Introduction
Autobiographical memory (AM) is a memory system that consists of episodes recollected from an individual’s life, that is, personal semantic and episodic information (Tulving Reference Tulving, Tulving and Donaldson1972, Reference Tulving1983), with the former referring to facts about the self, such as information on where the individual was born, and the latter referring to unique events, such as the first time riding a bicycle (Brewer & Rubin, Reference Brewer and Rubin1996). Dysfunctions such as impaired or biased AM recall have been observed across mood and anxiety disorders (Lenaert et al., Reference Lenaert, Boddez, Vervliet, Schruers and Hermans2015; Moscovitch et al., Reference Moscovitch, Vidovic, Lenton-Brym, Dupasquier, Barber, Hudd, Zabara and Romano2018; Weiss-Cowie et al., Reference Weiss-Cowie, Verhaeghen and Duarte2023), with accumulating evidence for an important role in the development and maintenance of unipolar depression (Weiss-Cowie et al., Reference Weiss-Cowie, Verhaeghen and Duarte2023). AM dysfunction in depressed individuals has been characterized by generally impaired recollection, as well as valence-specific effects, that is, impaired memory for positive events in the context of a better memory for negative events (Burt et al., Reference Burt, Zembar and Niederehe1995; Matt et al., Reference Matt, Vázquez and Campbell1992). Within the context of cognitive models of depression such as Beck’s negative cognitive triad model (Reference Beck2008; Beck et al., Reference Beck, Rush, Shaw, Emery, DeRubeis and Hollon2024) proposing that negative beliefs about the self, the world, and the future shapes depression, a negative AM bias can subserve a vicious cycle of dysfunctional and self-enforcing negative schemas that contribute to the development and maintenance of depression.
In experimental settings the behavioral and neural basis of AM and its alterations in mental disorders have been examined by combining the retrieval of general and emotional autobiographical memories, for example, by cued recall, personalized scripts or prospection (St. Jacques & Cabeza, Reference St. Jacques, Cabeza, Ghetti and Bauer2012) with functional magnetic resonance imaging (fMRI, for details on the specific experimental paradigms, see also Brewer & Rubin, Reference Brewer and Rubin1996; Gilboa, Reference Gilboa2004; Young et al., Reference Young, Erickson, Nugent, Fromm, Mallinger, Furey and Drevets2012; Young, Bellgowan, Bodurka, & Drevets, Reference Young, Bellgowan, Bodurka and Drevets2013). Despite a certain level of inconsistency between the studies, recent meta-analyses confirm impairments in retrieving specific autobiographical memories in individuals with depression and increased risk for depression (Hallford et al., Reference Hallford, Rusanov, Yeow and Barry2021, Reference Hallford, Rusanov, Yeow and Barry2022; Liu et al., Reference Liu, Li, Xiao, Yang and Jiang2013), with some original studies suggesting valence-specific effects, for example, better memory for negative events (Kuyken & Dalgleish, Reference Kuyken and Dalgleish2011).
The neurobiological basis of AM has been examined in numerous fMRI studies suggesting that AM relies on circuits encompassing prefrontal regions as well as the hippocampal formation, retro splenial cortex and posterior cingulate cortex (PCC; Shepardson, Dahlgren, & Hamann, Reference Shepardson, Dahlgren and Hamann2023; Svoboda et al., Reference Svoboda, McKinnon and Levine2006), with some evidence for differential involvement of the basal ganglia and amygdala/insula depending on the emotional content of the memories (Testa et al., Reference Testa, Sotgiu, Rusconi, Cauda and Costa2024). The widespread engagement may reflect the complex interaction between mental processes during AM encompassing memory recall, self-referential processes, executive function, imagery and semantic contextualization. Within this network the hippocampal formation plays a pivotal role in the memory formation and recall (Fink et al., Reference Fink, Markowitsch, Reinkemeier, Bruckbauer, Kessler and Heiss1996; Gardini et al., Reference Gardini, Cornoldi, De Beni and Venneri2006; Greenberg et al., Reference Greenberg, Rice, Cooper, Cabeza, Rubin and LaBar2005; Ryan et al., Reference Ryan, Nadel, Keil, Putnam, Schnyer, Trouard and Moscovitch2001) as well as in detailed and immersive recollection of memory details for AM, and is further supported by the medial prefrontal cortex (PFC) involved in the processing of self-referential stimuli (St. Jacques & Cabeza, Reference St. Jacques, Cabeza, Ghetti and Bauer2012). Furthermore, the lateral PFC (specifically the left ventrolateral PFC) has been identified as a key region for memory search and retrieval in AM recall (Shepardson et al., Reference Shepardson, Dahlgren and Hamann2023). Regions such as the amygdala or the basal ganglia have been identified to mediate the emotional content of AM, such that the amygdala has been involved in recalling negative events (Doré et al., Reference Doré, Rodrik, Boccagno, Hubbard, Weber, Stanley and Ochsner2018; McCrory et al., Reference McCrory, Puetz, Maguire, Mechelli, Palmer, Gerin and Viding2017) or the subjective sense of remembering visual (Greenberg & Rubin, Reference Greenberg and Rubin2003) and emotional (Rubin & Berntsen, Reference Rubin and Berntsen2003) re-experiencing of the event (Piolino et al., Reference Piolino, Desgranges and Eustache2009). Affectively colored AMs are evoked by stimulation of the amygdala (Vignal et al., Reference Vignal, Maillard, McGonigal and Chauvel2007), and the activity of amygdala single neurons were associated with familiarity and recollection (Rutishauser et al., Reference Rutishauser, Schuman and Mamelak2008), while the globus pallidus may mediate the emotional experience of positive AM (Testa et al., Reference Testa, Sotgiu, Rusconi, Cauda and Costa2024).
More recent approaches examining the network-level underpinnings of AM further indicate that the default mode network (DMN) – a large-scale brain network associated with self-referential processes, mind wandering, and memory (Faustino, Reference Faustino2022; Philippi et al., Reference Philippi, Tranel, Duff and Rudrauf2015) plays a significant role in AM. A recent fMRI meta-analysis on AM by Shepardson et al. (Reference Shepardson, Dahlgren and Hamann2023) found that there is a large degree of overlap between the DMN and brain regions involved during AM retrieval, particularly in ‘core DMN’ regions, including frontal and posterior cortical midline structures and the bilateral angular gyrus (Andrews-Hanna, Smallwood, & Spreng, Reference Andrews-Hanna, Smallwood and Spreng2014; Shepardson et al., Reference Shepardson, Dahlgren and Hamann2023).
While fMRI meta-analyses have allowed to robustly determine the neural systems underlying AM in healthy individuals (Shepardson et al., Reference Shepardson, Dahlgren and Hamann2023), results on the neural systems mediating dysfunctional AM in depression are based on single studies that are characterized by a level of inconsistency inherent to case–control fMRI studies (Etkin, Reference Etkin2019; Klugah-Brown et al., Reference Klugah-Brown, Zhou, Pradhan, Zweerings, Mathiak, Biswal and Becker2021; Köhler et al., Reference Köhler, Carvalho, Alves, McIntyre, Hyphantis and Cammarota2015). The development of neuroimaging meta-analytic approaches facilitates quantitative integration of findings from case–control MRI studies, offering critical advancements toward determining more robust structural and functional alterations in depression within a behavioral domain or in comparison to other mental disorders (Bore, Liu, Huang, et al., Reference Bore, Liu, Huang, Kendrick, Zhou, Zhang, Klugah-Brown and Becker2024; Liu et al., Reference Liu, Klugah-Brown, Zhang, Chen, Zhang and Becker2022; Zhou et al., Reference Zhou, Chen, Shen, Li, Chen, Zhu, Castellanos and Yan2020).
Against this background, the present preregistered neuroimaging meta-analysis aims to compensate for single-study deficits in aspects such as sample size and specific AM recall tasks that differ across studies. Neuroimaging meta-analyses have been developed to pool data extracted from original studies, which allows for examination of brain activity across studies with a higher statistical power. As such a neuroimaging meta-analysis on AM recall in depressive and at-risk individuals can increase the robustness and generalizability of the results and allow to quantitatively determine common brain regions associated with AM recall in depressive individuals. Similar approaches have been recently applied to other domains and have indicated, for example, robust striatal alterations during reward processing in depression across domains and at-risk populations (Bore, Liu, Gan, et al., Reference Bore, Liu, Gan, Wang, Xu, Ferraro, Li, Zhou, Zhang, Vatansever, Biswal, Klugah-Brown and Becker2024; Bore, Liu, Huang, et al., Reference Bore, Liu, Huang, Kendrick, Zhou, Zhang, Klugah-Brown and Becker2024; Zhou et al., Reference Zhou, Gao, Bao, Liang, Cao, Tang, Li, Hu, Zhang, Sun, Roberts, Gong and Huang2024). These findings may also help to inform interventions that aim at targeting neural alterations in depression, for example, by closed-loop real-time neuroimaging interventions (Li et al., Reference Li, Jiang, Gong, Zhao, Zhao, Liu, Kendrick, Zhu and Becker2019; Misaki et al., Reference Misaki, Tsuchiyagaito, Guinjoan, Rohan and Paulus2024; Young, Siegle, et al., Reference Young, Siegle, Zotev, Phillips, Misaki, Yuan, Drevets and Bodurka2017).
Based on previous studies, we hypothesized that (1) neural alterations during AM in depression will be observed in the hippocampus and amygdala, in particular decreased activity in the hippocampus, and increased amygdala response in depressive individuals when retrieving negative autobiographical memories (eg, Doré et al., Reference Doré, Rodrik, Boccagno, Hubbard, Weber, Stanley and Ochsner2018; Young, Bodurka, and Drevets, Reference Young, Bodurka and Drevets2016; Young, Siegle, Bodurka, & Drevets, Reference Young, Siegle, Bodurka and Drevets2016) and that (2) the identified regions will connect on the network level with the DMN.
To test our hypotheses we capitalized on previous case–control neuroimaging studies on AM in depression and performed a coordinate-based meta-analysis on Seed-based d mapping with Permutation of Subject Images (SDM-PSI), a novel and robust meta-analytic technique that produces unbiased estimation of effect sizes and generation of neurofunctional maps (Albajes-Eizagirre et al., Reference Albajes-Eizagirre, Solanes, Fullana, Ioannidis, Fusar-Poli, Torrent and Radua2019), with subsequent meta-analytic co-activation and connectivity analyses determining the network-level communication of the identified regions.
Methods
Search strategy and selection criteria
The current preregistered meta-analysis adhered to the guidelines of conducting a coordinate-based meta-analysis. A pre-registration was submitted on the Open Science Framework (https://osf.io/35xtf) platform to increase accountability and transparency prior to the commencement of the meta-analysis. A comprehensive literature search was conducted independently according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines by C.C.C.C. First, a broad search of original fMRI studies on fMRI case–control studies AM in depression was conducted on PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Web of Science (https://www.webofscience.com/wos/). Suitable studies from the reference lists of review articles were additionally included. The literature was screened referring to the returned titles and abstracts. Studies that were written in English, reporting whole-brain results and were published between 1998 and 2023 were included. Only peer-reviewed, original case–control studies comparing patients and healthy controls were included. The following search items were applied: ‘functional magnetic resonance imaging OR fMRI’ AND ‘autobiographical memory’ AND ‘depression OR major depressive disorder OR at-risk of depression’ have to co-occur with any of the following keyword: ‘memory recall.’ Data selection process was double checked by H.L.M.T. and M.C.B., with any discrepancies settled by B.B. This search yielded 150 unique studies as shown in the PRISMA flow diagram freely available (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennan, Chou, Glanville, Grimshaw, Hróbjartsson, Lalu, Li, Loder, Mayo-Wilson, McDonald and Moher2021; https://www.prisma-statement.org/prisma-2020-flow-diagram), see Supplementary Table S2. The systematic literature review identified 13 suitable studies.
Coordinate-based meta-analytic approach
A coordinate based meta-analysis of AM in individuals with or at the risk of depression was conducted using SDM-PSI (https://www.sdmproject.com) which performs statistical inferences on results from multiple studies (Albajes-Eizagirre et al., Reference Albajes-Eizagirre, Solanes, Fullana, Ioannidis, Fusar-Poli, Torrent and Radua2019) and has been successfully employed to determine robust neurofunctional alterations within or across mental disorders (Bore, Lui, Huang, et al., Reference Bore, Liu, Huang, Kendrick, Zhou, Zhang, Klugah-Brown and Becker2024; He et al., Reference He, Bore, Jiang, Gan, Wang, Li, Xu, Wang, Fu, Li, Zhou, Kendrick and Becker2025). Peak coordinates and effect sizes of case–control differences during AM were extracted from the original studies reporting results on the whole-brain level. The meta-analysis additionally included results from studies in populations at the risk of depression. These studies commonly defined at risk as high familial risk for depression, that is, having a first-degree relative with major depression (Macdonald et al., Reference MacDonald, Murray, Moutsiana, Fearon, Cooper, Halligan and Johnstone2016; Young et al., Reference Young, Bellgowan, Bodurka and Drevets2013; Young, Bellgowan, Bodurka, & Drevets, Reference Young, Bellgowan, Bodurka and Drevets2015; Young, Siegle, et al., Reference Young, Siegle, Bodurka and Drevets2016). Given that previous studies reported that AM impairments can be observed in patients with major depression disorder (MDD), at-risk individuals and in individuals with remitted depression (eg, Köhler et al., Reference Köhler, Carvalho, Alves, McIntyre, Hyphantis and Cammarota2015; Kuyken & Dalgleish, Reference Kuyken and Dalgleish2011; Weiss-Cowie et al., Reference Weiss-Cowie, Verhaeghen and Duarte2023) and preliminary results indicate overlapping neural alterations (eg, Young et al., Reference Young, Bellgowan, Bodurka and Drevets2013). Hence, this study pooled such studies. The low number of studies including at-risk individuals (4), however, did not allow to run comparative meta-analyses. This practice was in line with previous meta-analyses, which article searches included both at risk and depressed patients (Bore, Liu, Gan, et al., Reference Bore, Liu, Gan, Wang, Xu, Ferraro, Li, Zhou, Zhang, Vatansever, Biswal, Klugah-Brown and Becker2024; Bore, Lui, Huang, et al., Reference Bore, Liu, Huang, Kendrick, Zhou, Zhang, Klugah-Brown and Becker2024).
Our major aim was to identify brain functional alterations during AM recall and its directionality in depression by implementing the Seed based d mapping extractions and mean analyses described in Supplementary Methods. Z-values representing between-group differences of depressed or at-risk individuals and healthy controls were transformed to t-values using the SDM statistical converter (https://www.sdmproject.com/utilities/?show=Coordinates). MNI coordinates (x, y, z) of activation clusters with statistically significant differences across tested contrasts will be reported.
Supplementary analyses were conducted on Neurosynth to explore the functional connectivity and meta-analytic co-activation patterns of the identified regions. Functional connectivity reflects brain regions that are coactivated across the resting-state fMRI time series with the seed voxel (Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead, Roffman, Smoller, Zöllei, Polimeni, Fischl, Liu and Buckner2011), while meta-analytic coactivation employs all of the fMRI studies available in the Neurosynth database and serves as the meta-analytic analogue of the functional connectivity map (Rottschy et al., Reference Rottschy, Caspers, Roski, Reetz, Dogan, Schulz, Zilles, Laird, Fox and Eickhoff2013; Schnellbächer et al., Reference Schnellbächer, Hoffstaedter, Eickhoff, Caspers, Nickl-Jockschat, Fox and Dogan2020) (details in Supplementary Methods).
Analyses of the effects of valence AM recall
Given the frequently reported valence-specific effects in depression (Köhler et al., Reference Köhler, Carvalho, Alves, McIntyre, Hyphantis and Cammarota2015; Liu et al., Reference Liu, Dai, Chen, Qi, Xin, Zhuang, Zhou, Zhou, Luo, Huang, Wang, Zou, Chen, Kendrick, Zhou, Xu and Becker2021), we conducted further exploratory analyses (Whalley et al., Reference Whalley, Rugg and Brewin2012; Wu et al., Reference Wu, Chen, Huang, Chen, Yue, Yang and Gong2020). To identify possible effects of valence AM on brain region activation in AM recall, three sub analyses were performed based on evidence from the literature on valence effects on brain activity – one with all types of valenced AM recall, one with positive AM recall, and one with negative AM recall (McCrory et al., Reference McCrory, Puetz, Maguire, Mechelli, Palmer, Gerin and Viding2017; Young et al., Reference Young, Erickson, Nugent, Fromm, Mallinger, Furey and Drevets2012, Reference Young, Bellgowan, Bodurka and Drevets2013, Reference Young, Bellgowan, Bodurka and Drevets2014) (see Supplementary Methods).
Analyses of the effects of depression risk in AM recall
Eighty-two people at risk of depression were included in our main analysis to increase the statistical power of our meta-analysis. However, to ensure that our results were not driven by at-risk groups, we also ran a separate analysis with depressed patients. This led to a subanalysis that included 12 studies, with one study being fully excluded (MacDonald et al., Reference MacDonald, Murray, Moutsiana, Fearon, Cooper, Halligan and Johnstone2016) and some datapoints from 2 studies were further excluded (Young et al., Reference Young, Bellgowan, Bodurka and Drevets2013, Reference Young, Bellgowan, Bodurka and Drevets2015).
Additional analyses
Using SDM values and other confounding variables such as age and sex, we performed meta-regression analyses.
Tests for heterogeneity and publication bias
Heterogeneity and publication bias tests were performed with standard procedures. Publication bias was statistically evaluated by Egger’s test and funnel plots, where p values <0.05 were interpreted as significant.
Results
Demographic and clinical data summary of all included studies
A total of 13 studies comprising of 602 participants (n = 261 healthy controls, n = 341 patients) were included in the meta-analysis. There were 12 unique participant pools identified, as Young et al. (Reference Young, Bellgowan, Bodurka and Drevets2013); Young, Bodurka et al. (Reference Young, Bodurka and Drevets2016) used the same participants in two separate studies. With exception of some studies which did not provide sufficient information (Gillard et al., Reference Gillard, Werner-Seidler, Dalgleish and Stretton2023; MacDonald et al., Reference MacDonald, Murray, Moutsiana, Fearon, Cooper, Halligan and Johnstone2016; Whalley et al., Reference Whalley, Rugg and Brewin2012; Wu et al., Reference Wu, Chen, Huang, Chen, Yue, Yang and Gong2020), participants had a mean age of 34.41 (SD = 10.761). Healthy controls (n = 156) had a mean age of 34.17 (SD = 11.169), and depressive participants (n = 384) (including high risk, depressive and remitted depressive patients) had a mean age of 34.52 (SD = 10.604). There were no significant group differences between healthy controls and patients (t = .342, p = .732). The prisma flow diagram as well as a complete list of included studies and their design characteristics are shown in Figure 1 and Table 1.
Table 1. Demographic and clinical characteristics of included studies

Note: HC, healthy control; HR, high-risk; MDD, major depressive disorder; rMDD, remitted major depression disorder.

Figure 1. PRISMA flow diagram showing the identification of included studies.
Main meta-analytic findings
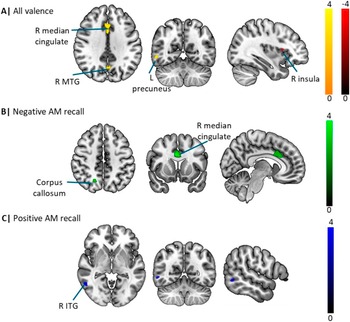
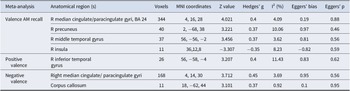
The overall meta-analysis encompassing both positive and negative valence AM recall revealed three significant clusters of increased activation in depression relative to controls located in the right anterior cingulate/paracingulate, right precuneus and the right middle temporal gyrus, and a single cluster of decreased activity in the right anterior insula. Sub-group meta-analyses investigating the effects of negative and positive valence AM recall further demonstrated that patients exhibited increased activity in the cingulate/paracingulate cingulate and right precuneus during negative recall but increased activity in the right inferior temporal gyrus during positive recall (Figure 2; Table 2).

Figure 2. Illustration of the main meta-analytic findings. (A) Meta-analysis of all valence studies. (B) and (C) Meta-analysis of negative and positive autobiographical memory studies, respectively.
Functional connectivity and behavioral-level analyses
Additional analyses were conducted on the network and behavioral levels. Network analyses were performed utilizing the Neurosynth database (https://www.neurosynth.org/) to explore the functional connectivity and meta-analytic coactivation patterns of the paracingulate and precuneus regions identified in the general valence meta-analysis as shown in Figure 3A and B, respectively. The top panel shows the strong functional connectivity of the paracingulate with core regions of the salience network, including the ACC as well as the bilateral anterior insula (extending into the inferior frontal gyrus). The overlap of the functional connectivity and meta-analytic coactivation maps confirmed that core connectivity profiles encompassed the anterior insula and anterior cingulate cortex (ACC; Figure 3A). The functional connectivity for the seed voxel in the precuneus showed interactions with core cortical midline regions of the default mode network, in particular medial frontal regions as well as the precuneus and PCC. The overlap of the functional connectivity and meta-analytic coactivation maps confirmed functional interactions with the cortical midline regions (Figure 3B).

Figure 3. (A) and (B) Functional connectivity and meta-analytic co-activation of the regions identified of being altered in depression during AM recall for paracingulate regions and precuneus. The top panel shows the functional connectivity, the middle panel shows the meta-analytic co-activation patterns, and the bottom panel shows the combination of both functional and meta-analytic co-activation patterns. Behavioral terms of the paracingulate and precuneus are shown in (C) and (D), respectively.
The additional behavioral characterization indicated that the paracingulate region is strongly associated with negative affective (eg, pain) and executive (conflict) functions, while the precuneus regions is associated with social cognitive and mnemonic functions, including autobiographic and theory (of) mind as shown in Figure 3C and D, respectively.
Additional analyses: Meta-regression
Meta-regression analyses of age and gender as possible confounding variables were conducted. The analysis found no significant associations.
Tests for heterogeneity and publication bias
Between-study heterogeneity was generally low across the three analyses. With reference to the bias p-values and symmetric funnel plots, publication bias analysis was not statistically significant as shown by smaller p-values in the Egger’s p column, of which the test significance threshold was set to p < .05 (see Table 2).
Table 2. Meta-analytic results of depression patients versus healthy controls in autobiographical memory recall at p < .0025 uncorrected

Note: AM, autobiographical memory; L, left; MNI, Montreal Neurological Institute; R, right.
Discussion
Our main meta-analysis identified increased activation in the right median cingulate/paracingulate gyri (overlapping with the dACC), left precuneus and right inferior temporal gyrus and concomitantly increased activity in the right anterior insula in depressive individuals compared with healthy controls during AM recall. These results were consistent when running a separate analysis excluding the at-risk individuals suggesting that these do not bias the estimation. Moreover, exploratory valence-specific meta-analyses revealed increased activity in an overlapping network during negative recall (ie, dACC and precuneus) and positive recall (inferior temporal gyrus) suggesting potentially valence-specific effects. On the network level, the core regions encompassing the dACC and precuneus exhibited functional interactions with the salience network and the DMN, respectively. Behavioral characterization further revealed an engagement in negative affect and executive functions of the dACC and AM and social cognition of the identifies precuneus region respectively.
While the functional and behavioral characterization aligns with the corresponding deficits in these domains in depression (eg, Xu et al., Reference Xu, Xin, Liu, Chen, Yao, Zhou, Zhou, Huang, Dai, Wang, Zou, Kendrick, Zhou and Becker2022; Young et al., Reference Young, Erickson, Nugent, Fromm, Mallinger, Furey and Drevets2012), the identified regions did not align with our hypothesized alterations, including reduced activation of the hippocampus, and increased activation of the amygdala during AM in depression. Both of these neural alterations have been conceptualized and integrated into memory and cognitive theories on depression. For instance, overarching theories posit that the valence-specific memory deficits in depression are related to stress-associated suppression of hippocampal functioning and sensitization of the amygdala (Dillon & Pizzagalli, Reference Dillon and Pizzagalli2018). Moreover, the cognitive reactivity posits that sadness or stress activates dysfunctional cognitive schemata in depressed patients, which in turn maintains depressive processing styles (Lau et al., Reference Lau, Segal and Williams2004) via the DMN and the hippocampus (Marchetti et al., Reference Marchetti, Koster, Sonuga-Barke and De Raedt2012; Mulders et al., Reference Mulders, van Eijndhoven, Schene, Beckmann and Tendolkar2015). Despite the conceptual framework results in original fMRI studies on AM were not unequivocal and may depend on the analyses (region focused versus whole-brain), valence of the tasks (ie, recalling positive, negative, and neutral AM), as well as ambiguity in certain paradigms (ie, recalling specific or any AM). While some of the original studies reported elevated amygdala activity and greater amygdala-hippocampal connectivity when recalling negative AM (Doré et al., Reference Doré, Rodrik, Boccagno, Hubbard, Weber, Stanley and Ochsner2018; Young et al., Reference Young, Bellgowan, Bodurka and Drevets2014; Young, Bodurka, et al., Reference Young, Bodurka and Drevets2016; Young, Siegle, et al., Reference Young, Siegle, Bodurka and Drevets2016), these effects may not have been robust enough to generate consistent results across the heterogenous studies. Furthermore, an increasing number of recent studies challenges the valence-specific function of the amygdala, indicating rather a general role in arousal or salience (Zhang et al., Reference Zhang, Gan, Xu, Yu, Wang, Song, Jiao, Liu, Zhou and Becker2025) and recent large-scale meta-analyses reported null findings in tasks traditionally related to amygdala activation, such as the emotional Stroop task (Chen et al., Reference Chen, Becker, Camilleri, Wang, Yu, Eickhoff and Feng2018; Feng et al., Reference Feng, Becker, Huang, Wu, Eickhoff and Chen2018).
The identified dACC region in the present study has traditionally not been considered to play a key role in AM per se. In the context of mnemonic functions this region has been associated with remote AM recall, which together with a recent meta-analysis suggests a potential contribution to AM (Daviddi et al., Reference Daviddi, Pedale, Jacques, Schacter and Santangelo2023; Steinvorth et al., Reference Steinvorth, Corkin and Halgren2006) (see also Martinelli et al., Reference Martinelli, Sperduti, Devauchelle, Kalenzaga, Gallarda, Lion and Piolino2013). However, in line with the meta-analytic functional and network-level characterization the dACC plays a key role in affective and executive functions, including conflict processing and negative emotional appraisal (Etkin et al., Reference Etkin, Egner and Kalisch2011; Li et al., Reference Li, Yang, Zhou, Liu, Wei, Xin, Daumann, Daumann, Kendrick and Becker2020), with recent work from Gillard et al. (Reference Gillard, Werner-Seidler, Dalgleish and Stretton2023), further suggesting that the dACC could be linked to activations across the affective salience network as a response to the psychological experience of social pain and affiliation (Dalgleish et al., Reference Dalgleish, Walsh, Mobbs, Schweizer, van Harmelen, Dunn and Stretton2017; Eisenberger et al., Reference Eisenberger, Lieberman and Williams2003), which aligns with previous findings indicating altered activity in this region during negative AM recall in depression (Young, Bodurka, & Drevets, Reference Young, Bodurka and Drevets2017).
Previous studies on dACC in depression have reported robust alterations in the functional intrinsic network architecture of this region (Zhou et al., Reference Zhou, Gao, Bao, Liang, Cao, Tang, Li, Hu, Zhang, Sun, Roberts, Gong and Huang2024) and an association with different symptom domains in depression (Sheline et al., Reference Sheline, Price, Yan and Mintun2010). During task engagement this region has demonstrated altered functional communication during social cognitive processes, including, for example, social working memory (Xu et al., Reference Xu, Xin, Liu, Chen, Yao, Zhou, Zhou, Huang, Dai, Wang, Zou, Kendrick, Zhou and Becker2022), which together with the stronger contribution of this region in the negative AM studies on depression may reflect that dysregulations in this area may contribute to an overgeneralized memory in depression (Falco et al., Reference Falco, Peynircioğlu and Hohman2015; Raes et al., Reference Raes, Griffith, Craeynest, Williams, Hermans, Barry and Hallford2023) or mediate affective emotional aspects as well as general cognitive deficits in depression leading to emotionally biased re-experience of social affective experiences (Gillard et al., Reference Gillard, Werner-Seidler, Dalgleish and Stretton2023).
The precuneus has been strongly associated with AM retrieval, with research on the specific functions indicating a contribution to autobiographical reminiscence (Daviddi et al., Reference Daviddi, Pedale, Jacques, Schacter and Santangelo2023; Shepardson et al., Reference Shepardson, Dahlgren and Hamann2023; Sreekumar et al., Reference Sreekumar, Nielson, Smith, Dennis and Sederberg2018), or first person visual and memory-based navigation (Wilson et al., Reference Wilson, Berry, Gracey, Harrison, Stow, Macniven and Young2005). It further shows its association with self-referential processing and self-consciousness (Freton et al., Reference Freton, Lemogne, Bergouignan, Delaveau, Lehéricy and Fossati2014). Interestingly, Freton et al. (Reference Freton, Lemogne, Bergouignan, Delaveau, Lehéricy and Fossati2014) found that the spontaneous tendency to recall AM is positively correlated to precuneus volume which may imply a contribution to spontaneous AM retrieval. Within recent pathological models of negative emotional experiences, for example, in Post Traumatic Stress Disorder, posteromedial regions such as the precuneus have been related to dysregulations in visual imagery processes (Thome et al., Reference Thome, Terpou, McKinnon and Lanius2020). The contribution of this region to autobiographical, self-referential processing, and social cognitive functions is further underscored by or behavioral characterization and the meta-analytic characterization as central part of the default mode network (see also Xin et al., Reference Xin, Zhou, Zhou, Ma, Geng, Zhao, Yao, Dong, Biswal, Kendrick and Becker2021). Together the findings may reflect that alterations in this region may mediate the mnemonic deficits in the patients, potentially reflecting overgeneralized memory recall or dysfunctional imaginary and self-referential processes in depression. Decreased spontaneous activity in the precuneus has been demonstrated in depression – yet not related disorders such as bipolar disorder (Gong et al., Reference Gong, Wang, Qiu, Chen, Luo, Wang, Huang and Wang2020) – with recent evidence indicating a role of altered precuneus engagement during aberrant self-referential AM processing adolescents with depression (van Houtum et al., Reference van Houtum, van Schie, Wever, Janssen, Wentholt, Tailby, Grenyer, Will, Tollenaar and Elzinga2023) as well as less vividness in AM retrieval in depression (van Schie et al., Reference van Schie, Chiu, Rombouts, Heiser and Elzinga2019). This further supports the notion that depressed individuals are less likely to retrieve vivid AM and may do so in the context of aberrant self-referential appraisal.
The third significant cluster of increased activation was in the inferior temporal gyrus, a subregion of the DMN. Although the specific role of the inferior temporal gyrus is not well explored, Sheline et al. (Reference Sheline, Barch, Price, Rundle, Vaishnavi, Snyder and Raichle2009) proposed that depressed individuals do not inhibit DMN activity while looking at negative pictures, which could possibly be linked to negatively valenced material. Lemogne et al. (Reference Lemogne, Delaveau, Freton, Guionnet and Fossati2012) further supported this finding by indicating that depressive self-focus was related to the lack of DMN inhibition. This implies that there are underlying mechanisms regarding depressive individuals’ possible fixation on negative AM contributing to the maintainence of depressive symptoms.
Conversely, the SDM-PSI analysis found reduced activation of the right anterior insula. The insula is important in supporting subjective feeling states or interoceptive processes (Critchley et al., Reference Critchley, Wiens, Rotshtein, Ohman and Dolan2004; Zhang et al., Reference Zhang, Zhang, Wang, Zhou, Qing, Zou, Li, Yang, Becker, Kendrick and Yao2023) and more vivid memories are associated with the activation of the insula (van Schie et al., Reference van Schie, Chiu, Rombouts, Heiser and Elzinga2019). With this in mind, it could be proposed that depressive patients are more likely to have reduced vividness in AM recall, which is indicative of decreased awareness of oneself. This implies that depressive patients are more distant when recalling AM, which is supported by Beck (Reference Beck1979), who posits that distancing oneself from a dissonant past self, or evaluating the self with a distance, are processes that are central to proposed theoretical accounts of depression. This was also supported by a study led by Kuyken and Howell (Reference Kuyken and Howell2006), which found that depressive patients have a more distanced view of their AM as compared to healthy individuals. The decreased activation of the insula could further suggest that depressive patients are more likely to have lowered awareness of the self, and an affected AM recall.
Functional connectivity analysis of the seed voxel in the paracingulate identified associations with the brain regions in the salience network, including the anterior insula, and the ACC (Uddin et al., Reference Uddin, Yeo and Spreng2019). The anterior insula plays an important role in supporting both, subjective feeling states of emotional experience and autonomic reactivity (Ferraro et al., Reference Ferraro, Klugah-Brown, Tench, Bazinet, Bore, Nigri, Demichelis, Bruzzone, Palermo, Zhao, Yao, Jiang, Kendrick and Becker2022) which may reflect that depressive individuals are more likely to fixate on negative emotional and autonomic experiences of AM (Dillon & Pizzagalli, Reference Dillon and Pizzagalli2018). In line with this interpretation previous studies indicate an engagement of the insula during recollection or reflection on personal distress (Eisenberger, Lieberman, & Williams, Reference Eisenberger, Lieberman and Williams2003; Wager et al., Reference Wager, Phan, Liberzon and Taylor2003) and evaluation of negative emotional states (Sanfey et al., Reference Sanfey, Rilling, Aronson, Nystrom and Cohen2003). Associations with the ACC supports previous research proposing that AM retrieval relates to subjectively painful experiences (Dalgleish et al., Reference Dalgleish, Walsh, Mobbs, Schweizer, van Harmelen, Dunn and Stretton2017; Eisenberger et al., Reference Eisenberger, Lieberman and Williams2003; Kelly et al., Reference Kelly, Lloyd, Nurmikko and Roberts2007). Together, the involvement of the salience network may reflect a more aversive affective experience during AM in depression or a dysregulated attentional balance between internally and externally focused attention (Gillard et al., Reference Gillard, Werner-Seidler, Dalgleish and Stretton2023; Xin et al., Reference Xin, Zhou, Zhou, Ma, Geng, Zhao, Yao, Dong, Biswal, Kendrick and Becker2021).
Furthermore, the functional connectivity analysis for the seed voxel in the precuneus points to correlations to the DMN. The DMN, more specifically the anterior medial PFC (amPFC) and the PCC, is crucial in AM retrieval as it facilitates self-referential processing (Andrews-Hanna et al., Reference Andrews-Hanna, Smallwood and Spreng2014). Individuals with MDD, or individuals with high risk of developing depression show alterations in the activation of DMN (Chou et al., Reference Chou, Deckersbach, Dougherty and Hooley2023; Posner et al., Reference Posner, Cha, Wang, Talati, Warner, Gerber and Weissman2016). Depressed individuals tend to show differential engagement in the DMN and self-referential processes during task and rest conditions (Chou et al., Reference Chou, Deckersbach, Dougherty and Hooley2023). Due to its core function and possibly causal contribution to free but not cued AM (Bonnici et al., Reference Bonnici, Cheke, Green, FitzGerald and Simons2018; Lanius et al., Reference Lanius, Terpou and McKinnon2020), alterations in the DMN may reflect impaired-free AM recall in depression. Finally, the corresponding behavioral characterization supports the engagement of both large-scale networks with the large-scale networks and suggests that dysbalanced engagement of the salience network and DMN facilitate AM deficits in depression.
Findings need to be considered in the context of limitations. First, several studies utilize specific AM recall (Young et al., Reference Young, Erickson, Nugent, Fromm, Mallinger, Furey and Drevets2012, Reference Young, Bellgowan, Bodurka and Drevets2013, Reference Young, Bellgowan, Bodurka and Drevets2014, Reference Young, Bellgowan, Bodurka and Drevets2015; Young, Bodurka, et al., Reference Young, Bodurka and Drevets2016; Young, Siegle, et al., Reference Young, Siegle, Bodurka and Drevets2016), posing difficulties in determining the valence of AM retrieved. While we aimed at disentangling valence-specific effects utilization of the valence-specific results should be interpreted with caution due to the comparably low number of studies (Dahlgren et al., Reference Dahlgren, Ferris and Hamann2020; Ding et al., Reference Ding, Chen, Chen, Li, Li, Castellanos and Guo2022). Second, there has been increasing support of semantic and episodic AM retrieval having independent neural networks and brain regions involved (Levine et al., Reference Levine, Turner, Tisserand, Hevenor, Graham and McIntosh2004; Maguire et al., Reference Maguire, Mummery and Büchel2000), and an increasing number of studies meta-analyses can focus on depression-related dysfunctions in episodic and semantic AM components. The studies were mostly conducted in western populations (exception: Wu et al. (Reference Wu, Chen, Huang, Chen, Yue, Yang and Gong2020) with participants from Southwest China). Studies have proposed that cultural contexts modulate the development of episodic AM (Harbus, Reference Harbus, Christensen, Schier and Sutton2010), and an increasing number of studies employ a cross-cultural neuroimaging approach (Yang et al., Reference Yang, Zhou, Xin, Becker, Linden and Hernaus2023).
In summary, the present meta-analysis does not support prevailing models that suggest amygdala and hippocampal engagement underlying AM deficits in depression. Rather, results support the role of core nodes in the salience (dACC) and default mode network (precuneus) as a pathological substrate of AM dysfunctions among people with depression or those at risk. With the dACC being linked to affective and executive functions, results suggest that its activation could be associated with activations in the salience network as a response to the psychological experience of social pain and affiliation. Findings indicate that activation in the precuneus is in line with current research, further emphasizing its role in AM retrieval, specifically in overgeneralised memory recall or dysfunctional imaginary and self-referential processes in depression.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0033291725102833.
Data availability statement
All included studies have been cited within the paper. Data supporting the results of this paper are available at the Open Science Repository platform (OSF) (https://osf.io/35xtf/).
Funding statement
The study was supported by the National Natural Science Foundation of China, NSFC, Grant No. 82271583 and start-up grants from The University of Hong Kong (2407102536).
Competing interests
The authors declare none.