Introduction
Amino acids are essential components that not only condense into peptides and proteins but also maintain the homeostasis of the immune system. Their influence on immune cell fate and functionality is multifaceted (Tomé Reference Tomé2021). On the one hand, amino acids provide the essential structural components and energy sources required for immune cell proliferation, differentiation, and functioning. On the other hand, immune cells have specific demands for amino acids. Processes like amino acid mobilization, uptake, and sensing drive metabolic reprogramming in immune cells, which affects their fate and functionality (Hope and Salmond Reference Hope and Salmond2021; Pearce and Pearce Reference Pearce and Pearce2013). Studies have revealed that metabolic pathways of various immune cell types during origin, proliferation, differentiation, maturation, activation, and senescence differ significantly from those in resting states. For example, during an immune response to infections or environmental changes, immune cells transition into a highly active state characterized by elevated expression of amino acid transporters (Song et al. Reference Song, Li and Tao2020). T cells serve as a representative example. When they become activated, they rapidly proliferate and upregulate the transcription and translation of key immune-related genes. This heightened activity accelerates amino acid metabolism to support the synthesis of essential macromolecules like proteins and nucleotides (Kaech et al. Reference Kaech, Hemby and Kersh2002).
Emerging research has revealed the link between dysregulated amino acid metabolism and various pathological conditions, such as metabolic disorders and immune dysfunction. In animal husbandry, amino acids are important feed components for livestock and poultry, effectively regulating immune dysfunction triggered by external or internal factors, thereby influencing disease resistance and survival rates (Bai and Plastow Reference Bai and Plastow2022). This is especially relevant under policies restricting antibiotic use and in the context of African swine fever, where amino acids, as key nutritional regulators, have become increasingly important in improving health and immune function in livestock such as pigs. Previous studies have suggested that serine and glutamine can influence the porcine T cells activity, improving host defense against pathogens (Chen et al. Reference Chen, Xia and He2020; Ma et al. Reference Ma, Zhang and Li2022; Ren et al. Reference Ren, Rajendran and Zhao2018; Shan et al. Reference Shan, Hu and Ni2022; Yu et al. Reference Yu, Tu and Yin2022; Zheng et al. Reference Zheng, Zhu and Zhao2023a). Research on amino acid metabolism pathways further highlights their role in regulating redox balance, gene expression in immune cells, and lymphocyte proliferation (Han et al. Reference Han, Ge and Ho2021; He et al. Reference He, Liu and Guan2023; Muri and Kopf Reference Muri and Kopf2021; Zhang et al. Reference Zhang, Wang and Yin2020). For example, arginine supports the growth and proliferation of immune cells while contributing to the synthesis of key immune mediators, such as nitric oxide and cytokines, which are vital for modulating inflammation and controlling autoimmune disorders (Martíi Líndez and Reith Reference Martíi Líndez and Reith2021). These findings illustrate the dynamic interplay between amino acid metabolism and immune cells, driven by signal transduction and metabolic reprogramming, which adapts to physiological and pathological conditions in animals. This provides an essential theoretical foundation for an in-depth understanding of how amino acid metabolism affects the immune cell fate and function and also opens new ways for the development of nutritional strategies to enhance animal immunity. Therefore, this review aims to focus on three key areas: (1) the mechanisms by which immune cells sense and selectively utilize amino acids; (2) the influence of amino acids on metabolic reprogramming in immune cells; and (3) the impact of amino acids on the function and fate of immune cells. Finally, we discuss future directions for research on how amino acid metabolism impacts immune cells, aiming to establish a theoretical basis that fosters integration between immunology and nutrition and expands the existing nutritional theories on amino acids.
Sensing and uptake of amino acids by immune cells
Mechanisms of amino acid sensing in immune cells
Rapamycin, a compound isolated from soil, exhibits antifungal and antitumor properties (Ghoname et al. Reference Ghoname, Ghozlan and Sabry2022). In mammals, the mammalian target of rapamycin (mTOR) is a conserved serine/threonine kinase that senses environmental changes to regulate eukaryotic cell metabolism and growth (Mota-Martorell et al. Reference Mota-Martorell, Jove and Pradas2020). This kinase forms two distinct complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). The primary difference between these complexes lies in their unique scaffold proteins, with mTORC1 showing higher sensitivity to macrolide drugs like rapamycin (Fu et al. Reference Fu, Fu and Su2023). Activation of mTORC1 involves key metabolites, such as amino acids, glucose, and nucleotides, through signaling pathways involving small GTPases, such as Ras homolog enriched in brain (RHEB) GTPases (Kim and Kim Reference Kim and Kim2016; Nguyen et al. Reference Nguyen, Frank and Jewell2017; Zhu and Wang Reference Zhu and Wang2020). At the molecular level, the Rag complex, consisting of heterodimers formed by RagA or RagB with RagC or RagD, collaborates with the Ragulator complex, which acts as a guanine nucleotide exchange factor for RagC or RagD. Together, these complexes facilitate the lysosomal translocation of mTORC1, a critical step that enables its activation by RHEB on the lysosomal surface (Groenewoud Marlous and Zwartkruis Fried Reference Groenewoud Marlous and Zwartkruis Fried2013; Lama-Sherpa et al. Reference Lama-Sherpa, Jeong and Jewell2023; Sancak et al. Reference Sancak, Bar-Peled and Zoncu2010; Tsujimoto et al. Reference Tsujimoto, Takamatsu and Kumanogoh2023). The regulatory mechanism of the Rag complex is further refined by the GTPase activating protein activity toward Rags 1 (GATOR1), which specifically targets RagA and RagB, modulating their activity (Shen et al. Reference Shen, Valenstein and Gu2019). Its localization to the lysosomal membrane is mediated through its interaction with the Rag complex. This process is further regulated by the KICSTOR complex, a key assembly that includes SZT2, and by the GTPase activating protein activity toward Rags 2 (GATOR2) complex, which acts upstream to fine-tune the Rag complex activity (Cui et al. Reference Cui, Joiner and Jansen2023; Valenstein et al. Reference Valenstein, Lalgudi and Gu2024; Zhao et al. Reference Zhao, Guan and Xu2023).
Some studies have demonstrated that immune cells sense amino acids through mTORC1, which relies on vacuolar-type ATPase on lysosomes (Wang et al. Reference Wang, Schianchi and Neumann2021d). Under conditions of sufficient amino acid availability, the vacuolar-type ATPase activates the guanine nucleotide exchange function of Ragulator, facilitating nucleotide exchange and activating Rag GTPases (Brady et al. Reference Brady, Diab and Puertollano2016; Hertel et al. Reference Hertel, Alves and Dutz2022). The activated Rag GTPases then recruit mTORC1 to the lysosomal membrane, positioning it near RHEB, which triggers mTORC1 activation (Carroll Reference Carroll2020; Gan et al. Reference Gan, Seki and Shen2019; Sancak et al. Reference Sancak, Bar-Peled and Zoncu2010, Reference Sancak, Peterson and Shaul2008). Following its activation, mTORC1 localizes to the lysosome, where it phosphorylates 4EBP1. This phosphorylation event releases eIF4E, which initiates protein synthesis (Battaglioni et al. Reference Battaglioni, Benjamin and Wälchli2022; Clemens et al. Reference Clemens, Elia and Morley2013; Grosso et al. Reference Grosso, Pesce and Brina2011; Wang et al. Reference Wang, Lei and Zhang2022). GATOR2 can inhibit GATOR1, but when arginine or leucine is present, the cellular arginine sensor for mTORC1 (CASTOR1) or Sestrin can bind GATOR2 in response to arginine or leucine, which relieves this inhibition (Jiang et al. Reference Jiang, Dai and He2023). Additionally, some studies have shown that a sensor of S-adenosylmethionine (SAM) (a metabolic product of methionine) upstream of mTORC1 (SAMTOR) can suppress mTORC1 by interacting with GATOR1, and binding of SAM to SAMTOR destroys this interaction (Kitada et al. Reference Kitada, Xu and Ogura2020). Amino acids such as arginine and methionine play important roles in activating mTORC1 across various T-cell subsets (Abdullah et al., Reference Abdullah, Zeng and Margerum2022). Key transporters, including L-type amino acid transporter 1 (LAT-1/SLC7A5) and SLC1A5, are critical for initiating mTORC1 signaling in naive, activated, and regulatory T cells (Tregs) (Grosso et al. Reference Grosso, Pesce and Brina2011; Huang et al. Reference Huang, Long and Zhou2020; Yang et al. Reference Yang, Lu and Piao2022). During the first division of CD8+ T cells, the asymmetric distribution of amino acid transporters changes how much mTOR accumulates in proximal and distal daughter cells, which eventually determines whether CD8+ T cells become memory cells or effector cells (Arsenio et al. Reference Arsenio, Kakaradov and Metz2014, Reference Arsenio, Metz and Chang2015; Cai et al. Reference Cai, Li and Wang2022; Chen et al. Reference Chen, Xu and Sun2023; Pollizzi et al. Reference Pollizzi, Sun and Patel2016). Additionally, the Rag complex is indispensable for detecting amino acid levels and has been found to suppress regulatory T-cell function (Delmonte et al. Reference Delmonte, Villa and Notarangelo2020; Shi et al. Reference Shi, Chapman and Wen2019; Thangavelu et al. Reference Thangavelu, Andrejeva and Bolivar-Wagers2022; Zhu et al. Reference Zhu, Wu and Li2024). Thus, immune cells sense environmental amino acid levels by regulating mTORC1 activity, reshaping their fate and functionality.
Immune cells also sense amino acids through general control nonderepressible 2 (GCN2), which detects tRNA that is not fully loaded with amino acids (Kim et al. Reference Kim, Sundrud and Zhou2020). Under normal conditions, tRNA that carries amino acids accumulates at ribosomes during protein translation, ensuring that the growing peptide chain receives enough amino acids (Englander et al. Reference Englander, Avins and Fleisher2015). When amino acids are scarce, uncharged or unloaded tRNA accumulates in the cell. This accumulation leads to an overall slowdown in protein translation to save energy and resources, accompanied by a selective reduction in the translation of mRNAs that restore cellular homeostasis (Darnell et al. Reference Darnell, Subramaniam and O’Shea2018). Excessive accumulation of uncharged tRNAs interacts with GCN2, causing a structural rearrangement that triggers downstream signaling pathways (Wu et al. Reference Wu, Zinshteyn and Wehner2019). Upon activation, GCN2 phosphorylates eIF2α at serine 51, which disrupts the assembly of the eIF2/tRNAiMet/GTP ternary complex, a crucial step in initiating protein translation (Kedersha et al. Reference Kedersha, Chen and Gilks2002) (Fig. 1). Previous studies have suggested that GCN2 activation negatively affects T-cell proliferation and Treg differentiation (Rashidi et al. Reference Rashidi, Miska and Lee-Chang2020; Sonner et al. Reference Sonner, Deumelandt and Ott2016; Zheng et al. Reference Zheng, Yao and Ge2023b). Studies on amino acid deprivation, such as those using indoleamine 2,3-dioxygenase to degrade tryptophan, have revealed that indoleamine 2,3-dioxygenase increases IL-10 production while reducing IL-12 expression in macrophages via a GCN2-dependent mechanism that inhibits protein synthesis (Battu et al. Reference Battu, Minhas and Mishra2017; Yan et al. Reference Yan, Zhang and Gran2010). During apoptosis or exposure to apoptotic antigens, macrophages suppress IL-12 mRNA expression while increasing IL-10 transcript translation (Filardy et al. Reference Filardy, Pires and Nunes2010). Additionally, the amino acid starvation response activated by halofuginone can suppress IL-1β production in macrophages through GCN2 activation (Battu et al. Reference Battu, Afroz and Giddaluru2018). Collectively, immune cells can sense amino acids through both the GCN2 and mTORC1 pathways, which allows them to regulate their fate and function based on amino acid availability.
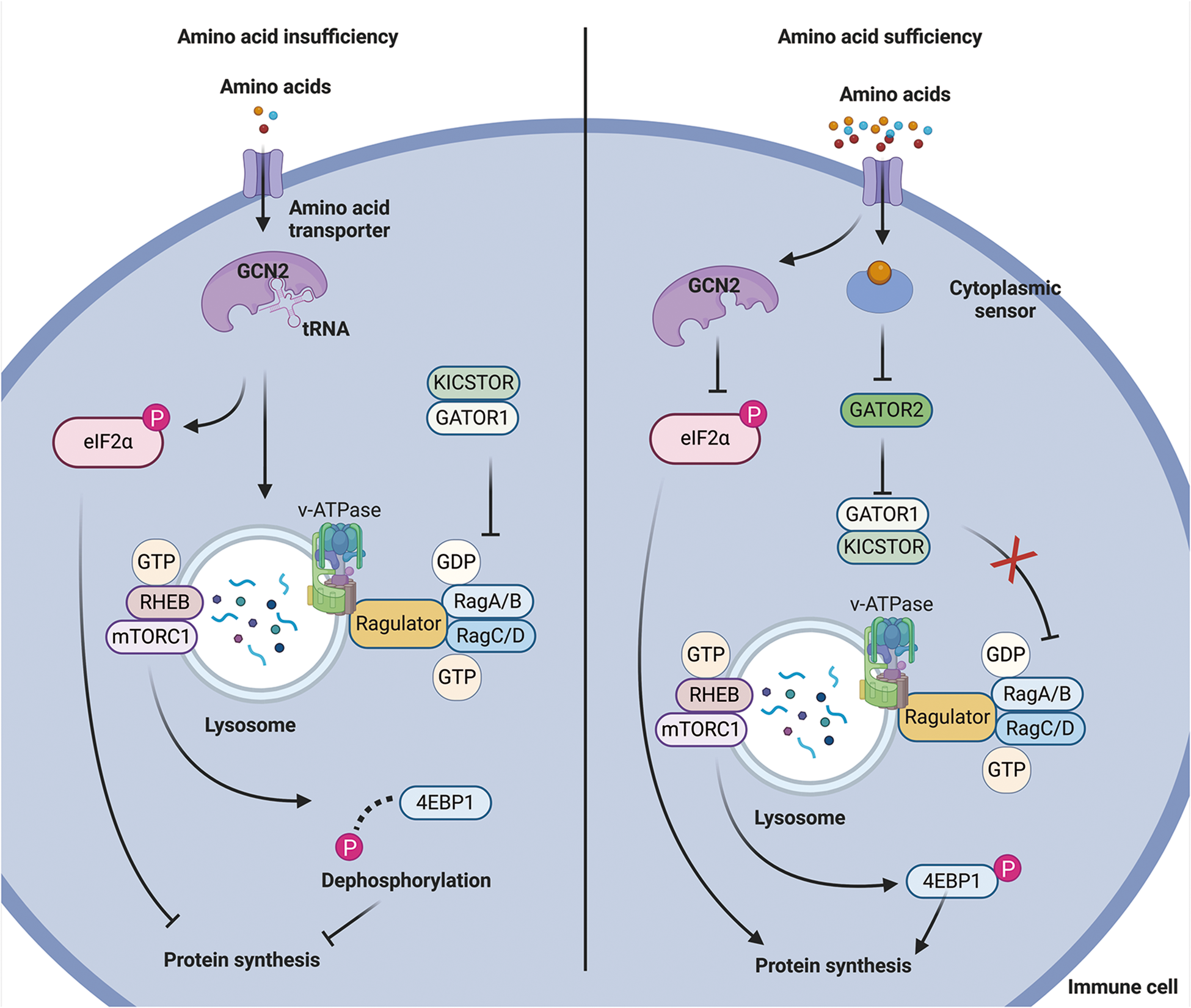
Figure 1. Mechanism of amino acid sensing by immune cells. When amino acid levels are low, uncharged tRNA activates GCN2, which interferes with the recruitment of mTORC1 substrates and blocks protein synthesis. In contrast, when amino acid levels are sufficient, cytoplasmic amino acid sensors inhibit GATOR1 through GATOR2, ultimately activating mTORC1 and promoting protein synthesis.
Mechanisms of amino acid mobilization and uptake in immune cells
Amino acid mobilization and uptake are critical regulatory points that impact the significantly influence immune cell function and fate. These cells primarily acquire amino acids through transporters that move amino acids from the microenvironment into the cells (Almeida et al. Reference Almeida, Lochner and Berod2016; Ma et al. Reference Ma, Ming and Wu2024; Tae et al. Reference Tae, Kim and S.-w.2023; Yang et al. Reference Yang, Chu and Liu2023). Activation of the T-cell receptor (TCR) has been shown to trigger metabolic reprogramming in T cells, leading to changes in glycolysis, oxidative phosphorylation, and fatty acid β-oxidation pathways (Cammann et al. Reference Cammann, Rath and Reichl2016; Marelli-Berg et al. Reference Marelli-Berg, Fu and Mauro2012; Patsoukis et al. Reference Patsoukis, Bardhan and Weaver2016; Xuekai et al. Reference Xuekai, Yan and Jian2024). These metabolic changes rely heavily on amino acid availability and the corresponding transporters. The large amino acid transporter 1, also referred to as SLC7A5, forms a heterodimeric complex with the transmembrane protein CD98 (also known as SLC3A2) to transport large hydrophobic amino acids (Bröer et al. Reference Bröer, Gauthier-Coles and Rahimi2019). This transporter facilitates the uptake of seven essential amino acids, excluding lysine and threonine (Brunocilla et al. Reference Brunocilla, Console and Rovella2023). T-cell activation dramatically increases the expression of the LAT-1/CD98 complex, supporting antigen recognition and rapid clonal expansion (Cantor and Ginsberg Reference Cantor and Ginsberg2012; Hayashi et al. Reference Hayashi, Jutabha and Endou2013). Some studies have found that CD4+ T cells with a knockout of the Slc7a5 gene exhibit impaired antigen responses, with an inability to proliferate or differentiate into Th1 and Th17 subsets (Sinclair et al. Reference Sinclair, Rolf and Emslie2013; Yeramian et al. Reference Yeramian, Martin and Serrat2006). These findings suggest the critical role of LAT-1 not only in early T-cell activation but also in guiding T-cell differentiation. In animal models of lupus and psoriasis induced by imiquimod (a TLR7 agonist), the absence or inhibition of LAT-1 significantly reduces IL-17 secretion and suppress the expansion of γδ T cells and CD4⁺ T cells (Zhang et al. Reference Zhang, Zhang and Yu2021b). Other amino acid transporters, such as SLC7A7, which forms complexes with CD98, are responsible for the transport of lysine, arginine, and other amino acids, highlighting the diversity and complexity of amino acid transporters in immune function regulation (Dai et al. Reference Dai, Feng and Hu2021). Once amino acids enter immune cells, the cells can recycle certain chemical groups to synthesize new amino acids (Yang et al. Reference Yang, Chu and Liu2023). Lysosomes within immune cells also contain amino acid transport mechanisms as part of the self-protection system during starvation (Wang et al. Reference Wang, Wan and Du2021a). These lysosomal transporters are also crucial for immune functions such as the production of type I interferons in dendritic cells and immunoglobulin G synthesis in B cells (Akkaya et al. Reference Akkaya, Akkaya and Miozzo2017; Jiang et al. Reference Jiang, Yan and Wang2018; Li et al. Reference Li, Wu and Jiang2022; Montoya et al. Reference Montoya, Schiavoni and Mattei2002).
The asymmetric distribution of amino acid transporters is crucial in determining T-cell fate (Arsenio et al. Reference Arsenio, Kakaradov and Metz2014). When interacting with antigen-presenting cells, SLC7A5 displays a notable asymmetric distribution (Kedl et al. Reference Kedl, Rees and Hildeman2000). This distribution results in differing amino acid concentrations and metabolic activities between proximal and distal T-cell subsets, ultimately influencing immune cell function and fate (Pollizzi et al. Reference Pollizzi, Sun and Patel2016). In proximal daughter cells, high SLC7A5 expression promotes increased amino acid uptake, particularly glutamine, which supports rapid proliferation and high energy demand (Huang et al. Reference Huang, Wang and Hong2023). Increased glutamine enhances glycolysis, providing abundant energy and carbon sources for biosynthesis. Additionally, glutamine contributes to α-ketoglutarate production, accelerating the tricarboxylic acid (TCA) cycle and enhancing metabolic activity and energy generation (Oh et al. Reference Oh, Sun and Zhao2020). Enhanced glycolysis and metabolic activity activate the c-Myc and mTORC1 signaling pathways, laying the molecular foundation for proximal daughter cells to differentiate into effector T cells. In contrast, distal daughter cells with lower SLC7A5 expression exhibit reduced amino acid uptake and metabolic activity, resulting in weaker metabolic activity. While this situation does not favor rapid cell proliferation, it does help the cells conserve energy and resources, preparing them for future reactivation and rapid responses. Consequently, these distal daughter cells tend to develop into memory T cells that survive long-term and can respond quickly when they encounter the same antigen again (Kaech and Cui Reference Kaech and Cui2012; Montacchiesi and Pace Reference Montacchiesi and Pace2022; Pollizzi et al. Reference Pollizzi, Sun and Patel2016; Verbist et al. Reference Verbist, Guy and Milasta2016). Overall, the uptake of amino acids and the asymmetric distribution of their transporters are decisive factors in T-cell function and fate, affecting both immediate immune responses and long-term functionality and survival.
T-cell development
The development of T cells occurs primarily in the thymus. Hematopoietic stem cells derived from the bone marrow differentiate into common lymphoid progenitor cells, which migrate to the thymus and further develop into progenitor T cells (Karsunky et al. Reference Karsunky, Inlay and Serwold2008). These progenitor cells undergo TCR rearrangement and selection processes, giving rise to mature conventional αβ T cells as well as unconventional subsets such as γδ T cells, natural killer T cells (NKT), mucosal-associated invariant T cells, and thymic-derived regulatory T cells (tTreg) (Hoebeke et al. Reference Hoebeke, De Smedt and Stolz2007; Liu et al. Reference Liu, Wang and Ding2015). T-cell maturation progresses through distinct developmental stages, including double-negative (DN) and double-positive (DP) phases. The DN stage is further classified into DN1, DN2a, DN2b, DN3a, DN3b, and DN4 phases (Guha et al. Reference Guha, Bhuniya and Shukla2020; Wang et al. Reference Wang, Qi and Yao2021b; Yui et al. Reference Yui, Feng and Rothenberg2010). The transition from DN2 to DN3 determines whether pro-T cells differentiate into αβ or γδ T cells (Ciofani and Zúñiga-Pflücker Reference Ciofani and Zúñiga-Pflücker2010). Pre-TCR and Notch signaling in DN3a cells are crucial for β-selection and the subsequent development of conventional αβ T cells (Ciofani and Zúñiga-Pflücker Reference Ciofani and Zúñiga-Pflücker2010). Conversely, DN2 and DN3 cells exposed to elevated interleukin-7 (IL-7) signaling pathways are driven towards the γδ T-cell lineage (Saba et al. Reference Saba, Kosan and Vassen2011). DP cells have the potential to differentiate into unconventional subsets, such as iNKT and tTreg cells (Wang et al. Reference Wang, Zhao and Jin2021c; Winter and Krueger Reference Winter and Krueger2019) (Fig. 2). In pigs, the composition of T-cell subsets differs significantly from other mammals. Some studies have shown that during late gestation in sows, the population of γδ T cells in embryonic blood and peripheral lymphoid tissues increases sharply, far exceeding the number of CD4⁺CD8⁻ and CD4⁻CD8⁺ T lymphocytes. This αβ-to-γδ T-cell ratio is distinct from what is observed in other mammals (Augustyniak et al. Reference Augustyniak, Czyżewska-Dors and Pomorska-Mól2023; Bianchi et al. Reference Bianchi, Zwart and Jeurissen1992; Le Page et al. Reference Le Page, Baldwin and Telfer2022; Schalk et al. Reference Schalk, Pfaffinger and Schmucker2019). Some T cells in pigs may originate outside the thymus (Licence and Binns Reference Licence and Binns1995). Previous studies identified mitotic T cells in the gastrointestinal epithelium of pigs, referred to as intraepithelial T cells (IEK) (Wiarda et al. Reference Wiarda, Trachsel and Bond2020). Currently, research on unconventional T cells in pigs remains limited. Further studies on these cells could enhance our understanding of their roles in amino acid metabolism, disease prevention, and vaccine responses.
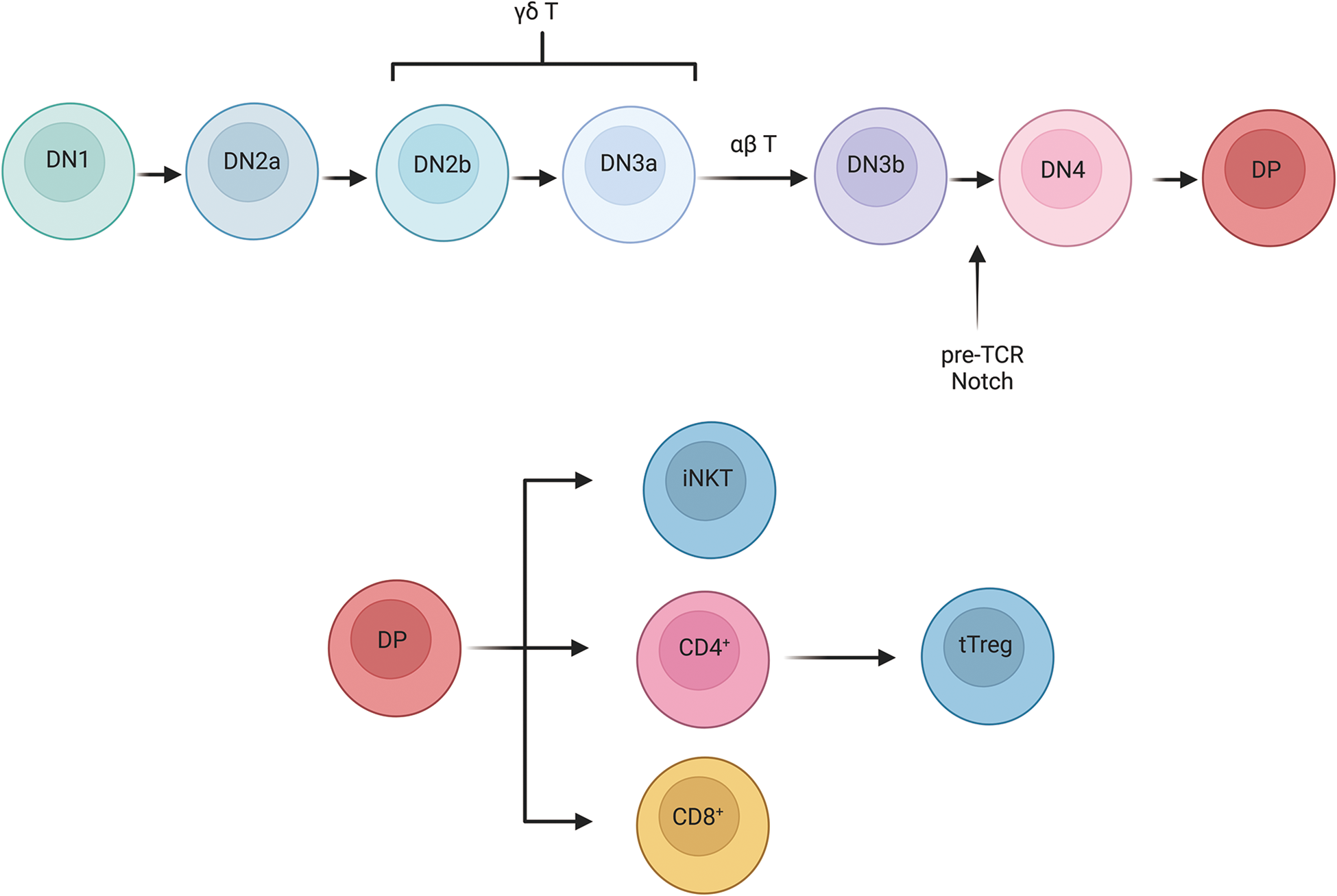
Figure 2. Development of T cells. T-cell development proceeds through a series of stages. DN1 cells can differentiate into B cells, myeloid cells, and innate T cells, while DN2b and DN3a cells can give rise to γδ T cells. At the DN3 stage, the pre-TCR complex-formed by TCRβ, pTα, and CD3 molecules-promotes β selection and drives the transition from DN3 to DN4. Both the pre-TCR and Notch signals are crucial for β selection and for the shift from the DN to the DP stage. After positive selection in the thymic cortex and negative selection in the thymic medulla, DP cells eventually differentiate into CD4+ T cells, CD8+ T cells, or iNKT cells.
Immune cell metabolism
Once immune cells mature, they leave the thymus. When T cells are in a resting state and have not been activated by receptor engagement or cytokine signals, their metabolic demands remain low. They rely mainly on fatty acid β-oxidation and TCA cycle. The maintenance of this resting metabolic state in T cells requires external signals such as IL-7. When T cells encounter an antigen, they undergo dynamic metabolic, differentiating into effector T cells in a process known as metabolic reprogramming. The activated T cells switch their primary energy source from oxidative phosphorylation to glycolysis (Almeida et al. Reference Almeida, Lochner and Berod2016; Kempkes et al. Reference Kempkes, Joosten and Koenen2019). Although glycolysis produces less ATP per cycle compared to oxidative phosphorylation, it can generate ATP at a faster rate, work effectively in low-oxygen or acidic environments, and provide higher biosynthetic efficiency to help maintain redox balance (Choudhury Reference Choudhury2021; Lees et al. Reference Lees, Gardner and Harvey2017; Wang et al. Reference Wang, Guan and Wang2020). Metabolites produced during glycolysis feed into the pentose phosphate pathway, which contributes to amino acid and nucleotide biosynthesis and generates nicotinamide adenine dinucleotide phosphate for reducing power (Ge et al. Reference Ge, Yang and Zhou2020). During the later stages of immune responses, a small subset of antigen-specific T cells persists as long-lived memory T cells (Xu et al. Reference Xu, Bhanumathy and Wu2016). These memory T cells are characterized by an increased mitochondrial mass, which enhances their spare respiratory capacity and prepares them for rapid responses upon re-exposure to antigens (Li and Zhang Reference Li and Zhang2020). Therefore, amino acid metabolism is an integral component of T-cell metabolism, providing intermediates that support these metabolic pathways and determining immune cell functionality (Almeida et al. Reference Almeida, Lochner and Berod2016; Kelly and Pearce Reference Kelly and Pearce2020; Wang and Zou Reference Wang and Zou2020) (Fig. 3).
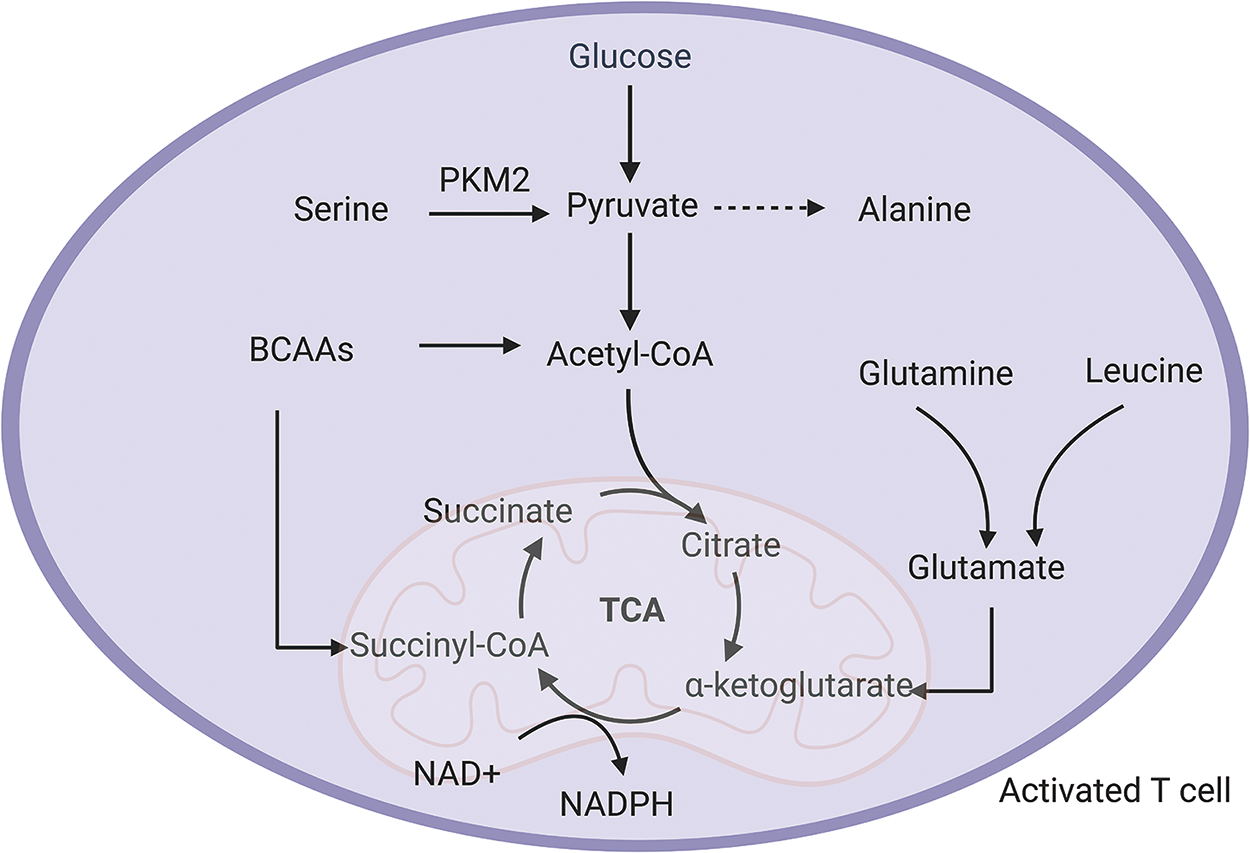
Figure 3. Amino acid metabolism in activated T cell. Serine is required for the production of cytokines in activated T cells with the help of the key glycolytic enzyme PKM2. Although pyruvate can be used to make alanine, activated T cells reduce the synthesis of alanine from pyruvate in order to conserve pyruvate metabolism and convert it into acetyl-CoA for TCA cycle activity. Branched-chain amino acids (BCAAs) provide the TCA cycle with the intermediate product CoA. Glutamine and leucine also contribute to the TCA cycle via glutamate to α-ketoglutarate.
In addition to the metabolic distinctions between effector and memory T cells, various T-cell subsets exhibit unique metabolic characteristics. The differentiation of Th1, Th2, Th17, and Tregs is heavily influenced by cytokines such as interferon-γ (IFN-γ), interleukin-4, interleukin-6, and transforming growth factor-β, respectively (Barnes and Powrie Reference Barnes and Powrie2009; Liu et al. Reference Liu, Cao and Feng2015; Li et al. Reference Li, Spolski and Liao2014, Reference Li, Wan and Flavell2007a; Maloy et al. Reference Maloy, Salaun and Cahill2003; Marie et al. Reference Marie, Letterio and Gavin2005; O’Shea et al. Reference O’Shea, Steward-Tharp and Laurence2009; Tang et al. Reference Tang, Wang and Xing2020). While Th1, Th2, and Th17 cells primarily rely on glycolysis for energy and biosynthesis, Tregs employ a mixed metabolic approach that integrates glycolysis, fatty acid oxidation, and oxidative phosphorylation (Kempkes et al. Reference Kempkes, Joosten and Koenen2019; Ma et al. Reference Ma, Ming and Wu2024) Interestingly, shifting the metabolic balance can alter T-cell differentiation. Inhibiting glycolysis during Th17 differentiation has been shown to favor the generation of Tregs (Barbi et al. Reference Barbi, Pardoll and Pan2013; Shi et al. Reference Shi, Wang and Huang2011; Sun et al. Reference Sun, Fu and Zhou2017; Zhang et al. Reference Zhang, Gang and Yang2021a). Similarly, the addition of exogenous fatty acids to T-cell cultures significantly suppresses the production of cytokines associated with Th1, Th2, and Th17 cells, while having minimal impact on Tregs. Notably, this suppression of effector T cells by fatty acids cannot be reversed by the addition of cytokines that typically promote their differentiation (Allen et al. Reference Allen, Fan and Monk2014; Michalek et al. Reference Michalek, Gerriets and Jacobs2011). Moreover, suppressing mTOR signaling to enhance fatty acid oxidation increases the number of memory T cells (Chi Reference Chi2012; Pearce et al. Reference Pearce, Walsh and Cejas2009; Waickman and Powell Reference Waickman and Powell2012). These findings underscore the fundamental metabolic differences between effector T cells and Tregs. Therefore, the study on amino acid metabolism-mediated T-cell fate and function has emerged as a hot and active topic in the present and future.
The impact of amino acid metabolism on immune cell fate and function
Glutamine metabolism-mediated immune cell fate
Glutamine and its metabolism are critical for the proliferation, differentiation, and activation of T cells by providing essential energy (Carr et al. Reference Carr, Kelman and Wu2010; Yaqoob and Calder Reference Yaqoob and Calder1997). Some studies have shown that in glutamine-free media, T cells predominantly differentiate into Tregs rather than Th17 cells, and prolonged glutamine deprivation exacerbates this effect (Edwards et al. Reference Edwards, Ngwa and Raybuck2021). Immune T cells rely on SLC38A2 to transport extracellular glutamine into the cell. Without this transporter, Th17 differentiation is significantly reduced, whereas the formation of Tregs remains unaffected (Zheng et al. Reference Zheng, Yao and Ge2023b). Within immune cells, glutamine metabolism requires the catalytic activity of glutaminase-1 (Gls1) to convert glutamine into glutamate. Th17 cells exhibit higher Gls1 expression levels than other T-cell subsets, with elevated intracellular levels of glutamate and α-ketoglutarate (a glutamate metabolite) (Kono et al. Reference Kono, Yoshida and Maeda2018). Previous studies have suggested that Gls1 deficiency reduces α-ketoglutarate levels, affecting chromatin states and gene expression, further inhibiting mTORC1 and IL-2 signaling, thereby impairing Th17 differentiation while promoting Th1 cell formation (Johnson et al. Reference Johnson, Wolf and Madden2018; Lin et al. Reference Lin, Ren and Xiong2024). The dependence of Th17 cells on glutamine has been further demonstrated in Gls1-deficient mice. Inhibition of Gls1, either through pharmacological means or siRNA-mediated approaches, significantly reduces Th17 differentiation in vitro (Kono et al. Reference Kono, Yoshida and Maeda2018).
Glutamine also influences the activation, proliferation, and development of other immune cells. In M1 macrophages, it enhances the production of α-ketoglutarate, a key metabolite that drives the synthesis of IL-1β and supports inflammatory responses (Kolliniati et al. Reference Kolliniati, Ieronymaki and Vergadi2021; Palmieri et al. Reference Palmieri, Gonzalez-Cotto and Baseler2020). In M2 macrophages, glutamine contributes to the TCA cycle, supporting anti-inflammatory responses (Gupta and Sarangi Reference Gupta and Sarangi2023; Jha et al. Reference Jha, Huang and Sergushichev2015; Liu et al. Reference Liu, Wang and Li2017; Zhao et al. Reference Zhao, Wang and Liu2020b). Additionally, glutamine is vital for the activation and functionality of NKT cells and B cells. NKT cells use glutamine to synthesize glutathione and hexosamines, while B cells depend on it for antibody production (Jiang et al. Reference Jiang, Yan and Wang2018; Loftus et al. Reference Loftus, Assmann and Kedia-Mehta2018). Overall, glutamine serves as a versatile metabolite that sustains the functionality and metabolic adaptability of diverse immune cell populations.
Branched-chain amino acids metabolism-mediated immune cell fate
In addition to glutamine, branched-chain amino acids (BCAAs) contribute to the TCA cycle and are involved in various biosynthetic processes. Through the generation of intermediates such as acetyl-CoA and succinyl-CoA, BCAAs provide essential substrates for the TCA cycle (Bo and Fujii Reference Bo and Fujii2025; Dimou et al. Reference Dimou, Tsimihodimos and Bairaktari2022; Neinast et al. Reference Neinast, Jang and Hui2019). During immune cell activation, neutral amino acid transporters, particularly SLC7A5/CD98, are significantly upregulated, serving as crucial mediators during pathogen infections and immune responses. In T cells, infections trigger elevated expression of SLC7A5, a process further maintained by IL-2 to ensure a constant supply of BCAAs (Almutairi et al. Reference Almutairi, Ali and He2019; Kang et al. Reference Kang, Song and Lee2024; Sinclair et al. Reference Sinclair, Rolf and Emslie2013). The inhibition of SLC7A5 has been shown to significantly reduce IFN-γ and IL-17 production, impairing the differentiation of Th1 and Th17 cells, while having minimal impact on the Tregs (Hayashi et al. Reference Hayashi, Jutabha and Endou2013; Song et al. Reference Song, Li and Tao2020). Loss of SLC7A5 in T cells also impairs mTORC1 and Myc-dependent glycolysis, affecting T-cell activation and proliferation (Marchingo et al. Reference Marchingo, Sinclair and Howden2020). In macrophages, lipopolysaccharide (LPS)-induced inflammatory responses also activate BCAA transporters, with leucine transported by SLC7A5 being essential for glycolysis (Yoon et al. Reference Yoon, Oh and Kang2018). Similarly, CD98 expression is closely linked to the proliferation of immune cells and cytokine production. Plasma cells with high CD98 expression demonstrate stronger immune responses, longer lifespans, and increased antibody production (Cantor et al. Reference Cantor, Browne and Ruppert2009, Reference Cantor, Slepak and Ege2011; Jensen et al. Reference Jensen, Potempa and Gotthardt2017; Nguyen et al. Reference Nguyen, Duan and Ali2021; Robinson et al. Reference Robinson, Dowling and Pitt2022). The enzyme BCAA aminotransferase 1 (BCAT1) is integral to BCAA metabolism, catalyzing the conversion of BCAAs into branched-chain keto acids, which serve as precursors for TCA cycle entry. Inhibition of BCAT1 effectively reduces glycolysis and oxidative phosphorylation, thereby reducing the production of anti-inflammatory metabolites (Papathanassiu et al. Reference Papathanassiu, Ko and Imprialou2017). These metabolic pathways not only drive short-term immune responses but also underpin the adaptive immune responses required for prolonged antigen exposure.
Serine metabolism-mediated immune cell fate
Serine regulates immune cell metabolic reprogramming, influencing their fate and function. It interacts with pyruvate kinase M2 (PKM2), enhancing glycolytic flux to provide energy for immune cells (Chaneton et al. Reference Chaneton, Hillmann and Zheng2012). Previous study has suggested that PKM2 activation in lipopolysaccharide-induced macrophages drives a shift toward glycolysis and speeds up IL-1β production (Bahiraii et al. Reference Bahiraii, Brenner and Yan2022; Palsson-mcdermott et al. Reference Palsson-mcdermott, Curtis Anne and Goel2015; Xie et al. Reference Xie, Yu and Kang2016). In CD4⁺ T cells, activation of the TCR promotes the nuclear translocation of PKM2, increasing glycolysis and facilitating differentiation into Th1 and Th17 subsets. Inhibiting PKM2 nuclear translocation can limit the differentiation of these cells and reduce cytokine production, which helps slow the progression of multiple sclerosis (Angiari et al. Reference Angiari, Runtsch and Sutton2020; Puckett et al. Reference Puckett, Alquraishi and Chowanadisai2021; Traba et al. Reference Traba, Sack and Waldmann2021). Additionally, limiting serine intake can reduce PKM2 activity, thereby lowering macrophage activation in atherosclerosis and decreasing LPS-induced IL-1β production (Liu et al. Reference Liu, Le and Chen2021; Shirai et al. Reference Shirai, Nazarewicz and Wallis2016; Tabas and Bornfeldt Reference Tabas and Bornfeldt2020). Similar findings from our lab have demonstrated that LPS-treated piglet serum exhibits elevated IL-1β levels, which are reduced by dietary serine supplementation (Zhou et al. Reference Zhou, Zhang and He2017). In a dextran sulfate sodium-induced colitis model, our study also indicated that adding serine lowered the levels or activity of proinflammatory cytokines in mice (Zhang et al. Reference Zhang, Hua and Zhang2018). Additional research highlights the role of phosphoglycerate dehydrogenase (PHGDH), a rate-limiting enzyme in the serine de novo synthesis pathway, in macrophages. Inhibiting PHGDH increases NAD⁺ levels and enhances the activity of NAD⁺-dependent SIRT1/3, which promotes IL-1β production. PHGDH also supports Toll-like receptor 4 transcription via H3K9/27 acetylation and activates the NLRP3 inflammasome by facilitating acetylation of inflammasome components (Wang et al. Reference Wang, Chen and Chen2024). Lack of serine can suppress IL-1β production in macrophages by inhibiting mTOR signaling (Chen et al. Reference Chen, Xia and He2020; Shan et al. Reference Shan, Hu and Ni2022). Moreover, our team was the first to discover that adding appropriate amounts of serine to sow diets during late pregnancy and lactation significantly increased antibody levels in both sows and piglets, raised the positive rate of CD4⁺/CD8⁺ cells, improved the growth performance of nursing piglets and the reproductive performance of sows, and enhanced immunity in both sows and piglets (He et al. Reference He, Liu and Long2020). This suggests that serine may be a promising new feed additive for improving swine immunity. Additionally, serine supports mitochondrial metabolism through serine hydroxy methyltransferase 2 (Shmt2), essential for mitochondrial translation and respiration. Shmt2-deficient models exhibit severe respiratory defects (Tani et al. Reference Tani, Ohnishi and Shitara2018). Serine-derived one-carbon units are crucial for nucleotide synthesis and methionine cycling, particularly in proliferating tissues. In Shmt2 null Jurkat cells, supplying one-carbon units can fix defects in mitochondrial respiration and translation, especially under low-glucose conditions, highlighting the vital role of this pathway in adjusting metabolic states (Minton et al. Reference Minton, Nam and McLaughlin2018). Moreover, serine is indispensable for T-cell proliferation and differentiation. The serine de novo synthesis pathway supports purine synthesis and one-carbon metabolism, while activated T cells upregulate key enzymes in this pathway to regulate immune responses and metabolism directly (Ma et al. Reference Ma, Bantug and Griss2017). In models of Pasteurella multocida infection, serine levels in the lungs significantly decrease. Supplementing serine in these mice reduces bacterial colonization and inflammatory responses, further demonstrating its critical role in immune regulation (He et al. Reference He, Yin and Wu2019).
Sulfur-containing amino acids metabolism-mediated immune cell fate
Methionine plays multiple roles in regulating immune cell function and fate, primarily through its involvement in methylation processes. By providing SAM, methionine drives the methylation of biomolecules, which can promote or inhibit transcription by changing how DNA is accessed by transcriptional machinery. During T-cell activation, both repressive and activating histone methylation events occur frequently, facilitating transcriptional remodeling (Henning et al. Reference Henning, Roychoudhuri and Restifo2018; Sinclair et al. Reference Sinclair, Howden and Brenes2019). Additionally, RNA methylation, particularly N6-methyladenosine modification, plays a pivotal role in maintaining T-cell homeostasis. The absence of this modification leads to defects in mRNA stability, splicing, and translation initiation, ultimately impairing T-cell proliferation and differentiation (Frye et al. Reference Frye, Harada and Behm2018; Galloway et al. Reference Galloway, Kaskar and Ditsova2021; Li et al. Reference Li, Tong and Zhu2017). Beyond transcriptional regulation, methionine metabolism has a profound impact on immune memory formation and function. The methylation of histones, RNA, and other cellular components relies on the availability of SAM. For example, Th17 cells starved of methionine or subjected to methionine cycle inhibition exhibit reduced H3K4 methylation, leading to decreased IL-17 production, while methionine restriction in Th1 cells similarly reduces IFN-γ expression. Additionally, dietary methionine restriction has been shown to reduce the number of IL-17- and IFN-γ-producing cells, thereby influencing immune responses in conditions such as experimental autoimmune encephalomyelitis (Roy et al. Reference Roy, Chen and Mamane2020).
Methionine not only participates directly in methylation reactions through its metabolite SAM but also supports immune cell function and proliferation through byproducts such as S-adenosylhomocysteine and further metabolic pathways. β-glucan-trained human peripheral blood mononuclear cells exhibit increased H3K4 trimethylation at cytokine and immune signaling gene promoters, enhancing cytokine production upon Candida albicans re-exposure (Quintin et al. Reference Quintin, Saeed and Martens Joost2012). Similarly, memory CD4⁺ T cells show enriched histone marks associated with cytokines such as IL-17 and IFN-γ and transcription factors like T-bet, establishing a “primed” chromatin state that enables rapid cytokine production upon stimulation (Durek et al. Reference Durek, Nordström and Gasparoni2016; Schmidl et al. Reference Schmidl, Delacher and Huehn2018). Methionine transport is critical for these processes. Following antigen stimulation, T cells upregulate methionine transporters, including SLC7A5, to meet the increased demand for methyl donor production and protein synthesis, which are essential for T-cell differentiation and function. The balance between SAM and its byproduct, S-adenosylhomocysteine, also modulates histone methylation levels. Accumulation of S-adenosylhomocysteine due to disruptions in methionine metabolism can inhibit histone methylation, further suppressing immune gene expression. This metabolic-epigenetic interplay ensures that immune cells efficiently integrate nutrient availability with functional adaptation, reinforcing the role of methionine metabolism in maintaining long-term immune memory (Kelly and Pearce Reference Kelly and Pearce2020).
Cysteine, another sulfur-containing amino acid, is vital for maintaining redox balance and methylation regulation. It serves as a key precursor for glutathione (GSH), one of the body’s main antioxidants that control reactive oxygen species levels and preserve intracellular redox balance. In immune cells, methionine metabolism can generate cysteine. Beyond the regulatory roles methionine plays, cysteine also modulates immune cell responses through specific mechanisms: (1) antioxidant and cellular protection: Upon activation, T cells, B cells, and macrophages upregulate GSH synthesis, with cysteine providing the sulfur atom essential for this process. For example, LPS-stimulated macrophages produce high ROS levels, which are neutralized by GSH to prevent oxidative damage. (2) sulfur metabolism and translational support: Cysteine also contributes to iron-sulfur cluster synthesis, vital for mitochondrial electron transport chains and various metabolic enzymes. These clusters play a vital role in sustaining energy production and metabolic activity in T cells and macrophages (Fig. 4). Additionally, cysteine plays a role in post-translational modifications. The sulfur from cysteine is critical for tRNA thiolation, which facilitates efficient translation, particularly for proteins required in immune activation. Furthermore, iron-sulfur clusters derived from cysteine support mitochondrial metabolism and electron transport chain function, ensuring energy production for highly active immune cells. Therefore, sulfur-containing amino acids play indispensable roles in regulating oxidative stress, cellular metabolism, epigenetic control, and long-term immune memory.
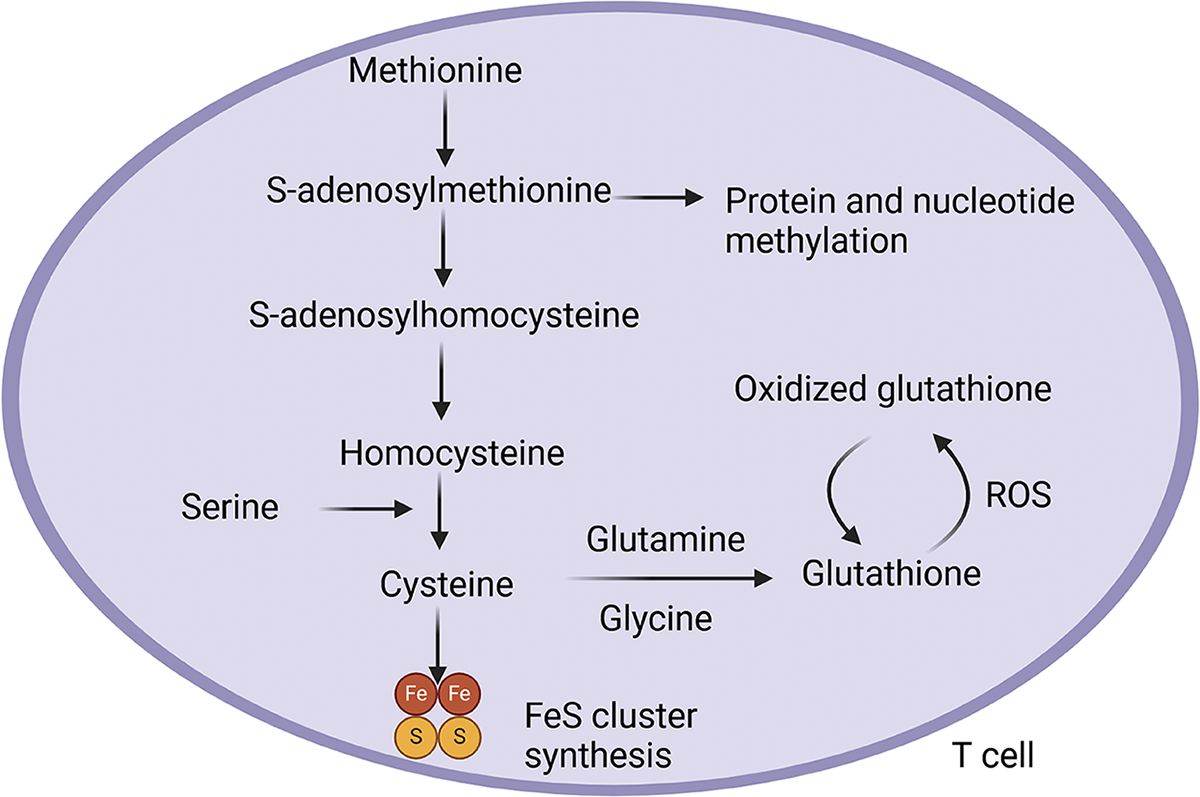
Figure 4. Sulfur-containing amino acids maintain redox status homeostasis in T cells. In T cells, methionine can be converted into cysteine. During this process, the intermediate S-adenosylmethionine donates methyl groups that modify immune effector proteins and nucleic acids, promoting cytokine gene expression in T cells and supporting innate and adaptive immune memory. Serine can also be transformed into cysteine, which contributes to the synthesis of the antioxidant glutathione and the formation of iron-sulfur (FeS) clusters.
Arginine metabolism-mediated immune cell fate
Arginine influences protein structure modifications and immune cell activity regulation through its metabolism. In the presence of SAM, arginine generates polyamines, highlighting the interdependence and centrality of arginine and SAM in immune cell metabolism (Puleston et al. Reference Puleston, Buck and Klein Geltink2019). Polyamine production signals sufficient nutrient supply, coordinates biosynthesis, and supports immune cell proliferation. Polyamines participate in producing rare amino acid derivatives critical for the post-translational modification of eukaryotic translation initiation factor 5a (eIF5a), a key regulator of translation elongation and termination (Pelechano and Alepuz Reference Pelechano and Alepuz2017; Schuller et al. Reference Schuller, Wu and Dever2017). Inhibition of the polyamine-eIF5A-taillessin pathway suppresses oxidative phosphorylation-dependent M2 macrophage polarization, leading to a shift towards glycolysis-dependent M1 polarization (Puleston et al. Reference Puleston, Buck and Klein Geltink2019).
Other amino acids metabolism-mediated on immune cell fate
During immune cell proliferation and differentiation, nucleotides synthesis relies on amino acids such as aspartate and glycine. Aspartate and serine-derived glycine provide carbon backbones for nucleotide formation (Lane and Fan Reference Lane and Fan2015). Glycine plays a particularly critical role in the early stages of T-cell activation (Ma et al. Reference Ma, Bantug and Griss2017). Although glycine can convert to alanine, T cells typically use alanine for protein synthesis rather than glycolysis (Ron-Harel et al. Reference Ron-Harel, Ghergurovich and Notarangelo2019). Tryptophan’s metabolic product, melatonin, can influence macrophage M1/M2 polarization (Xia et al. Reference Xia, Chen and Zeng2019). Additionally, melatonin regulates the activation and differentiation of T cells (Th17, Treg, and memory T cells) by activating calcineurin and the ERK1/2-C/EBPα signaling pathways (Ren et al. Reference Ren, Liu and Chen2017a). Gamma-aminobutyric acid promotes the differentiation of intestinal Th17 cells and the expression of IL-17 during E. coli infection (Ren et al. Reference Ren, Yin and Xiao2017b). These functions, along with the broader immunomodulatory roles of amino acids, have been extensively summarized in previous reviews (Bhandage and Barragan Reference Bhandage and Barragan2021; Castellano and Molinier-Frenkel Reference Castellano and Molinier-Frenkel2020; Liao et al. Reference Liao, Fan and Bin2022; Stone and Williams Reference Stone and Williams2023; Tantawy and Naguib Reference Tantawy and Naguib2019; Yang et al. Reference Yang, Chu and Liu2023; Zhao et al. Reference Zhao, Raines and Huang2020a).
Challenges and practical considerations in pig farming
Despite the potential benefits of amino acid supplementation in improving immune function and growth performance in pigs, several challenges must be addressed for successful application in pig farming. Amino acid bioavailability is a key determinant of immune cell function and fate, as immune cells require optimal amino acid availability to support proliferation, cytokine production, and immune signaling pathways (Yang and Liao Reference Yang and Liao2019). The absorption efficiency of amino acids depends on feed formulation, processing methods, and the presence of anti-nutritional factors. Arginine and glutamine play essential roles in immune regulation, supporting T-cell activation and macrophage function, but their bioavailability is highly dependent on feed processing and dietary composition (Fanimo et al. Reference Fanimo, Susenbeth and Südekum2006; Sá et al. Reference Sá, Moreno and Carciofi2020; Wu et al. Reference Wu, Bazer and Johnson2018). Feed formulation influences amino acid digestibility through ingredient selection, balancing essential and non-essential amino acids to meet metabolic demands (Buraczewska et al. Reference Buraczewska, Wasilewko and Fandrejewski1999). Processing methods, such as extrusion and pelleting, can enhance amino acid bioavailability by breaking down cell walls and denaturing anti-nutritional proteins, but excessive heat treatment may degrade heat-sensitive amino acids like lysine and methionine (Ohh et al. Reference Ohh, Han and Chae2002). Anti-nutritional factors, such as tannins and phytates in plant-based feedstuffs, can inhibit amino acid absorption by forming complexes with proteins, reducing enzymatic hydrolysis and intestinal uptake (Woyengo and Nyachoti Reference Woyengo and Nyachoti2013). Plant-derived protein sources contain fiber and phytates, which can lower amino acid digestibility and reduce their availability to immune cells, thereby impairing immune function (Myrie et al. Reference Myrie, Bertolo and Sauer2008). Pigs (13-35 kg) fed a low-protein diet (13.9% crude protein) also impair T-cell activation and proliferation, suggesting that protein restriction can compromise immune resilience (Peng et al. Reference Peng, Hu and Liu2016).
Amino acid metabolism also interacts with other nutrients, influencing immune homeostasis and inflammatory responses. Amino acid antagonism is a significant factor influencing nutrient bioavailability and immune regulation. For instance, excessive lysine intake can interfere with arginine absorption by competing for the same transporters, potentially reducing nitric oxide production and impairing macrophage-mediated immune responses (Li et al. Reference Li, Yin and Li2007b). Similarly, an imbalance in BCAAs, particularly excessive leucine, can suppress isoleucine and valine uptake. Valine deficiency can reduce lymphocyte proliferation and hinder the growth of lymphoid tissue (Wang et al. Reference Wang, Peng and Zhang2023). Deficiencies in key vitamins, such as vitamin B6, which is involved in amino acid metabolism, can impair amino acid transamination processes, leading to reduced protein synthesis and weakened immune responses (Li et al. Reference Li, Yin and Wang2019).
Although amino acid fortification enhances immune resilience and growth performance in pigs, its cost-effectiveness remains a significant consideration. While synthetic amino acid supplements can improve the bioavailability of nutrients, they can increase feed costs, so the cost-effectiveness must be carefully evaluated (Ju et al. Reference Ju, Yun and Jang2008). Future research should focus on optimizing dietary formulations that balance immune support with economic feasibility to maximize health and productivity outcomes in pig farming. Addressing these challenges through optimized feed strategies and targeted research will improve the practical implementation of amino acid-based nutritional interventions in pig farming, ensuring that immune cell function and fate are optimally regulated to improve disease resistance and overall health outcomes.
Gut microbiota, amino acid metabolism, and immune cell function in pigs
Gut microbes not only utilize dietary amino acids for microbial protein synthesis but also influence host amino acid availability through protein degradation and nitrogen recycling (Dai et al. Reference Dai, Wu and Zhu2011; Macfarlane and Macfarlane Reference Macfarlane and Macfarlane2012). Specific commensal bacteria, such as Bacteroides, Clostridium, Lactobacillus, and Streptococcus, contribute to protein fermentation and enhance amino acid bioavailability by synthesizing essential amino acids that the host cannot produce (Dalmasso et al. Reference Dalmasso, Nguyen and Yan2008). In the small intestine, microbial protein synthesis is predominant, whereas in the large intestine, amino acid catabolism dominates, generating various metabolites such as ammonia, short-chain fatty acids and biogenic amines. These metabolites not only impact on intestinal health but also modulate host immune responses (Blachier et al. Reference Blachier, Mariotti and Huneau2007; Collins et al. Reference Collins, Surette and Bercik2012).
Tryptophan is metabolized by gut bacteria into indole derivatives, such as indole-3-aldehyde, which activate the aryl hydrocarbon receptor in immune cells. Aryl hydrocarbon receptor activation induces IL-22 production, which enhances mucosal immunity and maintains gut homeostasis by promoting epithelial barrier integrity (Zelante et al. Reference Zelante, Iannitti and Cunha2013). Additionally, Tryptophan metabolism leads to the formation of kynurenine, a metabolite that modulates immune responses by interacting with dendritic cells and macrophages, affecting cytokine secretion and T-cell differentiation (Ma et al. Reference Ma, Guo and Zhang2018). Glutamine is a critical amino acid for immune cells and intestinal barrier function. Gut bacteria metabolize glutamine into glutamate, which influences the gut-microbiome-immune axis by supporting intestinal epithelial renewal and regulating immune responses (Blachier et al. Reference Blachier, Boutry and Bos2009). Arginine metabolism by gut microbiota also plays a key role in immune modulation. Certain bacteria convert arginine into ornithine and nitric oxide, both of which contribute to macrophage activation and pathogen clearance. Nitric oxide, produced by inducible nitric oxide synthase, is essential for immune defense, as it enhances macrophage antimicrobial activity and controls inflammatory responses (Kan et al. Reference Kan, Lee and Wilson2015). However, when gut microbiota composition is imbalanced, amino acid metabolism can generate harmful metabolites that impair immune function. For example, the overgrowth of proteolytic bacteria, such as certain Clostridium species, can lead to excessive production of ammonia and hydrogen sulfide, which negatively affect intestinal epithelial integrity and immune homeostasis (Blachier et al. Reference Blachier, Mariotti and Huneau2007). However, there is currently little understanding of the basic mechanisms of the interactions between amino acids, the microbiome and immune cells. Future research should clarify their relationship in order to improve pig health and productivity.
Conclusion and perspectives
In recent decades, the relationship between amino acid metabolism and T-cell development and function has gained increasing attention. Although significant progress has been made, many fundamental issues remain unresolved. A deeper understanding is needed to elucidate how amino acid metabolism shapes the fate and function of immune cells across different mammalian species, particularly in livestock and poultry, where research remains relatively limited. While numerous studies have explored the impact of amino acids on T-cell activity and differentiation, the role of amino acid metabolism in maintaining or reprogramming effector cells is still not fully understood. Some studies have confirmed that dietary amino acids must be digested in the intestine and then undergo intracellular transformations before the body can utilize them. Simply adding amino acids to the diet may not allow them to be directed to specific sites to exert their corresponding effects. Immune cells exhibit distinct responses to amino acid perturbations, emphasizing the need to decode metabolic requirements in various tissues and physiological contexts. Another major challenge is converting our knowledge of how amino acids affect immune cell fate and function into precise and targeted therapies. Future studies targeting amino acid-specific signaling pathways in immune cells under different conditions will facilitate the development of targeted metabolic strategies for combating pathogens, tumors, and related diseases.
Acknowledgements
This work was supported by the Key Fundamental Research Program of Hunan Province (2024JC0007), the National Natural Science Foundation of China (U23A20233, 32172755), the Hunan Science and Technology Innovation leading Talent Support Program (2023RC1054), Municipal scientific and technological innovation cooperation project of Changchun (24SH16), the Shandong Province Taishan Industry Leading Talents Project Blue Talents Project, and the China Agriculture Research System of MOF and MARA (CARS-35).
Author contributions
L.H. and S.J. contributed equally to this work. L.H. and S.J. wrote the manuscript. T.L. and Y.Y. edited the paper. All authors provided feedback on the writing.
Conflict of interests
The authors declare no competing interests.





