Introduction
Self-regulation is a critical capacity first evident in infancy and shaped by early experiences, which may include exposure to maternal depression. In this meta-analysis, we first review the construct of self-regulation and infants’ development of self-regulation and then overview theoretical and empirical evidence indicating maternal perinatal (pre- and postnatal) depression may be a particularly salient exposure affecting early self-regulation. We then present a meta-analytic review of the empirical literature examining associations between maternal perinatal depression and self-regulation (broadly construed) across the first 2 years of life. We close by considering the strengths and limitations of the literature, placing our findings in the context of existing conceptual models, and considering implications for research and policy or clinical work.
Self-regulation
Conceptualization of self-regulation
Self-regulation abilities predict many domains of adaptive functioning (Robson et al., Reference Robson, Allen and Howard2020), including academic and cognitive performance (Best et al., Reference Best, Miller and Naglieri2011), emotional health (Aldao et al., Reference Aldao, Gee, De Los Reyes and Seager2016), and relational functioning (Fitzsimons & Finkel, Reference Fitzsimons and Finkel2011; Ringoot et al., Reference Ringoot, Jansen, Kok, van IJzendoorn, Verlinden, Verhulst, Bakermans-Kranenburg and Tiemeier2021). There is notable variation in current conceptualizations of self-regulation (e.g., Blair et al., Reference Blair, Berry and Friedman2012; Nigg, Reference Nigg2017; Vink et al., Reference Vink, Gladwin, Geeraerts, Pas, Bos, Hofstee, Durston and Vollebergh2020). Some inconsistency in the conceptualization of this construct stems from its parallel emergence across sub-disciplines with overlapping but different perspectives on the construct, including the temperament literature, more cognitively focused areas of the literature, and more affectively focused areas (Erdmann & Hertel, Reference Erdmann and Hertel2019). Ongoing debates about the construct include its overlap with co-regulation or external regulatory processes (Eisenberg et al., Reference Eisenberg, Spinrad and Eggum2010), whether it is conceptualized as a stable trait or an in-the-moment process (Cole et al., Reference Cole, Ashana Ramsook and Ram2019; Diaz & Eisenberg, Reference Diaz and Eisenberg2015), and the separability of reactivity and regulation (Adrian et al., Reference Adrian, Zeman and Veits2011). Overall, based on a review of prominent models (Blair & Ku, Reference Blair and Ku2022; Nigg, Reference Nigg2017), we conceptualize self-regulation broadly as a domain-general umbrella term that includes interacting bottom-up/reactive/automatic and top-down/deliberate/control processes that are internally initiatedFootnote 1 and regulate physiology, cognition, emotion, and behavior. Self-regulation is thought to develop hierarchically, with later-developing, more complex and volitional components (e.g., executive function) building on earlier-developing, more basic and automatic components (e.g., physiological regulation). In line with this framework, we consider the broad construct of self-regulation to encompass constructs specific to the regulation of physiology (e.g., autonomic regulation, adrenocortical regulation), cognition (e.g., executive function, attention control), emotion (e.g., emotion regulation), and behavior (e.g., behavior regulation). We also include constructs described within the temperament literature (e.g., effortful control, behavioral inhibition).
While much of the self-regulation literature, and some previous reviews (e.g., Power et al., Reference Power, van IJzendoorn, Lewis, Chen and Galbally2021), have focused on specific constructs within the self-regulation domain, we aimed to consider the self-regulation construct more broadly for three reasons. First, the broad construct of self-regulation is implicated frequently in the field of developmental psychopathology as a core aspect of healthy socioemotional development, and challenges in self-regulation are central to theories of the development of psychopathology (Caspi & Moffitt, Reference Caspi and Moffitt2018; Goodman & Gotlib, Reference Goodman and Gotlib1999). Second, conceptualizations of constructs underlying self-regulation (e.g., effortful control) vary substantially across the literature (Nigg, Reference Nigg2017). Given this variation, parsing the self-regulation literature by specific construct does not yield a uniform construct.Footnote 2 Finally, taking a broad approach in our conceptualization of self-regulation allowed us to consider various more meaningful characteristics of the self-regulation construct (e.g., independent regulation versus co-regulation, trait-like or process-oriented conceptualizations) as moderators.
Self-regulation development
The development of self-regulation begins prenatally and continues throughout infancy, childhood, and adolescence (Cerritelli et al., Reference Cerritelli, Frasch, Antonelli, Viglione, Vecchi, Chiera and Manzotti2021; Feldman, Reference Feldman2009; Geva & Feldman, Reference Geva and Feldman2008; Kopp, Reference Kopp1982; Panagiotakopoulos & Neigh, Reference Panagiotakopoulos and Neigh2014). The earliest forms of physiological regulation, as indexed, for example, by fetal heart rate variability (Kinsella & Monk, Reference Kinsella and Monk2009), are supported by autonomic nervous system (ANS) and hypothalamic-pituitary-adrenal (HPA) axis development and can be detected prenatally with physiological assessments (Cerritelli et al., Reference Cerritelli, Frasch, Antonelli, Viglione, Vecchi, Chiera and Manzotti2021; Panagiotakopoulos & Neigh, Reference Panagiotakopoulos and Neigh2014). During the first 2 to 3 months after birth, neurophysiological processes and reflexive behaviors, which can be reliably assessed with structured clinician assessments (Brazelton & Nugent, Reference Brazelton and Nugent2011), regulate infant physiological homeostasis and arousal states (Feldman, Reference Feldman2009; Kopp, Reference Kopp1982). Other early regulatory behaviors include rudimentary self-comforting, such as hand- or thumb-sucking, and early bids for caregiver regulatory support, such as distress vocalizations (Fox & Calkins, Reference Fox and Calkins2003). As these behaviors are readily observable, even from this early developmental stage, observation-based and caregiver-report assessments of self-regulation become possible. Later in the first year, infants begin to employ attentional and behavioral strategies to regulate emotions, such as averting their gaze from distressing stimuli and attempting to engage caregivers with positive vocalizations. Rudimentary inhibitory motor control also emerges around this time. In the second year of life, increases in and integration of attentional and inhibitory motor control capacities provide the foundation for various developmental competencies, including delaying gratification and beginning to comply with external demands (Fox & Calkins, Reference Fox and Calkins2003). In toddlerhood and beyond, prefrontal cortex development further facilitates attention regulation as a self-regulatory strategy that enables increasingly independent regulation of behavior, attention, and emotions (Feldman, Reference Feldman2009; Vink et al., Reference Vink, Gladwin, Geeraerts, Pas, Bos, Hofstee, Durston and Vollebergh2020). Research supports the hierarchical nature of this development by demonstrating that difficulties in foundational domains (e.g., physiological regulation) contribute to difficulties in later-developing abilities (Feldman, Reference Feldman2009; Wu et al., Reference Wu, Yan and Cui2021).
Critically, the early development of self-regulation occurs in the context of social relationships, primarily the caregiver-infant relationship (Beeghly & Tronick, Reference Beeghly and Tronick2011; Fox & Calkins, Reference Fox and Calkins2003; Vink et al., Reference Vink, Gladwin, Geeraerts, Pas, Bos, Hofstee, Durston and Vollebergh2020). The Mutual Regulation Model emphasizes caregivers’ role in self-regulation development during infancy and suggests that self-regulation develops in the dyadic context, based on the contributions of an infant subsystem, a caregiver subsystem, and the dynamic interaction between them (Gianino & Tronick, Reference Gianino, Tronick, Field, McCabe and Schneiderman1988). Thus, understanding long-term self-regulation development requires examination of not only infant regulatory capacities, but also of caregiver contributions and contextual factors affecting them.
Maternal depression and infant self-regulation
A substantial literature demonstrates that infants, children, and adolescents of mothersFootnote 3 with depression show differences across domains of functioning, including difficulties with self-regulation (e.g., Babineau et al., Reference Babineau, Green, Jolicoeur‐Martineau, Bouvette‐Turcot, Minde, Sassi, St‐André, Carrey, Atkinson, Kennedy, Lydon, Steiner, Gaudreau, Levitan, Meaney and Wazana2015; Bates et al., Reference Bates, Salsberry, Justice, Dynia, Logan, Gugiu and Purtell2020; Pinto et al., Reference Pinto, Nogueira-Silva and Figueiredo2023), compared with those of mothers without depression (Gotlib et al., Reference Gotlib, Buthmann and Miller2023). Additionally, there are theoretical reasons maternal depression may influence infant self-regulation development. Specifically, maternal physiological processes that accompany elevated prenatal depressive symptoms may affect the development of fetal/infant physiological regulation (Field et al., Reference Field, Diego, Dieter, Hernandez-Reif, Schanberg, Kuhn, Yando and Bendell2004; Galbally et al., Reference Galbally, Watson, van IJzendoorn, Saffery, Ryan, de Kloet, Oberlander, Lappas and Lewis2020; Kinsella & Monk, Reference Kinsella and Monk2009; Monk et al., Reference Monk, Fifer, Myers, Bagiella, Duong, Chen, Leotti and Altincatal2011), which is foundational to broader infant self-regulation (Feldman, Reference Feldman2009). Additionally, maternal postnatal depressive symptoms may make it more difficult for mothers to engage in sensitive parenting behaviors that support the development of infant self-regulation (Bernard et al., Reference Bernard, Nissim, Vaccaro, Harris and Lindhiem2018; Bernier et al., Reference Bernier, Carlson and Whipple2010). Thus, we expect that higher maternal perinatal depressive symptoms will be associated with lower infant self-regulation.
The present meta-analysis
Maternal perinatal depression is remarkably prevalent, affecting up to one in five mother-infant dyads (Bauman et al., Reference Bauman, Ko, Cox, D’Angelo, Warner, Folger, Tevendale, Coy, Harrison and Barfield2020), with prevalence likely even higher since the onset of the COVID-19 pandemic (Iyengar et al., Reference Iyengar, Jaiprakash, Haitsuka and Kim2021). Understanding the association between maternal depression and infant self-regulation is critical due to the hierarchical nature of self-regulation development; factors affecting self-regulation in infancy continue to shape self-regulation into later childhood and beyond and may serve as a vulnerability for the development of psychopathology or other difficulties (Goodman & Gotlib, Reference Goodman and Gotlib1999). However, the literature on this association has been mixed. Some results suggest higher levels of maternal perinatal depression are associated with lower infant self-regulation (e.g., Bates et al., Reference Bates, Salsberry, Justice, Dynia, Logan, Gugiu and Purtell2020), some suggest no association (Conradt et al., Reference Conradt, Lester, Appleton, Armstrong and Marsit2013), and some suggest better infant self-regulation in the context of maternal depression (e.g., Khoury et al., Reference Khoury, Gonzalez, Levitan, Masellis, Basile and Atkinson2016). These discrepant findings may be due to various factors: imprecision related to sampling error in individual studies with modest sample sizes, important demographic and methodological characteristics of studies that contribute to systematic differences in findings, and variation in conceptualizations of self-regulation across studies.
While previous reviews have examined associations between maternal anxiety or stress and offspring self-regulation (Korja et al., Reference Korja, Nolvi, Grant and McMahon2017); maternal depression and broader child socioemotional development, temperament, psychopathology, or behavior problems (Beck, Reference Beck1998, Reference Beck1999; Connell & Goodman, Reference Connell and Goodman2002; Goodman et al., Reference Goodman, Rouse, Connell, Broth, Hall and Heyward2011; Harris & Santos, Reference Harris and Santos2020; Madigan et al., Reference Madigan, Oatley, Racine, Fearon, Schumacher, Akbari, Cooke and Tarabulsy2018; Martucci et al., Reference Martucci, Aceti, Giacchetti and Sogos2021; Parsons et al., Reference Parsons, Young, Rochat, Kringelbach and Stein2012; Petzoldt, Reference Petzoldt2018; Takegata et al., Reference Takegata, Matsunaga, Ohashi, Toizumi, Yoshida and Kitamura2021; Waxler et al., Reference Waxler, Thelen and Muzik2011); maternal depression and child executive function (Power et al., Reference Power, van IJzendoorn, Lewis, Chen and Galbally2021); and maternal depression and infant response to the Still Face Paradigm (Graham et al., Reference Graham, Blissett, Antoniou, Zeegers and McCleery2018), to our knowledge, no previous reviews have examined the association between maternal perinatal depression and infant self-regulation.
In this pre-registered meta-analysis, we systematically reviewed and quantitatively synthesized the empirical literature examining the association between maternal depression and infant self-regulation. Despite evidence of mixed findings in the literature, given theoretical links between maternal perinatal depression and infant self-regulation, we hypothesized that more symptoms of maternal depression or a depression diagnosis would be associated with lower infant self-regulation. We also examined heterogeneity and potential demographic and methodological moderating variables. Our demographic moderators (infant sex and infant age at self-regulation assessment) were exploratory. We had directional hypotheses for some of our methodological moderators (sample type, type of depression score, and reliability of depression and self-regulation measures), for which we expected stronger associations in clinical samples, with continuous scores, and with more reliable measures. Other methodological moderators (method used to assess maternal depression and infant self-regulation, timing of maternal depression, and moderators related to self-regulation conceptualizations) were exploratory.
Method
This study was pre-registered (https://osf.io/jw7t2/overview?view_only=76cb058fad0e42c9b584ec4c675ba0a1). Additional details, including deviations from preregistration, can be found below and in the Supplemental Methods.
Inclusion and exclusion criteria
Inclusion criteria for the meta-analysis were (a) assessment of self-regulation or an underlying construct (see Table 1) among human infants ages 0 through 23 months, (b) assessment of maternal depression prior to or concurrently with assessment of infant self-regulation, and (c) availability of sufficient information for calculating effect size(s) of the association between maternal depression and infant self-regulation. We defined infancy as 0 – 23 months to encompass a wider range of the developmental change occurring in early self-regulation and to allow us to examine infant age as a moderator of our findings. We also included only studies assessing maternal depression prior to or concurrentlyFootnote 4 with infant self-regulation to align with our broader theoretical model in which maternal depression influences infant self-regulation.
Table 1. Self-regulation-related constructs in the meta-analysis

a We excluded effect sizes for which the measure of self-regulation assessed only maternal contributions to infant regulation.
b We did not include assessments of (dys)regulation that clearly did not relate to self-regulation (e.g., regulation of laws, regulation of genes).
c Or (dys)regulation of a specific emotion (e.g., fear regulation).
Exclusion criteria were (a) assessment of maternal depression after assessment of infant self-regulation, (b) intervention studies that did not provide associations prior to the intervention or separately for the control group, (c) case studies or studies with fewer than 10 participants, (d) studies not in English, and (e) studies with insufficient effect size information. We did not apply any exclusion criteria related to study population or study geographic location, and included studies came from across the world.
Literature search
Our search and screening procedures were pre-registered, and deviations are noted. With the goals of capturing a comprehensive picture of early self-regulation and affording ourselves the ability to examine conceptual variations in the self-regulation construct as moderators, we conceptualized self-regulation as an umbrella term with many underlying constructs. We selected underlying constructs (Table 1) based on review of prominent models of self-regulation (Adrian et al., Reference Adrian, Zeman and Veits2011; Barkley, Reference Barkley2001; Baumeister & Vohs, Reference Baumeister and Vohs2007; Beeghly et al., Reference Beeghly, Perry, Tronick and Maltzmana2016; Blair & Ku, Reference Blair and Ku2022; Diamond, Reference Diamond2013; Eisenberg & Spinrad, Reference Eisenberg and Spinrad2004; Eisenberg & Sulik, Reference Eisenberg and Sulik2012; Feldman, Reference Feldman2009; Fox & Calkins, Reference Fox and Calkins2003; Hendry et al., Reference Hendry, Jones and Charman2016; Inzlicht et al., Reference Inzlicht, Werner, Briskin and Roberts2021; Kochanska et al., Reference Kochanska, Murray and Harlan2000; Kopp, Reference Kopp1982; Posner & Rothbart, Reference Posner and Rothbart1998; Rothbart & Derryberry, Reference Rothbart, Derryberry, Lamb and Brown1981; Rothbart et al., Reference Rothbart, Ellis, Rueda and Posner2003; Thompson, Reference Thompson1994; Zelazo & Carlson, Reference Zelazo and Carlson2020).
We searched four online databases (ERIC, Medline, PsycINFO, and ProQuest Dissertations). We searched titles, abstracts, and keywords of articles using a combination of search terms capturing the self-regulation-related constructs listed in Table 1 and depression (“depress*”) and targeting the infancy period (infan*, newborn*, neonat*, baby, or babies). We limited our search to studies in English and involving human subjects. To examine the past 10+ years of empirical literature, which have coincided with a substantial increase in interest in self-regulation, we searched for studies published between January 1, 2010 and November 30, 2023. Searches were conducted and records were exported on December 11, 2023.
The search produced 2,147 non-duplicate records (duplicates were identified manually by the first author). We first screened titles and abstracts of all records and excluded records that were not empirical, not in English, or did not include human subjects. We sought 1,036 records for full-text screening, of which five could not be retrieved. During full-text screening, we excluded 795 records that did not measure self-regulation or a related constructFootnote 5 in the first 2 years after birth. We further excluded 99 records that did not measure maternal depression or measured it after measurement of infant self-regulation. We excluded four records reporting intervention studies that did not provide information on the association between maternal perinatal depression and infant self-regulation prior to the intervention (i.e., at baseline) or separately for the control group. Further, we excluded 61 records for which we were unable to estimate effect sizes for the association between maternal depression and infant self-regulation.Footnote 6
We also examined reference lists of included records and records that cited included records for additional relevant records, which yielded three additional records that met inclusion criteria. Altogether, these search and screening procedures produced 68 reports that included 193 effect sizes and 16,722 unique mother-infant dyads that were included in the meta-analytic review. The search and screening procedure, which followed PRISMA guidelines (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennan, Chou, Glanville, Grimshaw, Hróbjartsson, Lalu, Li, Loder, Mayo-Wilson, McDonald and Moher2021) is documented in Figure 1.

Figure 1. Flow diagram for literature search and screening procedure. Note. This figure is adapted from the preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennan, Chou, Glanville, Grimshaw, Hróbjartsson, Lalu, Li, Loder, Mayo-Wilson, McDonald and Moher2021). a The search was limited to the years 2010 – 2023. However, the years of three articles were indexed incorrectly in the databases and thus removed during title/abstract screening. b When we identified two or more records that presented the association of the same variables within the same (or a subset of the same) sample, we retained the record presenting the effect size with the largest n, or the most recent record if ns were identical. If the non-retained record(s) provided additional information not presented in the retained record about any of our coded variables, we retained this information for analyses.
Twenty to twenty-five percent of records were second-screened to evaluate interrater reliability of screening criteria. Percent agreement was greater than 95% for each of the three screening steps, which were determining if 1) the record included an assessment of self-regulation or an underlying construct, 2) the self-regulation-related construct was assessed in the age range, and 3) the record included an assessment of maternal depression/depressive symptoms prior to or concurrent with the self-regulation assessment.
Study coding
Included studies were coded by the first author (a doctoral candidate in developmental and clinical psychology) or a trained research assistant (an undergraduate in child development) for the following variables, which were used in moderator analyses or to characterize the studies: 1) study information and sample characteristics, 2) maternal depression information, 3) infant self-regulation information, and 4) data for calculation/estimation of effect sizes. Twenty percent of records were second-coded to determine interrater reliability of each code used in moderator analyses. Additional information on specific coding procedures is provided in the Supplemental Methods.
Study information and sample characteristics
Potential Moderators.Footnote 7
Infant Sex (Cohen’s κ = .84). Percent of infants in the sample who were female, centered at 50%.
Infant Age at Assessment of Self-Regulation (κ = .96). Infant age in months at the time of self-regulation assessment, mean-centered.
Type of Sample (κ = .72). Community samples were intended to represent the broader general community, i.e., the researchers did not aim to recruit mothers with psychopathology. Clinical samples comprised participants recruited for clinical levels of maternal psychopathology or heightened psychological distress.
Additional descriptive codes
Mother Race and Ethnicity. Mother race and ethnicity, however it was reported in each study.
Maternal Age. Mean and/or range of maternal age in years.
Parity. Percent of mothers who were primiparous.
Country of Study. Country where data collection occurred.
Maternal depression information
Potential moderators
Maternal Depression Method (κ = 1.00). We coded the method as “self-report questionnaire,” “other-report questionnaire,” “diagnostic interview,” “extracted from medical chart,” “observational coding”, or “other.”
Maternal Depression Timing (κ = .95). We coded timing as “lifetime history,” “pre-pregnancy,” “pregnancy,” “postnatal,” or “other.” Only pregnancy and postnatal had sufficient frequency to be included in moderator analyses.
Type of Depression Score (κ = 1.00). We coded whether maternal depression scores were “binary” (e.g., presence or absence of a diagnosis of depression), “other categorical” (e.g., low, moderate, and high levels of depression), or “continuous.” The included effect sizes only used binary and continuous scores.
Reliability of Depression Measure (κ = .88). Reliability metrics (e.g., Cronbach’s α, Cohen’s κ) were not directly comparable, so we created a post-hoc binary (adequate/inadequate) reliability variable using the following thresholds: Cronbach’s α ≥ .70 (Tavakol & Dennick, Reference Tavakol and Dennick2011), Cohen’s κ ≥ .60 (McHugh, Reference McHugh2012), and intraclass correlation coefficient ≥ .75 (Koo & Li, Reference Koo and Li2016). All included effect sizes that reported reliability used measures with adequate reliability; thus, we could not examine this moderator.
Additional descriptive codes
Measure of Maternal Depression. The name of the maternal depression measure used.
Infant self-regulation information
Potential moderators
Self-Regulation Method (κ = 1.00). We coded the method as “questionnaire,” “observational coding,” “clinician assessment,” “interview,” “eye-tracking task,” or “other.”
Reliability of Self-Regulation Measure (κ = 1.00). We created a post-hoc binary (adequate/inadequate) reliability variable using the following thresholds: Cronbach’s α and McDonald’s ω ≥ .70 (Tavakol & Dennick, Reference Tavakol and Dennick2011), Cohen’s κ ≥ .60 (McHugh, Reference McHugh2012), Intraclass Correlation Coefficient ≥ .75 (Koo & Li, Reference Koo and Li2016), and percent rater agreement ≥ 70.
Independence of Self-Regulation Measure (κ = .96). We coded whether each self-regulation measure captured regulatory strategies employed independently by the infant or co-regulatory processes. See Supplemental Table S1 for details. Levels of this code, as well as the other codes indexing self-regulation conceptualization, are described with examples in Supplemental Table S1.
Trait- or Process-Oriented Measure of Self-Regulation (κ = .94). We coded whether self-regulation was conceptualized as a trait-like aspect of infant temperament or as a regulatory process.
Context of Self-Regulation Assessment (κ = 1.00). We coded whether regulation was assessed in the context of an acute stressor or not.
Additional descriptive codes
Self-Regulation-Related Construct Assessed. We coded which self-regulation-related construct was assessed for each effect size.
Measure of Self-Regulation. We coded the infant self-regulation measure for each effect size.
Effect size data
We coded unadjusted bivariate Pearson’s product moment correlation coefficients or the information needed to estimate them from the following unadjusted statistics: point-biserial correlation coefficients, standardized β, frequencies from crosstab frequency tables, means and standard deviations, t-tests, F-tests, contingency tables, χ-squares, or exact significance levels. We estimated effect sizes using the formulas provided by Lipsey & Wilson (Reference Lipsey and Wilson2001) via Wilson’s online effect size calculator (Wilson, Reference Wilson2023).Footnote 8 We also recorded the analytic sample size for each effect size.
Data analysis
Characteristics of included studies and effect sizes
We first examined characteristics of each included study and effect size, including study-level sample characteristics (e.g., sample type, demographics), characteristics of the measure of maternal perinatal depression, and characteristics of the measure of infant self-regulation.
Risk of Bias Assessment. As part of our characterization of included studies, we considered various study characteristics related to risk of bias. First, we considered demographics of the participants represented in the studies (race and ethnicity, country of study, ages of included mothers and infants) to assess specificity or generalizability of findings to the broader population. We also considered various methodological characteristics, including whether the selected measures were previously established in the field and reliable in the present sample. Further, we considered the methods used for both maternal depression and infant self-regulation, which allowed us to consider potential issues such as shared reporter bias.
Effect size estimation
To synthesize effect sizes, we used zero-order Pearson product moment correlation coefficients, which estimate the rank-order association between two continuous variables (Lipsey & Wilson, Reference Lipsey and Wilson2001). We transformed Pearson product moment correlation coefficients using Fisher’s Z r -transformation to correct bias in the standard errors used to estimate inverse variance weights (Hedges & Olkin, Reference Hedges and Olkin1985). We back-transformed the final pooled effect size for interpretation. We used the metafor package version 4.4-0 (Viechtbauer, Reference Viechtbauer2010) in R (R Core Team, 2021) to apply the Fisher’s Z r -transformation to effect sizes and estimate inverse variance weights.
Synthesis of the overall association between maternal perinatal depression and infant self-regulation
For our primary analysis, we used a three-level random effects multilevel model. We selected a random-effects model because we hypothesized there would be systematic heterogeneity in the underlying true effect sizes represented by the effect sizes included in the meta-analysis due to methodological and conceptual differences (Harrer et al., Reference Harrer, Cuijpers, Furukawa and Ebert2021). We selected a three-level model to account for interdependence of effect sizes, as many of the records included multiple effect sizes, thus violating the assumption of statistical independence. Including a third level accounted for this interdependence (effect sizes nested within studies) and allowed us to retain all effect sizes (Harrer et al., Reference Harrer, Cuijpers, Furukawa and Ebert2021). We estimated τ 2, the variance of the distribution of true effect sizes, using the restricted maximum-likelihood estimator, which performs well in meta-analyses synthesizing continuous effect sizes (Langan et al., Reference Langan, Higgins, Jackson, Bowden, Veroniki, Kontopantelis, Viechtbauer and Simmonds2019; Veroniki et al., Reference Veroniki, Jackson, Viechtbauer, Bender, Bowden, Knapp, Kuss, Higgins, Langan and Salanti2016). To quantify precision of the synthesized effect size, we calculated the 95% confidence interval (CI). Finally, we examined whether a three-level model provided a better fit to the data than a two-level model using a likelihood ratio test (Harrer et al., Reference Harrer, Cuijpers, Furukawa and Ebert2021). We used the metafor package version 4.4-0 (Viechtbauer, Reference Viechtbauer2010) in R for effect size synthesis.
Examine potential moderators of the association between maternal perinatal depression and infant self-regulation
After estimating an overall pooled effect size, we examined whether there was significant between-study and/or between-effect size heterogeneity in the estimate using Cochran’s Q, which tests whether the variation in the effect sizes in a meta-analysis exceeds the amount of variation expected if there were no between-effect size or between-study heterogeneity (e.g., if all variation were due to sampling error; Harrer et al., Reference Harrer, Cuijpers, Furukawa and Ebert2021). We also quantified the proportion of variance due to sampling error vs between-study and between-effect-size variation using a multilevel version of the I 2 statistic (Cheung, Reference Cheung2014). By convention, I 2 values of 25, 50%, and 75% represent low, moderate, and high heterogeneity, respectively (Migliavaca et al., Reference Migliavaca, Stein, Colpani, Barker, Ziegelmann, Munn and Falavigna2022). Further, we calculated the 95% prediction interval (PI), which provides a range into which we can expect the effects of future studies in similar contexts to fall (IntHout et al., Reference IntHout, Ioannidis, Rovers and Goeman2016). We used version 0.1.0 of the dmetar package (Harrer et al., Reference Harrer, Cuijpers, Furukawa and Ebert2021) in R.
For moderator analyses, we estimated separate three-level mixed-effects meta-analytic models with each moderator as a predictor using the metafor package version 4.4-0 (Viechtbauer, Reference Viechtbauer2010) in R. For each moderator model, effect sizes with missing data on the relevant moderator were excluded. For categorical moderators, we did not include categories with fewer than k = 5 effect sizes in moderator analyses. Results of moderator analyses can be interpreted as follows: For both continuous and categorical moderators, the intercept is the expected effect size when the value of the moderator is zero (for categorical moderators, the value for the reference group). The unstandardized regression coefficient provides the estimated difference in the effect size for a one-unit difference in the value of the moderator (for categorical moderators, the difference in expected effect size between reference and comparison groups). We did not conduct a priori power analyses for overall or moderator effects.
Examination of publication bias and influential cases
To evaluate publication bias, we created a funnel plot and used Egger’s regression asymmetry test (Egger et al., Reference Egger, Davey Smith, Schneider and Minder1997) in the metafor package version 4.4-0. Additionally, to explore our data for possible influential cases (Viechtbauer & Cheung, Reference Viechtbauer and Cheung2010), we calculated Cook’s distance for effect sizes and studies and standardized DFBETA values. As a priori guidelines, we considered Cook’s distances of > .45 or standardized DFBETA values >
![]() $2/\sqrt k$
to be evidence of potentially influential statistics (Harrer et al., Reference Harrer, Cuijpers, Furukawa and Ebert2021; Viechtbauer & Cheung, Reference Viechtbauer and Cheung2010). In the case of influential cases, we conducted sensitivity analyses without potential outliers to examine the robustness of our conclusions to potential influential cases.
$2/\sqrt k$
to be evidence of potentially influential statistics (Harrer et al., Reference Harrer, Cuijpers, Furukawa and Ebert2021; Viechtbauer & Cheung, Reference Viechtbauer and Cheung2010). In the case of influential cases, we conducted sensitivity analyses without potential outliers to examine the robustness of our conclusions to potential influential cases.
Results
Characteristics of included studies and effect sizes
Overview of included studies
A total of 68 records, which comprised 193 effect sizes, were included in this meta-analysis (see Table A1 in the Appendix for included records). Analytic sample sizes of included effect sizes ranged from 11 to 5,728, with an average analytic n of 244 mother-infant dyads.
Study information and sample characteristics
See Supplemental Table S2 for descriptive statistics of variables coded to characterize included records and for moderator analyses. Sample demographics and data collection country varied across studies. Specifically, of the 50 (74%) records that provided information on the race and/or ethnicity of their samples, 34 (68%) comprised 50% or greater White participants. While most records (54%) described studies conducted in the United States, 15 other countries (primarily European) were represented in the meta-analysis. Mothers in the studies ranged in age from 15 to 51 years. Most studies included only adult mothers; of the studies that reported the age range of their participants, only three included mothers under 18 years of age. On average, studies included slightly more than half (65%) primiparous mothers. Seven studies limited participation to only primiparous mothers. There was limited variability in the proportion of male and female infants in the included samples, with all samples approximately evenly split between male and female infants (range: 40 – 63% female). Across included effect sizes, infant age ranged from assessment within 24 hours of birth to an average age of to 16.10 months. Finally, most effect sizes (72%) were from community samples.
Maternal depression information
Next, we examined characteristics of the maternal depression assessments. Most (k = 109) effect sizes described associations between maternal postnatal depression and infant self-regulation. Most depression measures were self-report questionnaires (k = 156), and most used continuous measures of depression (k = 150). Among the 106 effect sizes for which reliability (internal consistency or interrater reliability) of the measure of maternal depression was reported, all reported adequate reliability. Finally, regarding measures used to assess maternal depression, the Edinburgh Postnatal Depression Scale (EPDS; Cox et al., Reference Cox, Holden and Sagovsky1987) was by far the most common (k = 87), followed by the Center for Epidemiologic Studies-Depression Scale (CES-D; Radloff, Reference Radloff1977; k = 26).
Infant self-regulation information
Next, we examined characteristics of infant self-regulation assessments. Emotion regulation and self-regulation were the most frequently assessed constructs (k = 37 each), followed by regulation (k = 34). For most effect sizes, infant self-regulation was assessed with parent-report questionnaires (k = 104), but assessment using observational coding of structured tasks was also common (k = 66). The most common measure used to assess infant self-regulation was the Infant Behavior Questionnaire-Revised (IBQ-R; Gartstein & Rothbart, Reference Gartstein and Rothbart2003; k = 87), although there was wide variability in the factors or subscales of the IBQ-R selected (e.g., Orienting/Regulatory Control factor, Negative Affectivity factor, Decreased Vocal Reactivity subscale). The most common observational coding paradigms were mother-infant structured- or free-play interactions (k = 25) and tasks from the Laboratory Temperament Assessment Battery (LabTAB; Goldsmith & Rothbart, Reference Goldsmith and Rothbart1992; k = 18). Self-regulation measure reliability was provided for 93 effect sizes, and of these, most (k = 71) showed adequate reliability. Regarding self-regulation conceptualizations, most effect sizes used measures that focused on combined approaches (k = 80) or independent infant contributions (k = 79) to self-regulation and did not assess self-regulation during stress tasks (k = 156). More than half of effect sizes used a measure that captured trait-like self-regulation (k = 107).
Synthesis of the overall association between maternal perinatal depression and infant self-regulation
As hypothesized, overall, higher levels of maternal perinatal depression were significantly associated with lower infant self-regulation (r = −.10, 95% CI = −.14, −.06, p < .001; Figure 2). The three-level model fit the data better than a two-level model with level-3 heterogeneity constrained to zero (χ 2(1) = 90.61, p < .001).
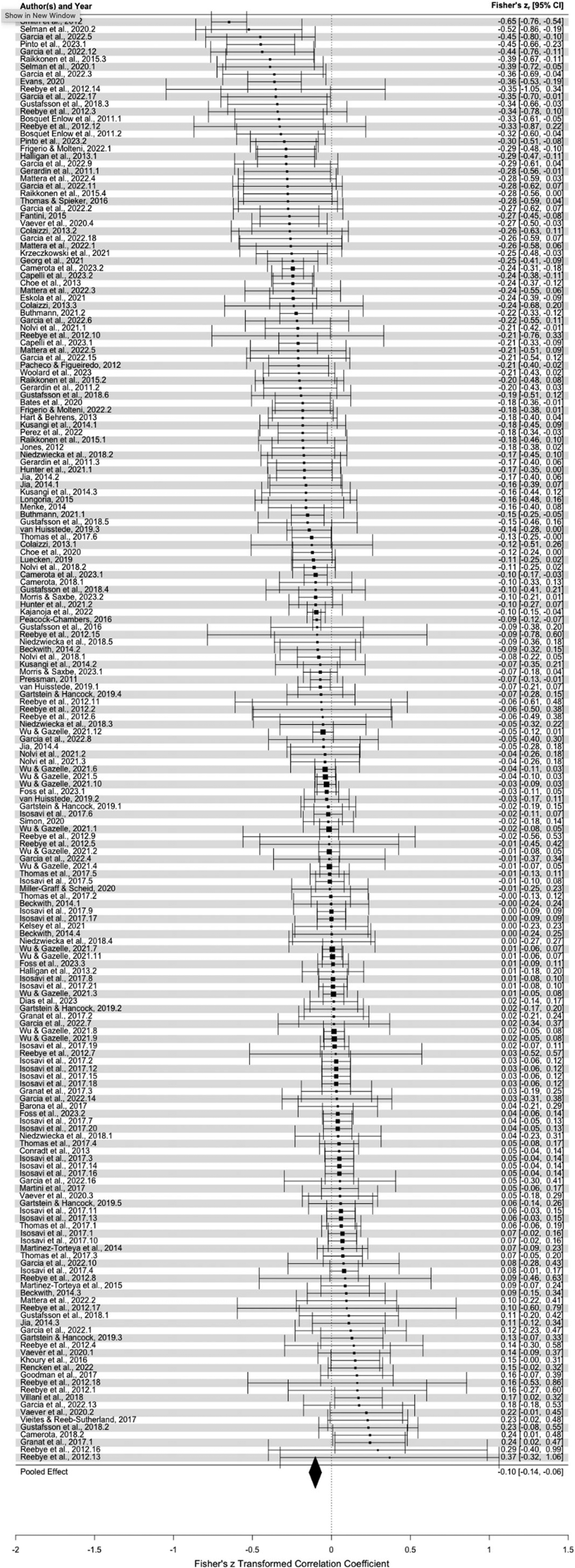
Figure 2. Forest plot of included effect sizes and overall pooled effect size of the association between maternal perinatal depression and infant self-regulation. Note. This figure shows estimated effect sizes (Fishers Zr-transformed correlation coefficients) and 95% confidence intervals of the included studies, as well as the overall pooled effect size. Stronger negative associations indicate that higher levels of maternal depression were associated with lower infant self-regulation.
Examine potential moderators of the association between maternal perinatal depression and infant self-regulation
We next examined heterogeneity in the overall effect size using multiple indices. The Q-statistic test for heterogeneity suggested true effect size differences in the data (Q (192) = 577.44, p < .001). Further, the PI ranged from r = −.36 to r = .17, indicating that levels of heterogeneity suggest that positive associations (i.e., in which more maternal depression is associated with more infant self-regulation) not due to sampling error cannot be ruled out for future studies (Harrer et al., Reference Harrer, Cuijpers, Furukawa and Ebert2021). The multilevel I 2 suggested about 18% of variance was due to sampling error, less than 1% of variance was due to within-study characteristics, and about 82% of variance was due to between-study characteristics. Thus, there was substantial heterogeneity not due to sampling error, and most of this heterogeneity was at the between-study level, indicating follow-up moderator analyses to examine this heterogeneity were warranted.
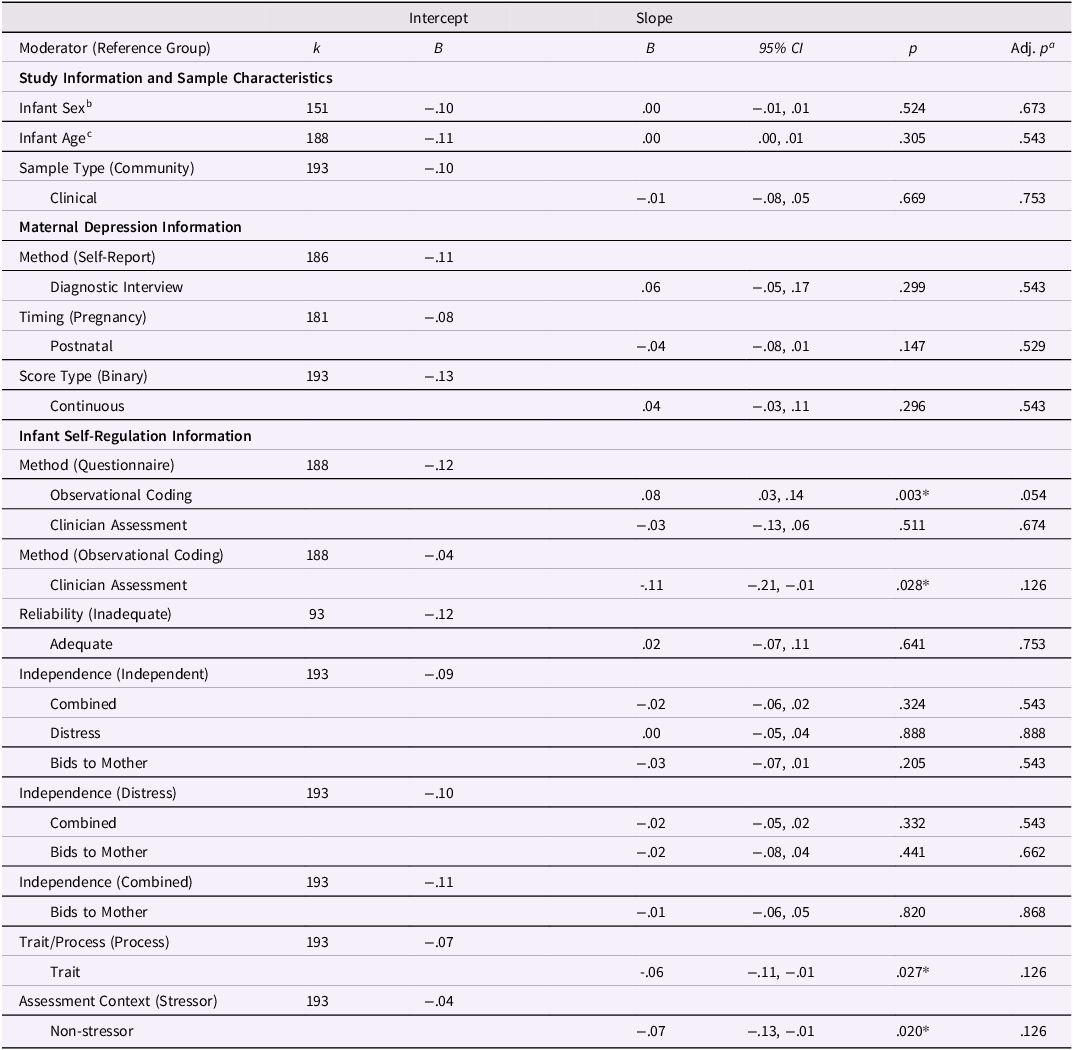
Results of moderator analyses are presented in Table 2.
Table 2. Results of moderator analyses

Note. All moderator analyses were conducted in a meta-regression framework, with categorical moderators entered using dummy coding. The unstandardized coefficient of the intercept is the effect size when the moderator is zero (for categorical moderators, the effect size for the reference group). The unstandardized coefficient of the slope is the estimated change in the effect size for a one-unit change in the value of the moderator (for categorical moderators, the estimated difference in effect sizes between the reference and comparison groups). For categorical moderators, the reference groups are indicated in parentheses. For categorical moderators with greater than two levels, multiple models with different reference groups were estimated to present all possible comparisons.
a p-values adjusted for multiple comparisons using the Benjamini-Hochberg adjustment.
b Percent of the sample that is female, centered at 50%.
c Mean age of the sample in months, mean-centered.
* p < .05.
Study information and sample characteristics
None of the variables describing study or sample characteristics (i.e., infant sex, infant age, or sample type) significantly moderated the association (see Table 2, all ps > .305).
Maternal depression information
Similarly, none of the maternal depression-related variables (i.e., method, timing, or score type) significantly moderated the association (see Table 2, all ps > .147).
Infant self-regulation information
Several infant self-regulation-related variables moderated the association, although none remained significant after multiple comparisons correction. First, the magnitude of the association between maternal depression and infant self-regulation depended on the method used to assess infant self-regulation. The estimated effect size was smaller and not significantly different from zero when infant self-regulation was assessed with observational coding (r IntObservational = −.04, 95% CI = −.09, .02, p = .185) compared to questionnaires (r IntQuestionnaire = −.12, 95% CI = −.16, −.08, p = < .001) and clinician assessments (r IntClinician = −.15, 95% CI = −.23, −.07, p = < .001). The association did not differ between questionnaires and clinician assessments. The other two significant moderators were among the exploratory codes aimed to capture self-regulation conceptualization. Effect sizes based on trait-focused self-regulation measures (r IntTrait = −.13, 95% CI = −.17, −.08, p = < .001) showed stronger associations than effect sizes based on process-focused self-regulation measures (r IntProcess = −.07, 95% CI = −.12, −.02, p = .005). Further, effect sizes based on self-regulation measures in stressor contexts (r IntStressor = −.04, 95% CI = −.10, .02, p = .221) showed weaker, non-significant associations than those based on measures in non-stressor contexts (r IntNotStressor = −.11, 95% CI = −.15, −.07, p = < .001).
In sum, overall, more maternal depression was associated with lower infant self-regulation, and this pooled effect showed substantial heterogeneity. Effect sizes differed across levels of three moderators: self-regulation method, whether the self-regulation method was trait- vs. process-focused, and whether self-regulation was assessed in a stressor context.
Examination of publication bias and influential cases
Figure 3 shows the funnel plot for the meta-analysis. Visual inspection did not suggest substantial asymmetry. Consistent with this visual inspection, the intercept of Egger’s regression test was not significantly different from zero (B 0 = 0.02, SE = .02, p = .197). These findings do not raise concerns for publication bias.

Figure 3. Funnel plot providing visualization of potential small study bias.
We used multiple methods to explore our data for cases that may have undue influence on our results. Based on Cook’s distance, we did not identify any such cases. However, standardized DFBETA values suggested eight effect sizes (Conradt et al., Reference Conradt, Lester, Appleton, Armstrong and Marsit2013; Evans, Reference Evans2020; Goodman et al., Reference Goodman, Bakeman, McCallum, Rouse and Thompson2017; Khoury et al., Reference Khoury, Gonzalez, Levitan, Masellis, Basile and Atkinson2016; Rencken et al., Reference Rencken, Govender and Uys2022; Smith et al., Reference Smith, Paz, LaGasse, Derauf, Newman, Shah, Arria, Huestis, Haning, Strauss, Della Grotta, Dansereau, Neal and Lester2012; Vieites & Reeb-Sutherland, Reference Vieites and Reeb-Sutherland2017; and Villani et al., Reference Villani, Ludmer, Gonzalez, Levitan, Kennedy, Masellis, Basile, Wekerle and Atkinson2018) may function as influential cases based on our a priori guidelines. We ran eight separate sensitivity analyses each leaving out one of these effect sizes. All sensitivity models indicated a significant negative association between maternal depression and infant self-regulation, consistent with the primary analysis, and suggesting limited impact of these cases on substantive conclusions.
Discussion
Meta-analytic findings
The overall meta-analytic effect suggested that higher levels of maternal perinatal depression were associated with modestly lower infant self-regulation. This finding is consistent with the leading theoretical model of the intergenerational transmission of depression (Goodman & Gotlib, Reference Goodman and Gotlib1999) positing that maternal depression undermines offspring self-regulation, and that these resulting self-regulation difficulties are a vulnerability increasing risk for offspring depression. Indeed, difficulties with self-regulation are implicated as a key process in depression onset and maintenance (e.g., Joormann & Stanton, Reference Joormann and Stanton2016), further underscoring the importance of identifying and intervening upon early exposures that may undermine self-regulation.
Importantly, multiple approaches for quantifying heterogeneity indicated significant heterogeneity in the overall effect. This heterogeneity motivates examining moderators of the overall association. For example, the PI suggests that some levels of some moderators may yield group estimates for which higher levels of maternal depression are associated with more infant self-regulation. None of our selected moderators produced such results, but others that we did not consider may, which would have important theoretical implications for understanding nuances of this association.
Several methodological variables did moderate the overall effect, although none survived multiple comparisons correction. First, the infant self-regulation assessment method moderated the strength of the association, with effect sizes based on observational coding showing smaller (null) associations than effect sizes using questionnaires and clincian assessments. Questionnaire measures of self-regulation were typically completed by mothers, and it is possible the associations based on these measures were inflated by a negative reporting bias among mothers with depression or by a more general shared informant bias. Alongside this potential bias, it is also important to consider a strength of questionnaire measures filled out by mothers; namely, when responding to questionnaire measures, mothers have a considerable amount of data, given the amount of time they spend with their infant, on which to base their ratings. Clinician assessments (which rely on the direct observations of trained clinicians who are often maskedFootnote 9 to maternal depression levels) and observational coding are less likely to be affected by shared informant biases. However, while observational approaches are often considered the gold standard for observable psychological constructs, these approaches also have limitations. For example, scores on one-time observational measures capture a very small sample of infant behavior in a highly specific context and are likely also influenced by factors other than self-regulatory capacities (e.g., infant state, time since sleep or feeding).
Thus, observational data collected by trained research staff typically include highly standardized protocols and rigorous standards of inter-rater agreement. These are clear strengths. However, they may sacrifice some ecological validity, given the typically more contrived observational context, the limited observations of infants over relatively short timeframes, and the idiosyncratic noise that can arise with such snapshots in time – particularly with infants. In contrast, parental reports of infant behavior have the benefits of extended observations, occurring across more varied contexts, yet may be less objective and potentially reflect reporter biases. Indeed, this is particularly concerning for studies of maternal depression, where mothers’ own symptoms may color their observations of their infants’ behavior. The fact that we observed very similar findings between maternal and clinical observations – the latter often masked to maternal depression – may help to somewhat mitigate these concerns. For example, in addition to being statistically indistinguishable (which could potentially reflect the imprecision of the estimates), the effect sizes were quite similar in absolute terms. Nevertheless, all findings should be weighed with respect to their methodological strengths and limitations.
The additional significant moderators were whether the effect size relied on a trait-like measure of self-regulation and whether the self-regulation measure was assessed in a stressor context. It is important to note that these two codes overlap in important ways. While it would be possible for a trait-like measure to be coded as occurring in a stressor context, this did not occur in our dataset. However, the effect sizes coded as process-oriented were split between stressor and non-stressor contexts. These findings suggest that maternal depression may be more strongly related to general, trait-like self-regulation than to in-the-moment regulatory strategies. As with the self-regulation assessment method moderator, it is possible that a shared-reporter bias could in-part explain this finding, as most trait-like self-regulation measures were mother-reported. However, it is also possible that there are real substantive differences in the way maternal depression shapes trait-like versus in-the-moment regulation. Indeed, previous research indicates that associations between self-regulation assessed in a trait-like manner and self-regulation assessed in the moment are modest (Aureli et al., Reference Aureli, Coppola, Picconi, Grazia and Ponzetti2015), suggesting that these two assessment approaches capture somewhat related but largely distinct phenomena, which further underscores the importance of separating these two constructs to better understand their development and sequelae.
It is noteworthy that many variables that we expected would moderate the association did not. There are several potential explanations for these null findings, some methodological and some substantive. One important methodological consideration is that, given the relatively modest number of studies we were able to include in the meta-analysis, we were likely underpowered to detect some moderating effects. Hempel and colleagues (Hempel et al., Reference Hempel, Miles, Booth, Wang, Morton and Shekelle2013) note that few meta-analyses, particularly those in which values of the moderator are not equally distributed across categories, are powered to detect moderator effects. The distributions of several of our null moderators may have limited our ability to detect effects (i.e., the range of infant sex was quite restricted and the distributions of sample type, maternal depression method, maternal depression score type, and reliability of self-regulation measure were very uneven). One moderator that demonstrated a more even distribution and yielded a null result was maternal depression timing. However, given considerable stability in maternal perinatal depression (e.g., DiPietro et al., Reference DiPietro, Costigan and Sipsma2008) and our use of unadjusted bivariate correlation coefficients, this meta-analytic study was likely not well-suited to isolate timing effects.
Notably, the overall pooled effect size and effect sizes within moderator subgroups were small (Cohen, Reference Cohen1988). However, when considering the many influences that shape development in the first years of life, it is unsurprising that one specific exposure would have only a small effect (Götz et al., Reference Götz, Gosling and Rentfrow2021). Importantly, many established, clinically important effects are small in magnitude (e.g., Meyer et al., Reference Meyer, Finn, Eyde, Kay, Moreland, Dies, Eisman, Kubiszyn and Reed2001; Owens et al., Reference Owens, Potter, Hyatt, Albaugh, Thompson, Jernigan, Yuan, Hahn, Allgaier, Garavan and Harezlak2021). Thus, small effect sizes such as this one, when estimated with precision, are expected and may still be practically meaningful.
Quality of existing literature
Sociodemographic considerations
While there was variability in sociodemographic characteristics of the included studies, there were also notable gaps. First, 68% of studies comprised majority White participants, and over half were conducted in the United States, with most others conducted in Europe. There were notable exceptions, including Luecken and colleagues’ (Reference Luecken, Crnic, Gonzales, Winstone and Somers2019) study with Mexican and Mexican American dyads and Isosävi and colleagues’ (Reference Isosävi, Diab, Kangaslampi, Qouta, Kankaanpää, Puura and Punamäki2017) study with Palestinian dyads. With the goal of producing a broadly generalizable literature and given that both experiences of maternal depression (e.g., Bashiri & Spielvogel, Reference Bashiri and Spielvogel1999; Maxwell et al., Reference Maxwell, Robinson and Rogers2019) and self-regulation development (e.g., LeCuyer & Zhang, Reference LeCuyer and Zhang2015) may differ across racial and ethnic groups and cultural contexts, examining this association in more diverse or non-White samples and in non-Western contexts is critical for informing conceptual models and more broadly applicable clinical considerations.
Another limitation of the literature includes a focus on adult mothers. While including adolescent mothers requires additional ethical considerations (e.g., parental consent), given the stressors of adolescent parenthood, this population may be particularly vulnerable to perinatal psychopathology (Jeon et al., Reference Jeon, Kent-Marvick, Sanders, Hanson and Simonsen2024), and understanding risk and protective processes for adolescent mothers and their infants has critical prevention/intervention implications. Further, we were unable to code the percentage of mothers in each sample who were their infants’ biological mothers because of limited reporting of this characteristic. While a few studies restricted their sample to biological mothers only, no other included studies reported this information. We assume most studies included primarily biological mothers, consistent with the broader perinatal mental health literature, which includes limited consideration of adoptive and foster parents’ mental health (McKay et al., Reference McKay, Ross and Goldberg2010). Clear reporting on this characteristic would aid understanding of mother-infant mental health, with implications for supporting adoptive and foster mothers – many of whom may be parenting infants with complex needs related to prenatal and early life trauma and stress – and for understanding mechanisms of intergenerational risk transmission.
Maternal depression assessment
The included literature used well-established and validated self-report questionnaires or diagnostic interviews to assess maternal perinatal depression. While diagnostic interviews are considered the gold standard for assessing clinical levels of psychopathology, considering depressive symptoms using psychometrically sound self-report questionnaires also provides important information about subthreshold symptoms. Previous research suggests both clinical psychopathology assessed diagnostically and subthreshold symptoms assessed with continuous rating scales have implications for parenting and offspring functioning (e.g., Goodman et al., Reference Goodman, Simon, Shamblaw and Kim2020; West & Newman, Reference West and Newman2003). Thus, it is important to continue examining subthreshold and clinical perinatal depression. Notably, we were only able to code reliability of the maternal depression measure for about half of the included effect sizes, limiting our ability to consider reliability in the included samples. However, consistent with the use of validated assessments, for those effect sizes with reliability information, all exceeded our a priori thresholds indicating adequate reliability.
Self-regulation assessment
Consistent with the broader literature on infant self-regulation and our search strategy, our search demonstrated use of a wide range of methodologically and conceptually variable infant self-regulation assessment approaches. There is disagreement about the extent to which different outcome measures can be meta-analytically combined (e.g., Lipsey & Wilson, Reference Lipsey and Wilson2001). We opted for an inclusive approach, given that self-regulation-related terminology is often used inconsistently and interchangeably across studies (e.g., within the included studies, the orienting/regulatory capacity factor of the IBQ-R was variously described as assessing regulation, self-regulation, emotion regulation, and effortful control). As such, in the present review, infant self-regulation was assessed with measures of regulation, stress regulation, emotion/mood regulation, mutual/co-regulation, self-regulation, attentional control, state regulation, behavioral regulation, fear regulation, and inhibition. Various methods were also used, including observational coding of infants’ attention, behavior, and emotion during stress tasks or social interactions; parent-report questionnaires; structured assessments conducted by trained clinicians; and parent interviews. Notably, self-regulation measure reliability was only reported for about half of included effect sizes (of those that did report reliability information, 77% met our a priori thresholds for adequate reliability). There was little consistency in which measures showed inadequate reliability. For example, in some studies the orienting/regulatory control factor of the IBQ-R showed adequate reliability, while in other studies it did not, emphasizing the importance of examining reliability within a given sample, rather than relying solely on previous evidence of reliability. Given the variability in approaches to measuring self-regulation and the frequent use of novel or recently developed measures, it is critical that authors provide information about the reliability of their measures, both for results interpretation and to guide future measure selection.
Limitations of the meta-analysis
While this meta-analysis addresses an important gap in the literature, several limitations must be considered. Given the high prevalence of maternal perinatal depression (Bauman et al., Reference Bauman, Ko, Cox, D’Angelo, Warner, Folger, Tevendale, Coy, Harrison and Barfield2020), the importance of self-regulation for functioning across several domains (e.g., Aldao et al., Reference Aldao, Gee, De Los Reyes and Seager2016; Best et al., Reference Best, Miller and Naglieri2011; Fitzsimons & Finkel, Reference Fitzsimons and Finkel2011), and the strong theoretical rationale for an association between the two, it is unsurprising that our search yielded a relatively large body of recent literature examining this association (129 records). However, we were unable to include 61 records because adequate information for effect size estimation was not reported. This underscores the importance of reporting descriptive statistics and zero-order correlations of all study variables to facilitate cumulative science.
An additional limitation of the current study, particularly in light of null moderator results, is that we did not conduct a priori power analyses to determine the scenarios under which moderator analyses would be powered to detect true effects, as this is not often done in meta-analytic reviews. However, Hedges & Pigott (Reference Hedges and Pigott2004) provide guidelines to conduct such power analyses that can be applied a priori to future work to help differentiate true null moderator effects from false negatives.
Implications and future directions
Despite these limitations, this meta-analysis has two important implications. First, the association between maternal perinatal depression and infant self-regulation emphasizes the interconnectedness of mother-infant dyads during this sensitive developmental period. Whether the identified association represents a causal pathway from maternal depression to infant functioning or arises from other familial and/or contextual risk factors, our finding indicates that efforts to support both maternal and infant outcomes are warranted among dyads experiencing maternal depression. Second, this finding suggests important future research directions. While clear and specific policy and clinical implications require causally informed data, establishing support for this association grounds future research examining causal processes and is thus a critical step for identifying policy and clinical implications.
Potential causal pathways linking maternal perinatal depression and infant self-regulation are described in Goodman and Gotlib’s (1999) seminal model of the intergenerational transmission of depression. The model conceptualizes self-regulation impairments as one vulnerability observable in infants and children of mothers with depression that increases later risk of depression. Indeed, children of parents experiencing depression are at three-fold risk of developing depression themselves (Weissman et al., Reference Weissman, Wickramaratne, Nomura, Warner, Pilowsky and Verdeli2006), underscoring the importance of understanding early risk indicators in this population.
Two pathways in Goodman & Gotlib’s model suggest a causal effect of maternal depression on infant self-regulation. First, physiological differences in the maternal and fetal environments in the context of maternal depression may impact developing fetal and infant stress systems. Stress regulatory systems that underlie early physiological regulation, such as the ANS and the HPA axis, begin to develop in-utero (Cerritelli et al., Reference Cerritelli, Frasch, Antonelli, Viglione, Vecchi, Chiera and Manzotti2021; Howland et al., Reference Howland, Sandman and Glynn2017; Irwin et al., Reference Irwin, Meyering, Peterson, Glynn, Sandman, Hicks and Davis2021). These systems develop rapidly and are sensitive to environmental impacts, such as levels of maternal and placental stress hormones or maternal inflammation (Aboustate & Baune, Reference Aboustate, Baune, Teixeira, Macedo and Baune2020; Henrichs & Van den Bergh, Reference Henrichs, Van den Bergh, Gendolla, Tops and Koole2015; Irwin et al., Reference Irwin, Meyering, Peterson, Glynn, Sandman, Hicks and Davis2021), which are amplified in the context of maternal depression. Indeed, fetuses and infants of mothers who experience prenatal depressive symptoms tend to show differences in physiological regulation (Field et al., Reference Field, Diego, Dieter, Hernandez-Reif, Schanberg, Kuhn, Yando and Bendell2004; Galbally et al., Reference Galbally, Watson, van IJzendoorn, Saffery, Ryan, de Kloet, Oberlander, Lappas and Lewis2020; Kinsella & Monk, Reference Kinsella and Monk2009; Monk et al., Reference Monk, Fifer, Myers, Bagiella, Duong, Chen, Leotti and Altincatal2011). Thus, the observed association of elevated maternal depression and lowered infant self-regulation may arise in part via maternal prenatal physiological differences that affect fetal and infant physiological regulation.
Second, after birth, the development of self-regulation continues in the context of caregiver-infant interactions (Gianino & Tronick, Reference Gianino, Tronick, Field, McCabe and Schneiderman1988). The co-regulatory social interactions that support the infant’s developing self-regulation rely on the infant’s physiological regulatory capacities and the caregiver’s ability to interpret and respond appropriately to their infant’s communicative signals (Beeghly et al., Reference Beeghly, Perry, Tronick and Maltzmana2016), often referred to as sensitive parenting/caregiving (Ainsworth, Reference Ainsworth1969). More sensitive caregiving is associated with more self-regulation in infancy, toddlerhood, and early childhood (Augustine et al., Reference Augustine, Leerkes, Smolen and Calkins2018; Bernier et al., Reference Bernier, Carlson and Whipple2010; Blair et al., Reference Blair, Granger, Willoughby and Kivlighan2006; Camerota et al., Reference Camerota, Willoughby, Cox and Greenberg2015; Fay-Stammbach et al., Reference Fay‐Stammbach, Hawes and Meredith2014; Hofstee et al., Reference Hofstee, van der Velde, Huijding, Endendijk, Kemner and Deković2022; Samdan, Reference Samdan2020). Notably, a large body of evidence also indicates that maternal depression is associated with more difficulty engaging in sensitive caregiving behaviors (Bernard et al., Reference Bernard, Nissim, Vaccaro, Harris and Lindhiem2018; Goodman et al., Reference Goodman, Simon, Shamblaw and Kim2020; Mah, Reference Mah2016). Infants of mothers experiencing depression during the postnatal period may thus show difficulties with self-regulation in part due to reduced sensitive caregiving.
Importantly, while these two mechanisms are most directly implicated in explaining a potential causal effect of maternal perinatal depression on infant self-regulation, other factors may explain the observed association between maternal perinatal depression and infant self-regulation (Goodman & Gotlib, Reference Goodman and Gotlib1999). For instance, shared genetic factors underlying a liability toward dysregulation (Bridgett et al., Reference Bridgett, Burt, Edwards and Deater-Deckard2015) or contextual stressors driving both maternal depression and infant dysregulation may also explain the association observed in this meta-analysis.
To better understand the association between maternal depression and infant self-regulation, it is necessary to consider all of these pathways and their likely co-occurrence. Given confounding of prenatal environmental effects, parenting behaviors, genetic effects, and stressful life circumstances in observational family studies, teasing apart the relative causal contributions of each of these pathways proves difficult. However, examining relevant questions with a combination of thoughtful and rigorous methodological approaches including twin, family, and adoptive designs; other natural experiments; intervention studies; and statistical approaches that control for potential confounders provides a way forward (e.g., Davis et al., Reference Davis, Hankin, Swales and Hoffman2018; Rutter, Reference Rutter2007; Wilson & Rhee, Reference Wilson and Rhee2022).
Conclusions
In sum, the present meta-analysis synthesized the association between maternal perinatal depression and infant self-regulation across 193 effect sizes. We found a small, negative association indicating higher levels of maternal perinatal depression are associated with lower infant self-regulation. While notable limitations in this literature highlight important future directions, this association underscores the importance of supporting mother-infant dyads as a strategy for bolstering mental health and well-being for future generations.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579425100837.
Data availability statement
We adhered to TOP Level 2 Guidelines. The data and code necessary to reproduce the analyses presented here, along with the coding documentation, are publicly accessible at the following URL: https://osf.io/jw7t2/overview?view_only=76cb058fad0e42c9b584ec4c675ba0a1.
Acknowledgments
The authors would like to thank Drs. Megan Gunnar and Monica Luciana for their helpful feedback on earlier versions of this project.
Funding statement
This work was supported by a National Science Foundation Graduate Research Fellowship awarded to ERP (grant 2237827) and the Masonic Institute for the Developing Brain Predoctoral Fellowship in Developmental Science awarded to ERP. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Competing interests
We have no known conflicts of interest to disclose.
Pre-registration statement
Analyses were pre-registered on 4/6/23. Data, code, materials, and the preregistration for this research are available at the following URL: https://osf.io/jw7t2/overview?view_only=76cb058fad0e42c9b584ec4c675ba0a1. Deviations from the pre-registered plan are detailed in the Supplement.