Highlights
Intravenous levetiracetam (LEV) is used as the second-line treatment option for status epilepticus, but there is still uncertainty regarding the optimal loading dose strategy.
Weight-based dosing ≥30 mg/kg of LEV seems to result in more favorable outcomes.
Future prospective studies are required to definitively guide optimal dosing.
Introduction
Status epilepticus (SE) is a critical neurological emergency where seizures persist and fail to terminate spontaneously. Prolonged seizures, lasting a minimum of 5 minutes, can lead to continuous seizure activity with potential for irreversible neuronal damage and fatality if not appropriately treated. Reference Trinka, Cock and Hesdorffer1 First-line treatment with benzodiazepines (BZD) is commonly administered to abort seizure activity. However, benzodiazepine-refractory SE (BRSE) can occur and is associated with mortality rates ranging from 35% to 60%. Reference Wylie, Sandhu and Murr2 As a result, an effective second-line antiseizure medication (ASM) such as levetiracetam (LEV) is essential in managing BRSE. A loading dose is recommended in emergency situations to critically achieve adequate seizure control. Reference Wheless, Clarke and Hovinga3 It can be administered as a weight-based dose ranging from 20 to 60 mg/kg or a fixed dose of 1000 to 3000 mg. Reference Brophy, Bell and Claassen4
Both fixed and weight-based LEV dosing strategies are commonly used in clinical practice. The Neurocritical Care Society Guidelines recommend a fixed LEV dosing strategy for SE based on observational data. Reference Uges, van Huizen and Engelsman5,Reference Eue, Grumbt, Müller and Schulze6 A study by Eue et al. demonstrated a 44.2% response rate (primary outcome of no BRSE) with up to 2000 mg IV LEV loading dose. Reference Eue, Grumbt, Müller and Schulze6 However, international guidelines recommend weight-based loading dose in patients with BRSE based on the results from the ESETT trial, which showed a LEV loading dose of 60 mg/kg to a maximum of 4500 mg effectively controlled seizures in 47% of refractory SE cases with minimal adverse events. Reference Vossler, Bainbridge and Boggs7,Reference Glauser, Shinnar and Gloss8,Reference Chamberlain, Kapur and Shinnar9 A recent systematic review suggested that 20–60 mg/kg weight-based LEV dosing is efficacious in 46.9–81.8% of cases; Reference Webb, Wanbon and Otto10 however, it lacks a direct head-to-head comparison of the safety and efficacy between different dosing strategies, leading to clinical ambiguity about the optimal LEV loading dose strategy for SE.
Two prior retrospective analyses have explored the efficacy and safety of different LEV loading doses in BRSE. In a 2023 study of 202 patients, a dosing strategy of 35 mg/kg was not associated with worse clinical outcomes. Reference Kuffer, Novy and Rossetti11 Similarly, a 2024 analysis of 218 patients found no significant differences in seizure termination or recurrence rates among LEV dosing groups of <20 mg/kg, 21–39 mg/kg and ≥40 mg/kg. Reference Schowe, Frick, Weitkamp and Jarboe12 However, these studies yielded negative results, contributing to ongoing uncertainty about the optimal LEV loading dose strategy. In this retrospective cohort study, we aimed to address this clinical question by comparing rates of successful BRSE termination in patients receiving low (<30 mg/kg) versus high (≥30 mg/kg) LEV weight-based dosing. The decision to use 30 mg/kg as a threshold to distinguish low versus high LEV dosing was arbitrary and reflects commonly observed practices in clinical settings (i.e., a fixed 2000 mg loading dose for a 70 kg patient correlates to an approximately 30 mg/kg weight-based load). The low weight-based dosing threshold would thus ideally capture most common fixed LEV doses seen in clinical practice. Reference Uges, van Huizen and Engelsman5
Methods
This was a single-center, retrospective cohort study of patients admitted to the emergency department of Vancouver General Hospital (VGH) between November 2022 and December 2023. The study included patients who were ≥18 years old with convulsive SE lasting at least 5 minutes despite receiving at least one dose of BZD. The use of electroencephalogram (EEG) monitoring was not considered as part of the study’s inclusion criteria. No EEG monitoring was involved. Patients were excluded if they did not receive BZD prior to LEV, received loading doses of other parenteral ASM prior to LEV, were intubated or admitted under critical care services prior to SE event, were pregnant or had traumatic brain injuries or toxicological seizures due to overdoses or alcohol withdrawal. The primary outcome of this study was to compare successful BRSE termination between the two groups. The need for additional ASM after receiving a LEV load was used as a surrogate marker for BRSE termination. This surrogate endpoint was selected as the best available objective measure to confirm clinical resolution of BRSE. The secondary outcomes were the proportion of patients who required endotracheal intubation, intensive care unit (ICU) admission or experienced adverse drug reactions in each group. Also 30-day all-cause mortality was captured in both groups. Ethics and operational approval for this study were obtained from the University of British Columbia Clinical Research Ethics Board and Vancouver Coastal Health Research Institute, respectively.
Data collection and assessment of outcomes
All patients presenting to the VGH Emergency Department between November 2022 and December 2023 who received intravenous LEV were screened for inclusion in the study. A total of 106 patients met the inclusion criteria. The electronic medical records of the included patients were reviewed for baseline demographics including age, weight, serum creatinine and estimated glomerular filtration rate (eGFR). Additionally, risk factors for seizures such as history and type of epilepsy, seizure etiology and electrolyte imbalances were collected for each patient. Electrolyte imbalances were defined for hyponatremia (serum sodium levels below 125 mmol/L), hypomagnesemia (serum magnesium levels below 0.8 mmol/L) and hypocalcemia (serum calcium levels below 1.9 mmol/L). Reference Nardone, Brigo and Trinka13 Finally, patient charts were reviewed for clinical outcome data including termination of BRSE, endotracheal intubation, ICU admission, 30-day all-cause mortality and adverse drug events. The majority of data were collected from consultation notes and electronic administration records. Details such as dosage and route of BZD administered, LEV loading dose, drug and dose of additional ASM(s), documented reasons for death and documented adverse drug events were manually extracted from each health record. All data were stored in a secure database using Research Electronic Data Capture (REDCap). Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde14 LEV loading doses administered were dichotomized using a built-in REDCap calculator that divided the administered dose by the patient’s documented weight, yielding a mg/kg dosage. This was further categorized as <30 mg/kg and ≥30 mg/kg.
Sample size
The sample size was calculated based on data from the study by Eue et al. Reference Eue, Grumbt, Müller and Schulze6 and ESETT trial, Reference Chamberlain, Kapur and Shinnar9 assuming a 1:1 recruitment ratio. With an expected moderate effect size of 0.5, power of 80% and an alpha of 0.05, a total of 314 patient encounters would be required.
Statistical analysis
Descriptive statistics were used to summarize patient characteristics. Normally distributed variables were reported as mean and standard deviation, and non-normally distributed variables were reported as median and interquartile range, based on the Shapiro-Wilk test. A multiple logistic regression was utilized to compare the proportion of patients with BRSE termination between the two dosing strategies. An adjusted odds ratio (aOR) with its 95% confidence interval for the association between type of LEV dose and the termination of BRSE was calculated. Age, history of epilepsy and eGFR were considered as potential adjustment variables. Final adjustment variables were selected by performing a likelihood ratio test. A few missing eGFR variables were addressed and imputed with their median. The secondary outcomes were examined using descriptive statistics to describe exploratory trends and observations. In addition, patients were randomly split into two groups and conducted win-ratio analyses based on a composite outcome consisting in order of mortality, ICU admission, endotracheal intubation and BRSE termination. A win ratio was calculated for both the unstratified sample and groups stratified by age of 65 years old and seizure history. Reference Pocock, Ariti, Collier and Wang15 All analyses were performed in R 4.1.2. 16
Results
A total of 106 patients who presented to the VGH Emergency Department and were treated with LEV for BRSE met the inclusion criteria. Patient baseline characteristics and relevant BRSE risk factors are presented in Table 1.
Table 1. Baseline characteristics
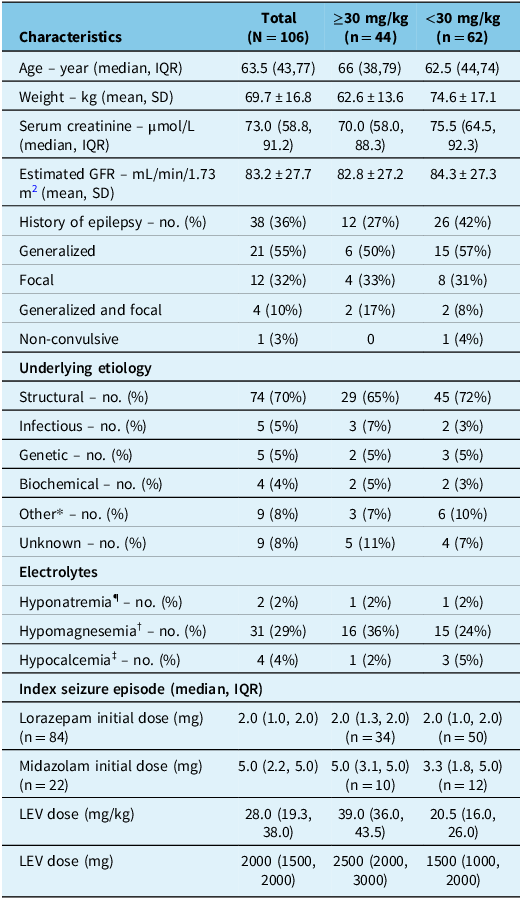
GFR = glomerular filtration rate; LEV = levetiracetam.
*Medication noncompliance, metabolic.
¶ Na < 125; † Mg < 0.8; ‡ Ca < 1.9.
The median age of patients was 63.5 years, with an average weight of about 70 kg. Thirty-eight patients (36%) had a history of epilepsy, and the seizure etiology was mainly structural (70%). Patients were initially treated with lorazepam (79%) or midazolam (21%), with median initial doses presented in Table 1. BZD was given either intravenously (90%), intramuscularly (7%) or sublingually (3%). The median LEV loading dose was 28.0 mg/kg (or median dose of 2000 mg).
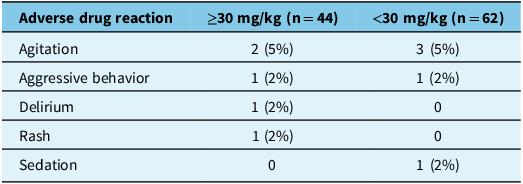
Out of 106 patients, 54 (51%) had termination of BRSE, not requiring additional ASM (Table 2). There was a larger proportion of patients with termination of BRSE in the higher (≥30 mg/kg) compared to the lower (<30 mg/kg) weight-based dosing group (66% vs 40%, respectively). The unadjusted OR was 2.86 (95% CI: 1.30–6.52). The significance of potential adjustment variables was assessed, and age and eGFR were included in the final model. eGFR was identified as a variable influencing patient response to each loading dose strategy (specifically, patients with better renal function were more likely to respond favorably to LEV). The aOR was 3.07 (95% CI: 1.36–7.2). There were lower rates for endotracheal intubation, ICU admission and all-cause mortality in the higher weight-based dosing group (Table 3). There was a total of 21 deaths (20%) recorded in this study, with higher rates observed in the lower weight-based dosing group. Among the reported causes, two patients in the higher weight-based dosing group died due to refractory SE, compared to one patient in the lower weight-based dosing group. The majority of these deaths were non-SE-related (Table 3). LEV was well tolerated in both groups, with adverse drug reactions being comparable between both dosing strategies (Table 4).
Table 2. Primary outcome (adjusted odds ratio * for termination of BRSE)

BRSE = benzodiazepine-refractory status epilepticus.
* Adjusted for age and estimated glomerular filtration rate.
Table 3. Secondary endpoints
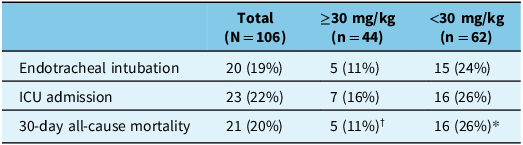
ICU = intensive care unit.
†Documented reasons in electronic records: refractory SE (n = 2), hydrocephalus (n = 1), end-stage cancer (n = 1), unknown (n = 1).
*Documented reasons in electronic records: end-stage cancer (n = 5), hemorrhagic stroke (n = 4), respiratory failure (n = 2), cognitive decline (n = 2), sepsis (n = 2), refractory SE (n = 1).
Table 4. Safety outcomes
Win ratio analysis revealed differences between higher and lower weight-based dosing strategies. In the overall analysis, the win ratio was 2.78 (95% CI: 2.70–2.86), reflecting a greater likelihood of favorable outcomes with higher weight-based dosing. Stratified analyses showed consistent patterns. When stratified by age (≥65 years), the win ratio was 1.91 (95% CI: 1.85–1.97), and when stratified by seizure history, the win ratio was 1.92 (95% CI: 1.86–1.97).
Discussion
In this retrospective cohort study, the use of a LEV loading dose of ≥30 mg/kg was more effective in terminating BRSE compared to the lower weight-based loading dose of <30 mg/kg. Despite existing studies with negative results, our study remains the first to demonstrate a positive association between a specific LEV dosing threshold (≥30 mg/kg) and improved clinical outcomes in BRSE. Notably, higher weight-based loading doses were linked to increased rates of seizure termination, lower rates of endotracheal intubation, ICU admission and all-cause mortality, while maintaining a tolerable safety profile. In contrast to the studies by Schowe et al. and Kuffer et al., Reference Kuffer, Novy and Rossetti11,Reference Schowe, Frick, Weitkamp and Jarboe12 our study employed a different statistical approach, utilizing regression analysis to calculate aORs for the primary outcome and a win-ratio analysis for hierarchical comparisons of the primary outcome and select secondary outcomes (Figure S1). These methodological differences may account for the discrepancy in findings and further highlight the importance of dosing strategies in optimizing BRSE management.
The observed association between higher weight-based LEV loading doses and BRSE termination is consistent with findings from other contemporary trials such as ESETT, where clinical termination of SE was achieved with high weight-based LEV dosing (60 mg/kg to a maximum of 4500 mg). Given linear kinetics, low protein binding (<10%) and relatively high volume of distribution (0.5–0.7 L/kg in adults), Reference Wright, Downing and Mungall17 the pharmacokinetic profile of LEV supports the use of higher initial doses to rapidly achieve therapeutic serum levels for effective acute seizure control. Current guidelines offer reference ranges for LEV trough level concentrations, but there is a lack of evidence to associate specific levels with seizure control. Reference Patsalos, Berry and Bourgeois18,Reference Hiemke, Bergemann and Clement19 A recent meta-regression and pharmacokinetic modeling analysis suggests that a weight-based loading dose of 40 mg/kg, up to 4500 mg, may achieve optimal LEV serum concentrations in refractory SE. Reference Lau, Haag and Maharaj20 This underscores the importance of weight-based dosing to achieve adequate drug concentrations after administration. This approach may mitigate the risk of under-dosing, which could necessitate additional ASM to manage refractory SE, as observed in this study with the lower weight-based dosing group.
While the median LEV weight-based loading dose in this study population was 28.0 mg/kg (or median dose of 2000 mg), the median weight-based loading dose was 20.5 mg/kg (or median dose of 1500 mg) in the lower dosing group and 39.0 mg/kg (or median dose of 2500 mg) in the higher dosing group. The maximum loading dose given in this study did not exceed the recommended maximum dose of 4500 mg (maximum weight-based loading dose observed was 62.5 mg/kg (or dose of 4300 mg)). Based on existing research and observational findings from this study, it can be suggested that initiating LEV treatment with a loading dose of at least 30 mg/kg, up to 60 mg/kg with a maximum of 4500 mg, might be an effective practice approach for improving BRSE management outcomes. The decision to adopt this threshold would require replication in research to further validate and establish optimal LEV weight-based dosing guidelines for managing BRSE effectively.
Lower rates for endotracheal intubation and ICU admission in the higher weight-based dosing group suggest that adequate loading doses of LEV are crucial for managing seizures effectively, as evidenced by the results of our win-ratio analyses. By achieving therapeutic concentrations promptly, LEV can terminate seizures and prevent invasive interventions such as intubation and admission to the ICU. Overall, there were more non-SE-related deaths compared to SE-related deaths in this patient population (Table 3). This study was not powered to compare mortality rates between the two dosing strategies; however, the persistent benefit seen in the win-ratio analysis showcases the benefits in other clinically important outcomes seen with higher weight-based LEV dosing. The adverse drug reactions observed in this study were consistent with the limited side effects reported in previous LEV studies, supporting its overall favorable safety profile.
Lorazepam was the most commonly administered BZD in both dosing groups, with a recommended dose of 4 mg for initial treatment of SE. The median initial lorazepam dose in both groups was subtherapeutic, although it was relatively closer to the recommended dose in the lower weight-based LEV dosing group, suggesting that despite a conventionally recommended BZD dose, the lower weight-based LEV dose group fared worse. The observation that patients received various initial doses of BZD may reflect clinical practice variability or individual patient factors such as the severity of seizure presentation. This variability limits the assessment of adequate dosing with BZD. While optimal BZD dosing should ideally be confirmed before considering LEV, the results of this study highlight the importance of a systematic approach to medication dosing in SE and underscore the equal importance of adequate LEV dosing in managing BRSE to ensure optimal patient outcomes.
There are several limitations to this study. First, the retrospective design limits the ability to control for unknown confounders and the resultant selection bias, although efforts were made to correct for this limitation by performing a regression analysis. Second, future studies with larger sample sizes would be beneficial to confirm these results and ensure that the observed effects are robust and applicable to a broader population. Third, data collection was limited to what was available and documented in the electronic health record. Given the lack of objective data to confirm clinical resolution of BRSE, additional ASM after LEV load was used as a surrogate endpoint for BRSE termination. There may be a possibility that patients who did not return to neurological baseline (such as patients with non-convulsive SE) were missed and not captured in the study.
Conclusion
The study demonstrated that patients who received ≥30mg/kg of LEV as a loading dose had greater odds of BRSE termination compared to those who received <30mg/kg. It also showed lower rates for clinically important outcomes of endotracheal intubation, ICU admission and 30-day all-cause mortality in the higher LEV dosing group. Dosing guidance provided by this study may help improve successful seizure control and patient outcomes in clinical practice. Prospective studies are warranted to further explore the role of higher weight-based dosing and treatment of BRSE in this clinical setting.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cjn.2025.56.
Author contributions
NT, AL, HH, TH and VC contributed to the study conception. All authors (NT, AL, HH, TH, DY, VC) contributed to the study methodology, formal analysis and investigation and manuscript writing, reviewing and revisions. All authors read and approved the final manuscript.
Funding statement
The authors received no financial support for the research, authorship and/or publication of this article.
Competing interests
The authors disclose the following potential conflicts of interest: Trana Hussaini has received a grant (Investigator Initiated Research Grant from Paladin Labs Inc.), honoraria for lectures/presentations/educational events (Oman Internation Pharmacy Congress from Oman Ministry of Health and Fellows Symposium Presentation from the Canadian Society of Transplantation), as well as unpaid leadership roles in committees such as the Canadian Liver Foundation/BC Yukon Chapter Medical Advisory and the Canadian Society of Transplantation Education Committee. However, these potential conflicts do not influence the research, authorship and/or publication of this article. All other authors (NT, AL, HH, DY, VC) declare no additional potential conflicts related to the publication of this article.





