Introduction
Staphylococcus aureus bloodstream infection (BSI) can result in serious morbidity and mortality, especially in neutropenic patients. Up to 8.4% of patients with febrile neutropenia have a S. aureus BSI, with mortality ranging from 15–45% at 3 months. Reference Freifeld, Bow and Sepkowitz1–Reference Ryu, Lee and Kim3 Narrow-spectrum anti-staphylococcal beta-lactam therapy is considered standard of care for the treatment of methicillin-susceptible S. aureus (MSSA) BSI; however, standard of care for patients with febrile neutropenia is treatment with broad-spectrum anti-Pseudomonal beta-lactam therapy. Reference Freifeld, Bow and Sepkowitz1,Reference Baddour, Wilson and Bayer4–Reference Averbuch, Orasch and Cordonnier6 A prior study found no significant difference in outcomes when comparing MSSA-targeted beta-lactam therapy to broad-spectrum anti-Pseudomonal beta-lactam therapy or combination therapy in neutropenic patients with MSSA BSI. Notably, the study did not describe therapy escalation or incidence of new gram-negative infections within the targeted therapy group, which is of interest with losing gram-negative coverage. Reference Aleissa, Gonzalez-Bocco and Zekery-Saad7 The purpose of our study was to compare broad-spectrum, anti-Pseudomonal beta-lactam therapy (broad-spectrum therapy) to MSSA-targeted beta-lactam therapy (targeted therapy) during the period of neutropenia to determine if targeting therapy prior to neutrophil recovery is safe.
Methods
Study design and population
This retrospective, single-center, cohort study included adult patients admitted to an oncology service at Barnes-Jewish Hospital with an index MSSA-positive blood culture between 6/1/2018 and 1/31/2024. Patients were included if they were neutropenic within 24 h of the index MSSA-positive blood culture. Patients were excluded if they had positive identification of any organism on culture or polymerase chain reaction from any source within 2 weeks prior to the index MSSA-positive blood culture except for MSSA, gram-positive organisms predicted to be covered by an antibiotic in both treatment groups, viruses, or Candida species in the respiratory tract or urine. Patients were also excluded if they received ≥24 h of vancomycin, linezolid, or daptomycin monotherapy while neutropenic, if they had antibiotics modified to targeted therapy and were concomitantly initiated on anti-Pseudomonal fluoroquinolone prophylaxis, if they received a combination of both therapies for ≥48 h, or if they did not receive either therapy for ≥24 h. The study was approved by the Washington University in St. Louis Institutional Review Board.
Study outcomes
The primary composite outcome was assessed from index MSSA blood culture positivity to neutrophil recovery, or hospital discharge if neutrophil recovery did not occur during hospitalization, and consisted of patients who met at least one of the following: all-cause mortality, new or recurrent fever, or identification of a gram-negative organism from any site. Secondary outcomes also assessed during this time frame included the components of the primary outcome, time to new or recurrent fever, new intensive care unit (ICU) admission, reason for new ICU admission, ICU length of stay (LOS), broadening of therapy, reason for broadening of therapy, and duration of broad-spectrum anti-Pseudomonal beta-lactam therapy. Additional secondary outcomes included 30-day all cause-mortality, 90-day MSSA BSI recurrence, length of antibiotic therapy for MSSA BSI, overall hospital LOS, and Clostridioides difficile infection.
Study definitions
Neutropenia was defined as an absolute neutrophil count (ANC) <500 cells/µL. The broad-spectrum therapy group received cefepime or meropenem for treatment of their MSSA BSI the entire time while neutropenic. The targeted therapy group included patients whose antibiotics were modified to oxacillin, cefazolin, or ceftriaxone for treatment of their MSSA BSI at any point while neutropenic. Broadening of therapy was defined as the use of a beta-lactam with a broader spectrum of activity as determined by a modified gram-negative spectrum score Reference Ilges, Ritchie, Krekel, Neuner and Micek8 (Supplemental Table 1) or the addition of a systemic anti-Pseudomonal fluroquinolone or aminoglycoside. Modification to another MSSA-targeted beta-lactam did not count as broadening of therapy. A complete list of study definitions is available in the Supplement.
Statistical analysis
A description of statistical analyses is available in the Supplement.
Results
A total of 263 patients were screened for eligibility (Supplemental Figure 1). Of the 40 patients included, 27 (67.5%) had antibiotics modified to targeted therapy and 13 (32.5%) remained on broad-spectrum therapy while neutropenic.
No statistically significant differences existed between groups with regards to baseline characteristics except significantly more patients received meropenem initially in the broad-spectrum therapy group (38.5% vs. 7.4%, P = 0.027). Patients in the broad-spectrum therapy group had a numerically longer median duration of neutropenia after index MSSA blood culture positivity (7.6 days vs. 4.8 days, P = 0.130). The median time to targeting therapy was 1.9 days, 81.5% of patients had achieved BSI clearance at the time of targeting, and patients received a median of 2.3 days of targeted therapy while neutropenic (Table 1).
Table 1. Baseline patient/treatment characteristics
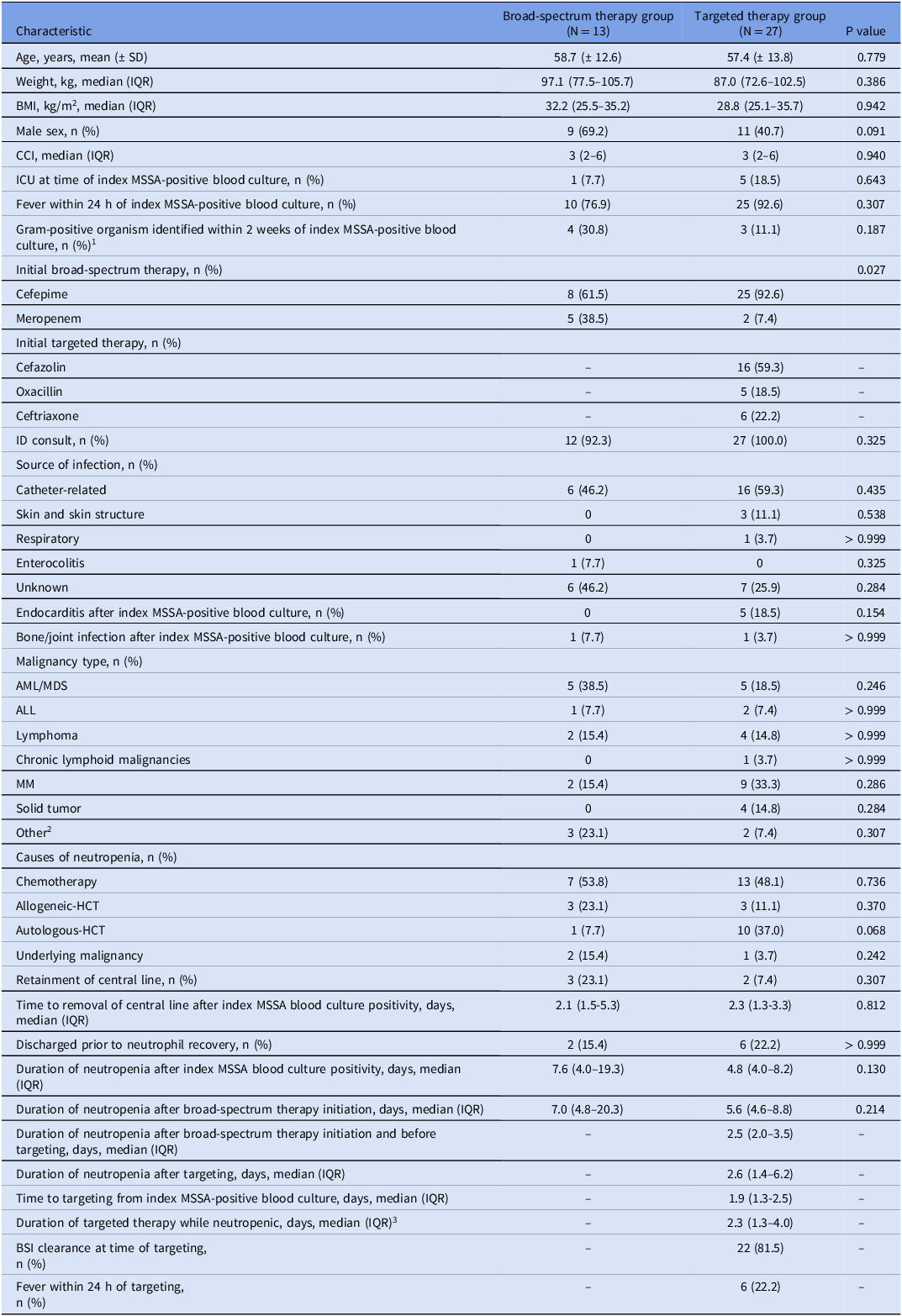
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BMI, body mass index; BSI, bloodstream infection; CCI, Charlson Comorbidity Index; HCT, hematopoietic cell transplantation; ICU, intensive care unit; IQR, interquartile range; MDS, myelodysplastic syndromes; MM, multiple myeloma; MSSA, methicillin-susceptible Staphylococcus aureus.
1 Gram-positive organisms identified: Streptococcus species (n = 9), Clostridium perfringens (n = 1).
2 Other malignancy types: chronic myelogenous leukemia (n = 1) and myeloproliferative neoplasms/disorders (n = 4).
3 Duration of targeted therapy while neutropenic includes duration of targeted therapy after re-targeting if patient was broadened and then received targeted therapy again prior to neutrophil recovery.
No statistical difference existed in the primary composite outcome between the broad-spectrum therapy and targeted therapy groups (38.5% vs. 22.2%, P = 0.451), which was met by experiencing new or recurrent fever in all patients. One patient in the broad-spectrum therapy group had a gram-negative organism identified on culture, compared to no patients in the targeted therapy group. This patient was receiving cefepime and had two gram-negative organisms, susceptible to cefepime, identified on blood culture the day after the index MSSA-positive blood culture. One patient in the targeted therapy group experienced a fatal intraparenchymal hemorrhage, deemed to be unrelated to infection. A shorter duration of broad-spectrum anti-Pseudomonal beta-lactam therapy was noted in the targeted therapy group (median 7.2 days vs. 2.3 days, P < 0.001). All other secondary endpoints were not statistically significant (Table 2).
Table 2. Primary and secondary outcomes

Abbreviations: BSI, bloodstream infection; ICU, intensive care unit; IQR, interquartile range; SD, standard deviation; LOS, length of stay; MSSA, methicillin-susceptible Staphylococcus aureus.
1 The primary composite outcome was assessed from index MSSA blood culture positivity to neutrophil recovery, or hospital discharge if neutrophil recovery did not occur during the hospital stay, and consisted of patients who met at least one of the following: all-cause mortality, new or recurrent fever, or identification of a gram-negative organism from any site. Patients meeting more than one component could only meet the primary composite outcome once.
2 One patient met the primary outcome (new or recurrent fever) while on both broad-spectrum therapy and targeted therapy (overlap of therapies <48 h).
3 All patients broadened for worsening/persistent infectious symptoms had a fever at the time of broadening.
4 Other reason for broadening of therapy: ciprofloxacin added to oxacillin due to intention to switch to targeted therapy with anti-Pseudomonal fluoroquinolone concomitantly that was delayed to the next day; patient received 3 doses of ciprofloxacin prior to switch to ceftriaxone monotherapy.
5 Duration of broad-spectrum therapy after index MSSA-positive blood culture while neutropenic includes duration of broadened therapy if patient broadened to a broad-spectrum anti-Pseudomonal beta-lactam.
Discussion
We found similar outcomes when broad-spectrum anti-Pseudomonal beta-lactam therapy was modified to MSSA-targeted beta-lactam therapy prior to neutrophil recovery in an oncology patient population. Importantly, no patients in the targeted therapy group had a new gram-negative identified on culture during their duration of neutropenia as loss of gram-negative, especially Pseudomonal, coverage is the primary concern with modification to targeted therapy in patients with neutropenia. The time to targeted therapy after identification of MSSA on blood culture was a median (IQR) of 1.9 (1.3–2.5) days in our study, demonstrating modification of antibiotics to targeted therapy can be done early in the treatment course.
Patients who were continued on broad-spectrum therapy had a numerically higher rate of 30-day all-cause mortality and readmission and an increased ICU LOS from index MSSA blood culture positivity to ANC recovery, though none of these differences were statistically significant. Differences in baseline characteristics, including 20% more patients with acute myeloid leukemia/myelodysplastic syndromes and significantly increased empiric meropenem use in the broad-spectrum therapy group, suggest this group was potentially a more unstable, complicated population compared to the targeted therapy group.
This study has several limitations, the main one being the small sample size, which may have reduced the ability to detect a statistical difference between groups. Data was collected via manual retrospective chart review and limited to the accuracy of documentation. Patients included in this study had a short duration of neutropenia after index MSSA blood culture positivity overall, and the median duration of neutropenia while on targeted therapy was only 2.3 days, which limits the generalizability to patients who have longer expected durations of neutropenia. Strengths of this study include the strict eligibility criteria that limited confounding variables and selected a truly high-risk patient population and assessment of safety outcomes, including broadening of therapy and identification of new gram-negative organisms, which were absent from prior literature. Reference Aleissa, Gonzalez-Bocco and Zekery-Saad7
In our study sample, worse outcomes were not found after modification of empiric broad-spectrum anti-Pseudomonal beta-lactam therapy to MSSA-targeted beta-lactam therapy in neutropenic patients with MSSA BSI. Modification of broad-spectrum therapy to target MSSA BSI in neutropenic patients prior to neutrophil recovery should be considered.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ash.2025.179
Data availability statement
Research data are not shared.
Acknowledgements
The authors thank Nicholas Hampton, PharmD, for his assistance with the collection of data, and Tristan Timbrook, PharmD, MPH, MBA, BCIDP, FIDSA, for his assistance with the statistical analyses, as well as their overall contributions to the project.
Financial support
None.
Competing interests
Lauren C. Russell, Jeff Klaus, Miguel A. Chavez, and Erik R. Dubberke have no actual or potential conflict of interest in relation to this manuscript. Tamara Krekel has received honoraria for serving as a speaker for AbbVie and Shionogi.



