3 M5, Canada; Iris Gutmanis, Sinai Health, 600 University Ave, Toronto, ON, M5G 1X5, Canada; Kanchan, Sinai Health, 600 University Ave, Toronto, ON, M5G 1X5, Canada; Sarah A Bennett, Sinai Health, 600 University Ave, Toronto, ON, M5G 1X5, Canada; Ayodele Sanni, Sinai Health, 600 University Ave, Toronto, ON, M5G 1X5, Canada; Julia Policelli, Sinai Health, 600 University Ave, Toronto, ON, M5G 1X5, Canada; Melanie Delgado-Brand, Lunenfeld-Tanenbaum Research Institute at Sinai Health, 600 University Ave, Toronto, ON, M5G 1X5, Canada; Robert G. Maunder, Sinai Health, 600 University Ave, Toronto, ON, M5G 1X5, Canada; and Dawn Bowdish, McMaster University, 1280 Main St W., Hamilton, ON, L8S 4 L8 Canada. We also thank the Pandemic Response Challenge Program of the National Research Council of Canada that provided antigens, protein standards, and secondary antibodies for ELISA assays and the Network Biology Collaborative Centre at the Lunenfeld-Tanenbaum Research Institute that provided facilities to perform ELISA assays.
Competing interests
The authors declare that they have no competing interests.
Ethical standard
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Research Ethics Boards of Sinai Health (20-0080-E, 17 April 2020), Sunnybrook Health Sciences Centre (1644, 13 April 2020), Michael Garron Hospital (807-2004-Inf-055, 9 April 2020), North York General Hospital (20-0017, 6 May 2020), University Health Network (20-5368, 21 May 2020), Unity Health Toronto (20-109, 1 June 2020), Oak Valley Health (121-2010, 4 November 2020), William Osler Health System (20-0028, 18 December 2020), Hamilton Health Sciences Centre (12809, 31 December 2020), St. Joseph’s Healthcare Hamilton (13044, 31 December 2020), University of Alberta (Pro00106776, 13 January 2021), University of Calgary (20-1950, 25 January 2021), Nova Scotia Health (1026317, 2 February 2021), The Ottawa Hospital (20210024-01H, 5 February 2021), and Centre Hospitalier Universitaire de Sherbrooke (MP-31-2021-4104, 9 June 2021). Informed consent was obtained from all participants involved in the study.
Funding statement
This work was supported by the Canadian Institutes of Health Research [173212 and 181116], the Weston Family Foundation, and Physicians’ Services Incorporated Foundation [6014200738]. Funders had no role in the collection, analysis, or interpretation of data, writing of the manuscript, or the decision to submit for publication.









