Background
Parkinson’s disease (PD) is a neurodegenerative pathology that affects 3% of individuals by the age of 65 and rises to 5% for those over 85 years of age. Reference Dexter and Jenner1 PD is characterized by the decrease of dopaminergic neurons in the substantia nigra pars compacta of the midbrain, as well as increases of alpha-synuclein positive cytoplasmic inclusions, referred to as Lewy bodies, in the remaining neurons. Reference Cerri, Mus and Blandini2 The physiological degeneration of the dopaminergic neurons in the substantia nigra pars compacta contributes to various motor pathologies, Reference Shafiq, Singh, Khan, Neary and Bardutz3 including resting tremors, rigidity, bradykinesia and gait problems. Reference Picillo, Amboni and Erro4 Furthermore, non-motor symptoms of PD involve cognitive deviations; psychiatric issues including apathy, anxiety and depression; sleep disorders including REM behavior disorder and insomnia; and autonomic dysfunctions. Reference Picillo, Amboni and Erro4
The default mode network (DMN) is an extensive brain system primarily activated during periods of rest, specifically when the brain is not primarily active in goal-directed activity. Reference Raichle, MacLeod, Snyder, Powers, Gusnard and Shulman5 The DMN plays multiple roles related to memory, self-referential processing, social cognition and consciousness and awareness. Reference Raichle, MacLeod, Snyder, Powers, Gusnard and Shulman5 The brain regions involved in the DMN include the medial prefrontal cortex (mPFC), the posterior cingulate cortex (PCC), the inferior parietal lobule (IPL), the precuneus and the lateral temporal cortex. Reference Buckner, Andrews‐Hanna and Schacter6 The PCC and precuneus play a key role in the DMN’s involvement in autobiographical memory retrieval. Reference Addis, Wong and Schacter7 The mPFC plays a key role in the DMN’s self-referential processing role, which is involved in reflection and evaluation of one’s past experiences, current emotions and self-thinking. Reference Qin and Northoff8 The IPL and mPFC are also involved in the DMN’s role in social cognition, which involves the “theory of the minds,” a process where individuals are aware and understanding of other people’s intentions and perspectives. Reference Mars, Sallet, Schuffelgen, Jbabdi, Toni and Rushworth9 Furthermore, DMN regulates human awareness and consciousness. Reference Raichle, MacLeod, Snyder, Powers, Gusnard and Shulman5 Studies suggest disruptions in DMN connectivity alter consciousness, and decreased DMN connections lead to decreased human awareness. Reference Vanhaudenhuyse, Noirhomme and Tshibanda10 These findings further suggest the importance of DMN for preserving a strong self-awareness and understanding of reality. Reference Boly, Tshibanda and Vanhaudenhuyse11
Studies suggest that various neurological pathologies have led to abnormalities in DMN connectivity. Reference Greicius, Srivastava, Reiss and Menon12 Many past articles have discussed the impact of PD on DMN activity using functional MRI (fMRI). This review aims to evaluate how fMRI has been used to assess alterations in DMN connectivity for individuals with PD, with particular focus on the relationship between DMN dysfunction and cognitive and motor symptom severity. We further note methodological limitations of current fMRI approaches and highlight key directions for future research.
Methodology
The following literature review was conducted to present current research findings using fMRI to study the effects of PD on the DMN. We included studies investigating the role of PD on DMN (until August 2024) in PubMed and EMBASE (including Medline). The keywords searched for this study include Default Mode Network, Parkinson’s Disease and Functional Magnetic Resonance Imaging. References obtained from studies included within the review were also incorporated. Emphasis was placed on only reviewing literature that utilized fMRI to conduct DMN testing for individuals with PD. Excluded studies include those not in English, as well as those utilizing alternative methods to measure DMN activity.
Results
Default mode network and Parkinson’s disease
The later-stage non-motor PD symptoms involving neuropsychiatric issues and cognitive problems are heavily linked to connectivity issues within the DMN. Reference Hacker, Perlmutter, Criswell, Ances and Snyder13 This decreased connectivity is associated with multiple cognitive issues for individuals with PD such as poorer executive functioning, worsening memory and diminished success in daily functions requiring memory. Reference Lucas-Jiménez, Ojeda and Peña14 Overall, these issues contribute to worsened cognitive functioning. Reference Ruppert, Greuel and Freigang15 Moreover, studies show that there is a negative link between the severity of PD motor symptoms and the level of connectivity in the DMN. Reference Chen, He and Zhang16 Individuals with greater PD motor symptoms have also been found to suffer larger alterations in DMN connections. Reference Chen, He and Zhang16 Findings such as these propose a link between the DMN and multiple symptoms of PD including both cognitive and motor issues. Reference Ruppert, Greuel and Freigang15
Studying default mode network and Parkinson’s disease using fMRI
Resting-state fMRI has been utilized to measure DMN activity and observe impaired DMN connectivity in individuals with PD. Reference Fox and Raichle24 Current studies utilizing fMRI technology have found that individuals with PD exhibit impaired DMN connectivity in specific regions, including the PCC, mPFC and the precuneus. Reference Tessitore, Esposito and Vitale25 Individuals with greater PD motor symptoms have also been found to suffer larger alterations in DMN connections anatomically within the frontal lobe and PCC. Reference Chen, Huang, Ma and Chen26 An overview of studies assessing alterations in DMN connectivity using fMRI for PD patients is included in Table 1.
Table 1. Observing alterations in DMN connectivity using fMRI for PD patients
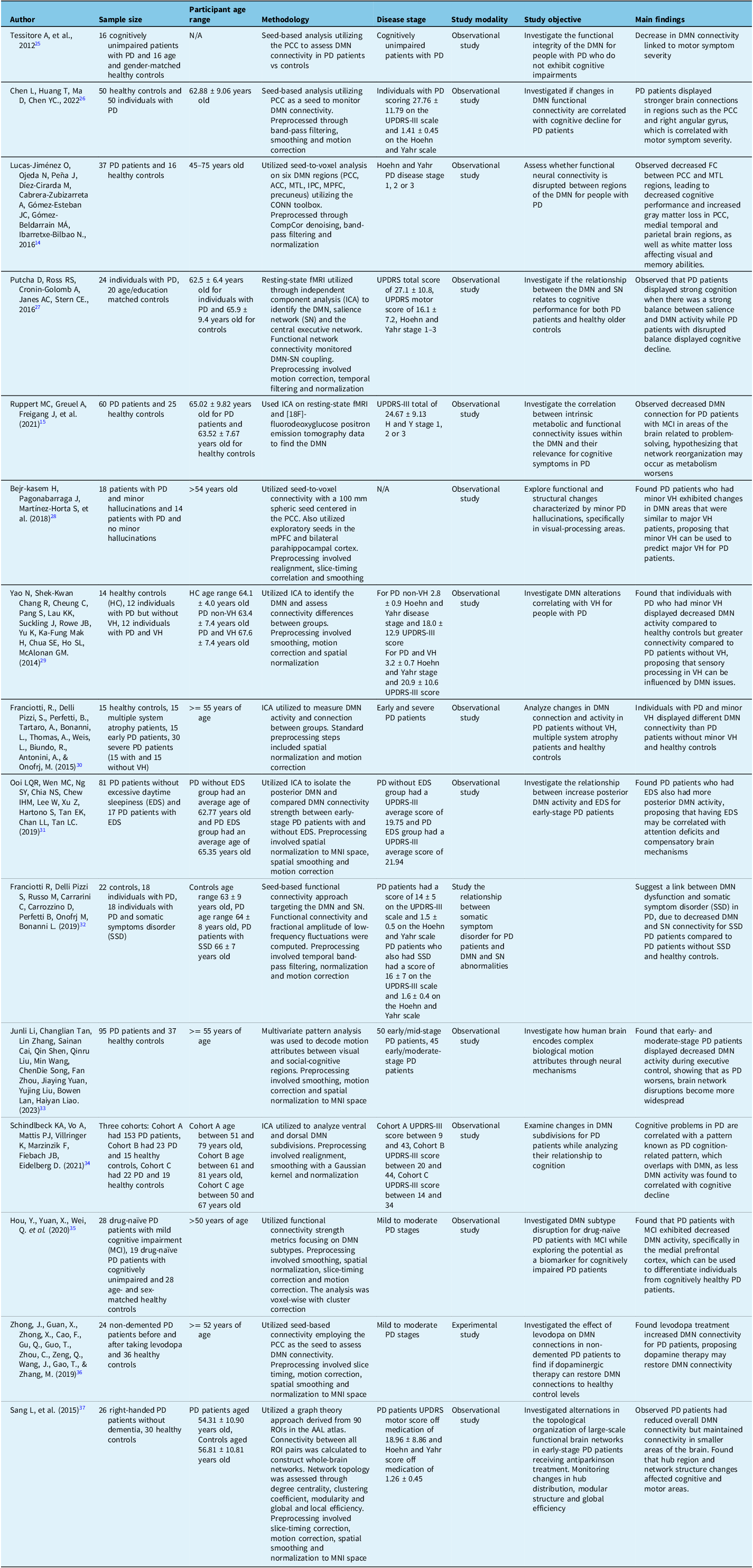
FC = functional connectivity; IPC = inferior parietal cortex; MTL = medial temporal lobe; PCC = posterior cingulate cortex; PD = Parkinson’s disease; VH = visual hallucinations; RIO = regions of interest; ACC = anterior cingulate cortex.
It is notable that throughout the reviewed studies, participant treatment conditions, whether treatment-naïve, actively partaking in levodopa or dopaminergic therapy, were not consistently reported. Dopaminergic medications enhance neural drive, which may influence DMN connectivity. Reference Zhong, Guan and Zhong36 Therefore, it is possible that there is some variability across the included results, an important factor to consider when interpreting the overall findings.
Discussion
Observing the relationship between PD progression and DMN connectivity using fMRI
Mechanisms underlying DMN disruption in PD
Several hypotheses have been proposed attempting to explain DMN connectivity changes for individuals with PD. Reference Helmich, Derikx, Bakker, Scheeringa, Bloem and Toni38 Although current research cannot identify the direct mechanism by which PD affects DMN connectivity, multiple hypotheses have been derived based on existing research. One such hypothesis suggests that the decrease in dopaminergic neurons in the brain for individuals with PD results in altered DMN connectivity. Reference van Eimeren, Monchi, Ballanger and Strafella17 Other theories highlight the potential roles of other neurotransmitters, such as serotonin and cholinergic systems, which are also affected by PD and may influence DMN connections. Reference Powell, Ireland and Lewis18
Another potential hypothesis for DMN connectivity issues proposes that the loss of dopaminergic neurons’ functioning and abundance as well as the observed neurochemical brain changes in PD patients contributed to the abnormal DMN connectivity found in individuals with PD. Reference Helmich, Derikx, Bakker, Scheeringa, Bloem and Toni38 Researchers suggest that when dopaminergic neurons are lost, a cascade of dopamine depletion is caused in PD patients in the striatum, leading to altered DMN connectivity. Reference Helmich, Derikx, Bakker, Scheeringa, Bloem and Toni38 Furthermore, the involvement of other neurotransmitter systems including the serotonergic and cholinergic systems has also been found to be linked to DMN connectivity issues for PD patients. Reference Powell, Ireland and Lewis18
An additional theory for DMN connectivity issues proposes alterations in biochemical pathways and concentrations as a possible explanation. Reference Braak, Tredici, Rüb, de Vos, Jansen Steur and Braak39 Researchers propose that neuroinflammation and mitochondrial dysfunction may have roles and serve as contributing factors that lead to DMN connectivity disruption for individuals with PD. Reference Schapira and Jenner40 Furthermore, scientists also propose the possibility that the uncharacteristic spread of alpha-synuclein pathology, which is a known symptom of PD patients, may also play a role in disrupting the normal connectivity of the DMN for individuals with PD. Reference Braak, Tredici, Rüb, de Vos, Jansen Steur and Braak39
DMN connectivity and disease progression
Despite these potential hypotheses, researchers have noticed a relationship between PD progression and DMN connectivity using fMRI measurement techniques. Reference Chen, He and Zhang16 Research shows that a decrease in DMN connectivity was linked to worsening PD motor symptoms by severity. Reference Chen, He and Zhang16 These cross-sectional study findings propose that PD has a large and varying effect on different brain networks, related to both motor and non-motor PD symptoms. Reference Chen, Huang, Ma and Chen26
Aside from these cross-sectional PD and DMN interventions, examinations utilizing longitudinal methods and fMRI have been conducted to better learn the mechanisms through which DMN connectivity is altered as PD conditions progress for individuals. Reference Chen, He and Zhang16 Interventions illustrate that as individuals with PD experience their condition worsen over time, DMN connections also progressively decrease among these individuals, specifically for PD patients who develop cognitive problems. Reference Chen, Huang, Ma and Chen26 These findings suggest that continuous DMN alteration and loss of connectivity over time may be a key contributor to worsening motor symptoms and cognitive symptoms for individuals with PD. Reference Chen, Huang, Ma and Chen26
Conflicting results regarding DMN connectivity in PD have been reported across studies. Tessitore and colleagues found diminished DMN connectivity in patients with PD, with lower connectivity correlating with an increase in motor symptom severity. Reference Tessitore, Esposito and Vitale25 Conversely, Chen et al. observed an increase in connectivity among certain DMN regions, specifically the PCC and the right angular gyrus, both of which are centers associated with motor symptom severity. These inconsistencies may reflect differences in study methodologies, such as variations in disease stage, medication statuses, seed placement strategies and data preprocessing approaches. Reference Chen, Huang, Ma and Chen26 These inconsistencies suggest that DMN connectivity alterations among patients with PD are likely region-specific, rather than reflecting a uniform pattern across the network, highlighting the influence of disease heterogeneity. Standardization of imaging protocols and clinical characterization across future investigations are essential to resolve these divergences.
Therapeutic modulation of DMN connectivity
Therapeutic interventions utilizing resting-state fMRI have been implemented to study the effects of clinical dopaminergic interventions on the connectivity of the DMN for individuals with PD. Reference Tahmasian, Eickhoff and Giehl41 A previous meta-analysis of fMRI examinations found that dopaminergic therapy was able to partially restore DMN connections, specifically in the regions of the mPFC and the PCC. Reference Tahmasian, Eickhoff and Giehl41 These findings suggest that dopamine replacement therapy could be implemented to modify DMN connectivity for individuals with PD by improving DMN connections to normal levels, possibly resulting in an improvement in both cognitive and motor symptoms for PD patients. Reference Tahmasian, Eickhoff and Giehl41
Variability across PD phenotypes
fMRI studies have also noticed that different subtypes of PD have exhibited varying effects on DMN connectivity. Reference Wang, Yu and Yan42 Studies examining different PD phenotypes show that alterations in DMN connectivity are not uniform across all subtypes. Reference Wang, Yu and Yan42 For instance, patients with the tremor-dominant subtype displayed abnormal connectivity patterns primarily within the posterior DMN regions, while patients with the postural instability and gait difficulty (PIGD) subtype exhibited further disruptions in frontal DMN regions. Reference Wang, Yu and Yan42 These findings suggest that motor phenotype influences the spatial pattern of DMN dysfunction, potentially reflecting differing underlying pathophysiology between tremor-dominant and PIGD phenotypes. This information proposes the idea that changes in DMN connectivity may be related and vary due to the different clinical PD phenotype an individual carries. Reference Wang, Yu and Yan42 These fMRI findings hint at PD being a heterogeneous and complex pathology, with varying effects on DMN and connectivity depending on different subtypes of the disease. Reference Wang, Yu and Yan42
Limitations
Employing resting-state fMRI as a measurement tool presents some limitations in practice. One issue presented by fMRI technology is the low temporal resolution provided as compared to other counterparts. Reference Logothetis, Pauls, Augath, Trinath and Oeltermann20 Due to fMRI measuring the hemodynamic response time needed for changes in blood flow based on neural stimulation, fMRI temporal resolution takes place in seconds, rather than milliseconds. Reference Logothetis, Pauls, Augath, Trinath and Oeltermann20 fMRI results may also be negatively altered due to participant head movements or physiological noise, which can promote faulty data if not corrected during analysis. Reference Goto, Abe, Miyati, Yamasue, Gomi and Takeda43 Another prominent issue is inter-tester variability when it comes to recording the BOLD signal. Although the BOLD signal is indicative of blood flow change, neural activity is not directly measured, making it difficult to differentiate between the mix of vascular and neural factors reflected in the signal. Reference Logothetis, Pauls, Augath, Trinath and Oeltermann20
Future directions
Although much research has been conducted related to PD and DMN, many drawbacks remain, which can be aimed to improve for the future. One problem is the lack of depth of understanding regarding the specific mechanisms through which PD alters DMN connectivity. While fMRI studies propose a correlation between the severity of PD and DMN connectivity, the exact physiological process and mechanism through which this occurs remains unknown. Future investigations should explore the specific mechanism of action through which decreased dopamine levels, neurotransmitter abnormalities, alpha-synuclein changes and neuroinflammation cause altered DMN connectivity for PD patients.
Furthermore, another concern is the lack of studies investigating the effect of PD on DMN connectivity related to neuropsychiatric PD symptoms as opposed to cognitive and motor symptoms. While some studies have been done exploring neuropsychiatric symptoms such as depression and their effect on DMN connectivity, many more studies have investigated the relationship between DMN abnormalities and motor and cognitive PD symptom severity. Future research should be conducted exploring other neuropsychiatric PD symptoms such as sleep disorders and hallucinations, to learn how and if these symptoms also relate to DMN abnormalities for PD patients. Moreover, while some research has been conducted exploring the different impact of differing PD subtypes on DMN connectivity, more research needs to be done to learn how each specific PD subtype differently affects DMN connectivity and if specialized clinical solutions can be tailored to cater to these different subtypes and improve DMN connectivity for PD patients.
The lack of longitudinal studies is also an issue. While most current studies are cross-sectional and provide an understanding of DMN connectivity at a specific time-point, further examinations should monitor longitudinal studies to observe changes in DMN connectivity over a long period of time as PD severity increases and cognitive and motor symptoms worsen.
Another weakness is the lack of research pertaining to utilizing DMN connectivity as a biomarker for disease progression. While literature proposes using fMRI to monitor DMN connectivity as a biomarker for PD severity progression and success of treatment interventions, further research is needed to create standard protocols to permit observing DMN connectivity as a biomarker for treatment interventions.
As DMN results have shown promise as a potential biomarker for disease progression, professionals should explore how these insights can be applied in clinical patient care rather than remaining restricted to research-based initiatives.
Conclusion
PD is a complex pathology exhibiting a myriad of motor, cognitive, neuropsychiatric and other symptoms. The DMN is an important brain network involved in resting-state functions as well as in brain tasks related to memory and executive functioning. fMRI has been used as a tool to understand the relationship between abnormal DMN connectivity and PD and the various symptoms correlated with the pathology. While current research implies a relationship between PD progression and motor and cognitive symptom severity and DMN dysfunction, future interventions should explore the concrete mechanisms of action leading to these problems, through the utilization of fMRI as an imaging tool to reach a more comprehensive understanding of the correlation between DMN dysfunction and PD progression for individuals with PD.
Availability of data and materials
Not applicable
Acknowledgments
We would like to thank everyone who assisted with this review project.
Author contributions
The paper was conceived, drafted and edited by ZAK, MAS, JS, ZR and HAB.
Funding statement
This paper was funded by private donations to the Brain Health and Wellness Research Lab situated at the University of Regina in Saskatchewan, Canada.
Competing interests
The authors report no conflicts of interest.
Ethical statement
Not applicable
Consent for publication
Not applicable


