Trigeminal neuralgia associated with a solitary pontine lesion, termed SPL-TN, is a recently characterized entity that is reported to be distinct from multiple sclerosis (MS) and may be under-recognized. Reference Tohyama, Hung and Cheng1 We herein describe two cases of SPL-TN, with the aim of raising awareness of this unique clinic-radiographic syndrome.
We report two men (patients 1 and 2) who developed right-sided trigeminal neuralgia (TN) at ages 59 and 65, respectively. Both had no history of demyelinating disease or herpetic infection and unremarkable neurologic examinations. On brain MRI, both had right-sided T2-weighted fluid-attenuated inversion recovery (T2-FLAIR)-hyperintense, T1-hypointense and non-gadolinium-enhancing pontine lesions extending linearly from the trigeminal nerve root entry zone toward the fourth ventricle and corresponding to the intrapontine trigeminal fibers (Figure 1), without other lesions to suggest demyelination. Patient 1 also had a cervical spine MRI that was unremarkable. Patient 1 had only mild abutment of the superior entry zone segment of the trigeminal nerve without sustained pain relief after microvascular decompression, while patient 2 had no evidence of neurovascular compression. Neither had CSF testing for oligoclonal bands or viral infection performed. Both had repeated brain MRIs over the years that showed persistence of solitary lesions, without the development of other lesions to suggest demyelinating disease. Patient 1 had refractory TN despite trials of multiple medications and neurosurgical treatments, while patient 2 responded to carbamazepine but had symptom recurrence following drug discontinuation due to intolerance.
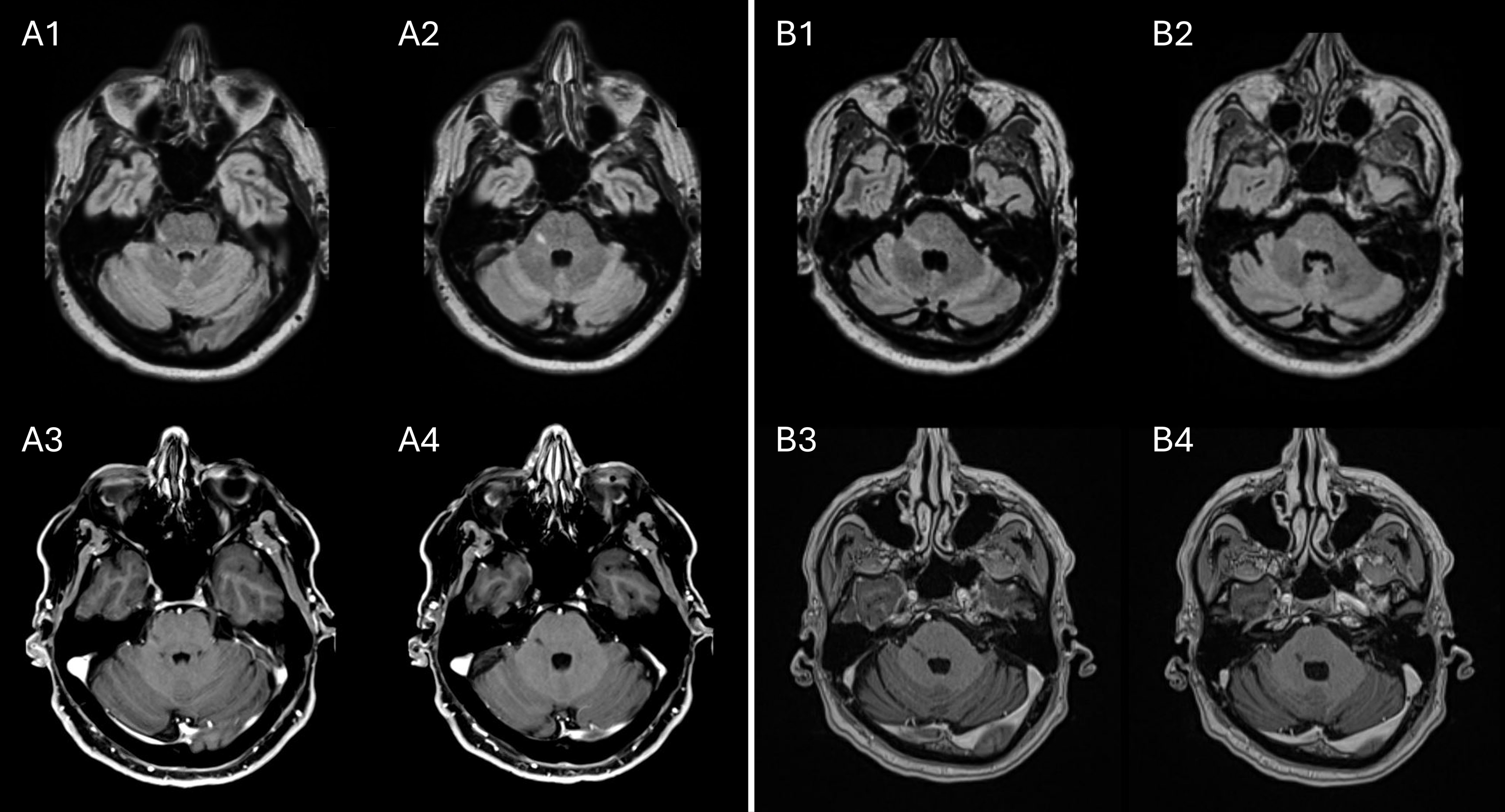
Figure 1. Solitary pontine lesions in two patients with trigeminal neuralgia. In patient 1, a T2-FLAIR-hyperintense (A1, A2), T1-hypointense and non-gadolinium-enhancing (A3, A4) lesion extending from the trigeminal nerve root entry zone toward the fourth ventricle is shown. In patient 2, a T2-FLAIR-hyperintense (B1, B2), T1-hypointense and non-gadolinium-enhancing (B3, B4) lesion is similarly present. Neither patient had other lesions suggesting demyelination, compatible with solitary pontine lesions. T2-FLAIR = T2-weighted fluid-attenuated inversion recovery.
The two patients we report herein are typical of SPL-TN, which has been differentiated from MS by its typically older age of onset and absence of other lesions to fulfill dissemination in space as required by the 2017 McDonald criteria. Reference Tohyama, Hung and Cheng1,Reference Thompson, Banwell and Barkhof2 Recognition of SPL-TN may aid in prognostication, given the frequently intractable nature of symptoms in these patients despite medical and surgical treatments. Reference Tohyama, Hung and Cheng1 While SPL-TN is reported to be a distinct entity, it is noteworthy that linear pontine lesions corresponding to the intrapontine trigeminal fibers have been reported in patients with MS, Reference Renard, Tubery and Castelnovo3 making the possibility of a demyelinating etiology challenging to exclude. It is plausible that SPL-TN represents a form of solitary sclerosis (SS), which is a demyelinating disease that may lie on the same pathologic spectrum of MS despite the absence of dissemination in space. Reference Schmalstieg, Keegan and Weinshenker4 While SS typically presents with progressive motor impairment, a critical demyelinating lesion involving the trigeminal fibers rather than corticospinal tracts could conceivably cause TN. Reference Schmalstieg, Keegan and Weinshenker4–Reference Jackson-Tarlton, Flanagan and Messina7 Neither of our patients had CSF testing for oligoclonal bands performed, which may have provided supportive evidence for this possibility. In addition to demyelinating disease, linear pontine lesions have also been reported in patients with herpetic infection. Reference D’Amico, Russo and Ugga8,Reference Wang and Abboud9 While neither patient had a reported history of herpetic infection, CSF testing for these pathogens was not performed, and central nervous system (CNS) herpetic infection may occur in the absence of dermatologic manifestations; for these reasons, the possibility that SPL-TN may relate to herpetic infection is similarly challenging to exclude. Improved recognition of SPL-TN may help facilitate the determination of its etiology and inform prognostic discussions in clinical practice.
Author contributions
TJ: Drafting of the manuscript; major role in the acquisition of data; analysis or interpretation of data; study concept or design. KS: Revision of the manuscript for content; major role in the acquisition of data; study concept or design. MS: Revision of the manuscript for content; analysis or interpretation of data; study concept or design. JL: Revision of the manuscript for content; major role in the acquisition of data. BS: Revision of the manuscript for content; major role in the acquisition of data. CC: Revision of the manuscript for content; major role in the acquisition of data. PM: Revision of the manuscript for content; analysis or interpretation of data; study concept or design. AB: Drafting of the manuscript; major role in the acquisition of data; analysis or interpretation of data; study concept or design; study supervision.
Funding statement
No funding was received for this manuscript.
Competing interests
Dr Jairam has nothing to disclose.
Ms. Sangam has nothing to disclose.
Dr Sharma has nothing to disclose.
Dr Lau has nothing to disclose.
Dr Shettar has nothing to disclose.
Dr Casserly has received consulting fees from Roche, Sanofi, Genzyme, EMD Serono, Alexion and Novartis and investigator-initiated clinical trial funding from Biogen and serves as a principal investigator (PI) for clinical trials sponsored by Roche and EMD Serono. Additionally, she has been awarded a Western Teaching Innovation Award and a Digital Open Access Medical Education Grant from Western Libraries in support of educational initiatives.
Dr Malik has nothing to disclose.
Dr Budhram holds the London Health Sciences Centre and London Health Sciences Foundation Chair in Neural Antibody Testing for Neuro-Inflammatory Diseases and receives support from the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario.
Informed patient consent was obtained for this manuscript. Statistical analysis was not required for the preparation of this manuscript.


