Introduction
Symptomatic thrombosis in neonates (arterial and venous) is extremely uncommon, occurring at approximately 5 per 100,000 live births, but can have devastating consequences, particularly when it involves the arterial system. Reference Nowak-Gottl, von Kries and Gobel1 Long-term complications such as hypertension, limb length discrepancy, amputation, claudication, paraplegia, or hypoplastic kidneys with impaired renal function can significantly impact morbidity and mortality. Reference Arici, Yaman and Ecevit2 The most prominent risk factor for neonatal aortic thrombosis is umbilical arterial catheter use, with sonographic evidence of arterial thrombosis seen in up to 30% of patients following catheter removal. Reference Boo, Wong and Zulkifli3 Additional risk factors include severe cardiac or pulmonary conditions, Reference Tugrul Kural, Tinaztepe and Yurdakul4 sepsis, Reference Szymankiewicz, Oszkinis and Uchman5 asphyxia, meconium aspiration syndrome, severe dehydration, Reference Suri, Ramji and Thirupuram6 extreme prematurity, or thrombophilic conditions such as antithrombin deficiency, factor V Leiden mutation, protein C/S deficiency, or prothrombin G20210A mutation. Reference Nowak-Gottl, von Kries and Gobel1,Reference Tugrul Kural, Tinaztepe and Yurdakul4–Reference Nagel, Tuckuviene and Paes7
Spontaneous aortic thrombi, occurring in the absence of intravascular devices, are exceedingly rare, with only 80 cases reported between 1963 and 2018. Reference Mulcaire-Jones, Bailly and Frank8 Since then, four additional cases have been published. Reference Amonkar, Gavhane and Kharche9–Reference Samji, Twiss and Chan11 Adding the three cases presented here, the total number of reported cases now amounts to at least 87, highlighting the ongoing rarity of this condition. The diagnosis of spontaneous aortic thrombosis can be challenging due to its clinical resemblance to aortic coarctation (CoA), as both conditions can present with absent pulses in the lower extremities, pallor, pre-stenotic hypertension, and decreased left ventricular function. Reference Rodriguez and Sosenko12,Reference Kohli and Lodha13 Although spontaneous thrombosis typically involves the abdominal aorta, whereas coarctation affects the thoracic aorta, this symptomatic overlap represents an important differential diagnosis to consider during clinical evaluation. Depending on the severity and location, complications such as acute renal, hepatic, and heart failure may rapidly ensue. Reference Wieland, Jack and Seidemann14
Current treatment guidelines recommend heparinisation for mild to moderate cases and thrombolytic therapy for severe cases. Surgical or interventional thrombectomy is considered when thrombolysis is contraindicated or unsuccessful. Despite advances in treatment, neonatal aortic thrombosis still carries a high mortality rate of approximately 23%. Reference Mulcaire-Jones, Bailly and Frank8 Recent reports suggest that interventional thrombectomy could be a viable first-line treatment in neonates experiencing critical cardiac decompensation or imminent organ failure. Reference Gutierrez, Alten and Law15–Reference Herron, Covi and Pappas17
Given the diagnostic ambiguity and rarity of neonatal aortic thrombosis, a structured clinical approach is essential. This includes (1) thorough diagnostic evaluation with echocardiography and abdominal Doppler sonography to differentiate thrombosis from congenital anomalies such as coarctation or interrupted aortic arch; (2) consideration of potential pathophysiologic contributors such as dehydration, perinatal asphyxia, and inherited or acquired thrombophilia; and (3) clear therapeutic decision-making guided by the severity of organ dysfunction and haemodynamic compromise. In this case series, we aim to provide clinical insight into each of these domains by describing three neonates with spontaneous aortic thrombosis and proposing a diagnostic and therapeutic framework to support timely and effective intervention.
Materials and methods
All patients treated at the Department of Paediatrics at Hannover Medical School between 2007 and 2023 were screened for ICD-10 code I74 (Arterial embolism and thrombosis). The data were exported to an Excel spreadsheet (Microsoft® Excel, version 16.90, Washington, USA) and evaluated for cases of spontaneous aortic thrombosis. Medical history was reviewed by 2 clinical experts (AR, SK). PubMed database was screened using the terms 'neonatal aortic thrombosis intervention' and 'neonate interventional thrombectomy'. English language articles reporting on the outcome of interventional therapy of neonatal aortic thrombosis were identified and included in the discussion. Separately, a systematic search was conducted in PubMed using the terms 'neonatal spontaneous aortic thrombosis' and ’spontaneous aortic thrombosis neonate' to identify reported cases. The total number of published cases was compiled.
Results
Of all 152 paediatric patients treated at the Department of Paediatrics at Hannover Medical School between 2007 and 2023 for arterial embolism and thrombosis, three neonates diagnosed with spontaneous aortic thrombosis during the neonatal period (age at diagnosis under 29 days) were identified.
Case 1
A hypotrophic male infant, born at 41 3/7 weeks of gestation (birth weight: 2980 g), presented to the paediatric emergency department in an external hospital on the seventh day of life after normal postnatal adaptation with refusal to feed and decreased urine output over the previous 1.5 days. The infant appeared severely ill, with pale, greyish skin, cold and pulseless lower extremities, and a core temperature of 33 °C. Suspecting late-onset sepsis, antibiotic treatment with ampicillin, cefotaxim and gentamicin was started immediately. Blood tests revealed liver and kidney failure, hypertonic dehydration, and lactic acidosis (creatinine: 199 µmol/L, alanine aminotransferase (ALT): 1240 IU/L, lactate: 7.7 mmol/L). Echocardiography showed impaired left ventricular function but no congenital heart defects or aortic arch anomalies, with a patent ductus arteriosus and a thrombus measuring 1.4 × 0.7 cm in the abdominal aorta, obstructing the celiac trunk and superior mesenteric artery. The kidneys appeared hyperechogenic with blurred corticomedullary differentiation, though flow in the renal arteries was still visualised. The patient’s respiratory and haemodynamic instability necessitated intubation and treatment with catecholamines (dobutamine, epinephrine). Due to the life-threatening condition and rapid clinical deterioration, the interdisciplinary team opted for interventional therapy. Given the significant coagulation disorder associated with acute liver failure and the difficulty in arterial access due to aortic obstruction, access was achieved via the patent ductus arteriosus using the femoral vein. Recanalisation was performed through balloon dilatation and local lysis with alteplase (recombinant tissue plasminogen activator, rt-PA), followed by therapeutic heparinisation for 24 hours (400 IU/kg/day), which successfully restored partial perfusion to the abdominal aorta, the celiac trunk, and the superior mesenteric artery. The clinical course, however, was complicated by a massive pulmonary haemorrhage on the third day post-admission, under therapeutic heparinisation, which led to severe respiratory decline, requiring intensified mechanical ventilation (high-frequency oscillation (HFO), FiO2 1.0). On the fourth day, the patient was transferred to Hannover Medical School. Unfortunately, by the time of arrival, the child had already progressed to multi-organ failure, with signs of severe neurological damage, including non-reactive pupils. Cranial sonography revealed pendulum flow in the proximal internal carotid artery, indicative of brain death. The patient passed away on the day of transfer, preventing any conclusive thrombophilia testing. As the patient was initially managed externally, detailed information on medications, laboratory monitoring, and devices used is limited.
Case 2
A eutrophic male neonate (39 2/7 weeks of gestation, birth weight: 3270g) was born in an external hospital with a paediatric cardiology department via vacuum extraction, exhibited delayed adaptation and required continuous positive airway pressure support. The lower half of the body remained pale, cold, and mottled, despite good perfusion in the upper body, in addition perinatal acidosis was present (umbilical artery pH: 7.01, BE: −10.4 mmol/L, APGAR 2/7/8). Echocardiography revealed normal left ventricular function and an unremarkable aortic arch, while abdominal ultrasound detected a thrombus at the aortic bifurcation with significantly reduced flow velocities in the iliac vessels (Figure 1A). Following consultation with Hannover Medical School, therapeutic heparinisation (400 IU/kg/day) was initiated, and the patient was promptly transferred to Hannover with continuous positive airway pressure support. Laboratory tests on admission showed no evidence of kidney dysfunction (creatinine: 76 µmol/L) but indicated impaired liver function with an ALT of 75 IU/L, an aspartate aminotransferase (AST) of 150 IU/L, and a Quick of 39%. The D-dimer was markedly elevated at 22.79 mg/L. The newborn was intubated on the day of transfer due to impending respiratory exhaustion. Given the patient’s overall condition, systemic thrombolysis with rt-PA was chosen as the primary therapy. In accordance with the German guidelines for thrombosis in children at that time, the child received rt-PA via continuous infusion for six days (starting at 1 mg/kg/day, with a maximum of 2 mg/kg/day), Reference Nowak18 followed by full heparinisation (400 IU/kg) for 24 hours before transitioning to subcutaneous enoxaparin for six months (1.5 mg/kg twice daily, targeting anti-Xa levels: 0.6–0.8 IU/mL). During thrombolysis, ultrasound examinations were performed three times a day to detect any signs of intracranial, adrenal, or abdominal bleeding, with no complications observed. Laboratory monitoring showed a decline in D-dimer from 22.79 mg/L on admission to 3.94 mg/L by discharge, and an improvement in Quick from 39 to 71%. The aortic thrombus regressed under systemic thrombolysis and was no longer detectable by the time of discharge on the eighth day of admission. Clinically, the sonographic findings correlated with strongly palpable inguinal pulses and normal skin perfusion in the lower extremities. The boy’s subsequent psychomotor development was entirely normal. Thrombophilia screening, performed partly during the acute phase and completed three months after initial hospitalisation, revealed no evidence of a prothrombotic disorder, including antithrombin deficiency, protein C/S deficiency, antiphospholipid syndrome, prothrombin G20210A mutation, factor V Leiden mutation, and dysfibrinogenemia.
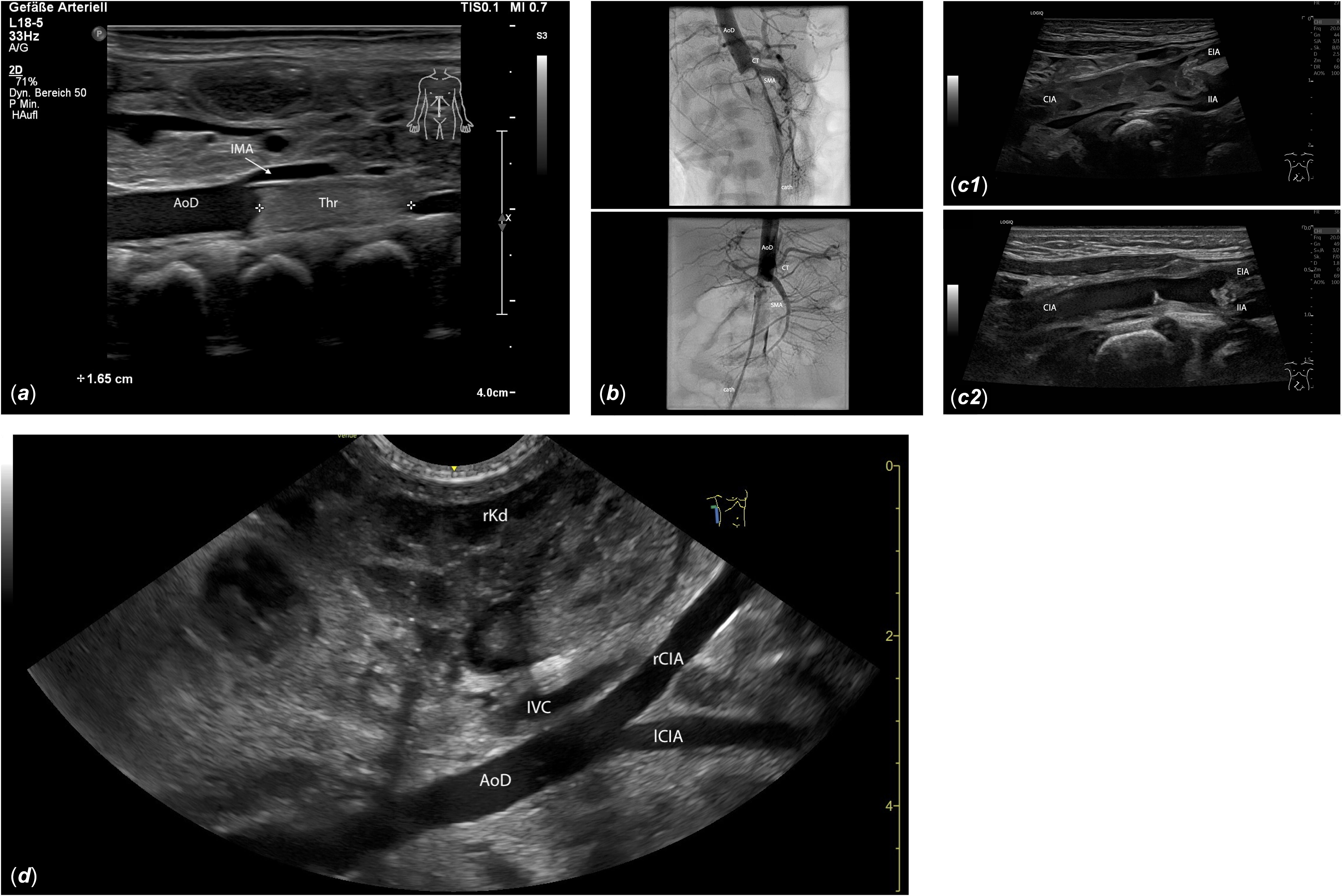
Figure 1. (a) Longitudinal midline view of the upper abdomen: A 1.65 cm hyperechogenic thrombus (Thr) is visible in the descending aorta (AoD), distal to the inferior mesenteric artery (IMA), extending into the aortic bifurcation. (b) The interventional angiography shows a contrast medium filling defect distal to the superior mesenteric artery (SMA), indicating a large thrombus obstructing blood flow to both renal arteries and the lower extremities. AoD: descending aorta; cath: catheter; CT: celiac trunk. For video see video supplement 2. (c) Oblique view of the right-sided inguinal region: C1, 10 days post interventional thrombectomy: inhomogeneous, dissolving thrombotic material extends from the common iliac artery (CIA) beyond the iliac bifurcation into the external iliac artery (EIA) and the internal iliac artery (IIA). C2, 17 days post interventional thrombectomy: The thrombus in the CIA is nearly dissolved, with only small hyperechogenic thrombus remnants visible on the vessel wall. The right EIA remains thrombosed, but the lumen of the proximal right IIA is largely restored. (d) View from the right flank: Imaging of the descending aorta (AoD) and the right and left common iliac arteries (rCIA, lCIA) is generally clearer from the flank than from the midline due to reduced intestinal gas interference. IVC: inferior vena cava; rKd: right kidney.
Case 3
The third patient, a full-term, eutrophic male infant (41 2/7 weeks gestation, birth weight 3565 g) was discharged from the maternity clinic on the day of birth, having normal postnatal adaptation (APGAR scores of 8/9/10, umbilical artery pH 7.2). In the following days, he experienced feeding difficulties. At a visit to the primary paediatrician on the seventh day of life, he exhibited significant weight loss (15% below birth weight), but his general condition remained otherwise stable, and he was discharged home. However, that night his condition worsened, with repeated episodes of vomiting and inability to feed, leading to admission to an external paediatric emergency department. Laboratory tests revealed pronounced metabolic acidosis (base excess: −15 mmol/L) and slightly elevated inflammatory markers (C-reactive protein: 28 mg/L, interleukin 6: 30 ng/l), prompting the initiation of antibiotic therapy with ampicillin, cefotaxime, and gentamicin for suspected late-onset sepsis.
Echocardiography, performed after hypertension was detected in the left arm (115/91 mmHg, mean arterial pressure 100) with no measurable blood pressure in the legs, showed reduced left ventricular systolic function without abnormalities in the aortic arch. As the clinical condition deteriorated, with respiratory failure and worsening lactic acidosis (maximum 7.2 mmol/L), he was intubated and transferred to Hannover Medical School. Upon arrival, at 10 days old, the infant was agitated, pale, and had a capillary refill time of 3 seconds. Femoral pulses were absent and could not be detected by Doppler sonography. Echocardiography revealed a significantly reduced biventricular systolic function, with no signs of coarctation (video supplement 1). The infant was also anuric, and laboratory results confirmed acute renal failure (creatinine peaked at 216 µmol/L). Coagulation parameters showed a Quick of 63% at admission and D-dimer levels of 3.47 mg/L, which decreased to 1.95 mg/L at discharge with a corresponding improvement in Quick to 91%.
With multi-organ failure imminent and a suspected diagnosis of an aortic thrombus, an emergency cardiac catheterisation via the right femoral artery was performed. Angiography confirmed a thrombus completely obstructing the descending aorta below the celiac trunk, extending partially into the superior mesenteric artery. The distal abdominal aorta, including both renal arteries and the lower extremities, was not perfused (Figure 1B). Additionally to local rt-PA thrombolysis (1 mg/kg), a technically complex mechanical thrombectomy was performed using off-label vascular plug devices (Amplatzer Vascular Plug II 6 + 8 mm, St Jude Medical, St Paul, Minnesota, USA) as an “alternative thrombectomy tool” and balloon angioplasty to address residual thrombus material in the iliac arteries bilaterally, resulting in successful reperfusion of the entire descending aorta and its branches, including both iliac arteries (video supplement 2–3). As small thrombus fragments persisted in the iliac vessels, systemic thrombolysis (rt-PA, 2 mg/kg) was administered, followed by therapeutic heparinisation (maximum 650 IU/kg/day) continued for six days, targeting an individualised partial thromboplastin time (PTT) range around 50–70 seconds. Subsequently, tinzaparin was administered at 275 IU/kg once daily, targeting anti-Xa levels between 0.6 and 1.0 units. Following the intervention, the patient’s ventricular function improved rapidly, allowing extubation after 4 days. Catecholamine therapy (epinephrine, norepinephrine) was discontinued after 5 days. Renal function recovered quickly, with creatinine levels returning to normal by the time of discharge. Follow-up sonography, performed every two days, revealed a marked reduction in the thrombus size in the iliac vessels, with successful reperfusion of the abdominal aorta, the superior mesenteric artery and the left internal and external iliac arteries (video supplement 4). The right internal iliac artery was also reperfused. The right external iliac artery remained thrombosed and obliterated, with good collateralisation compensating for the loss (Figure 1C). Aspirin was started shortly before discharge at a dose of 1–2 mg/kg once daily. Tinzaparin was discontinued after four months, and aspirin after eleven months.
Thrombophilia screening revealed no abnormalities and included the same parameters as in Case 2. Follow-up evaluations showed normal somatic and psychomotor development without signs of circulatory disturbance or leg length discrepancy.
Discussion
Epidemiology, aetiology, and diagnosis
Arterial thrombosis in newborns is rare but carries a high mortality rate, which decreased from approximately 83% between 1963 and 1979 to 23% after 2000. This improvement may reflect advances in diagnostics, earlier treatment, and supportive care. Spontaneous aortic thrombosis is typically diagnosed within the first few days of life; Reference Mulcaire-Jones, Bailly and Frank8 in our series, diagnoses were made on days 1, 7, and 10. Case 2 stands out because clinical suspicion of a circulatory disorder in the lower extremities arose in the delivery room. Prenatal detection is exceedingly rare, with few reported cases. Reference Cook, Weeks and Brown19 While the timing of thrombus formation in case 2 remains unclear, a perinatal origin appears likely given unremarkable prenatal screenings.
Approximately half of neonatal spontaneous aortic thrombosis cases have no identifiable cause, while around 25% may involve prothrombotic disorders. Reference Mulcaire-Jones, Bailly and Frank8 In cases 2 and 3, extensive thrombophilia testing revealed no abnormalities. In case 1, timely thrombophilia testing was not feasible, and it is unknown whether the parents underwent genetic testing. The diagnosis of thrombophilia in neonates is performed in a staged manner. Certain parameters, such as antithrombin, protein C, protein S, and antiphospholipid antibodies are assessed early, since antithrombin and protein C can be substituted, and antiphospholipid antibodies are usually maternally derived and thus detectable early after birth, but they frequently become undetectable by six months. Reference Mekinian, Lachassinne and Nicaise-Roland20 Genetic testing for inherited thrombophilia, such as factor V Leiden or prothrombin G20210A mutations, is typically performed during a more stable, post-acute phase. This staged approach to thrombophilia testing is incorporated as a component of the diagnostic algorithm presented in Figure 2.
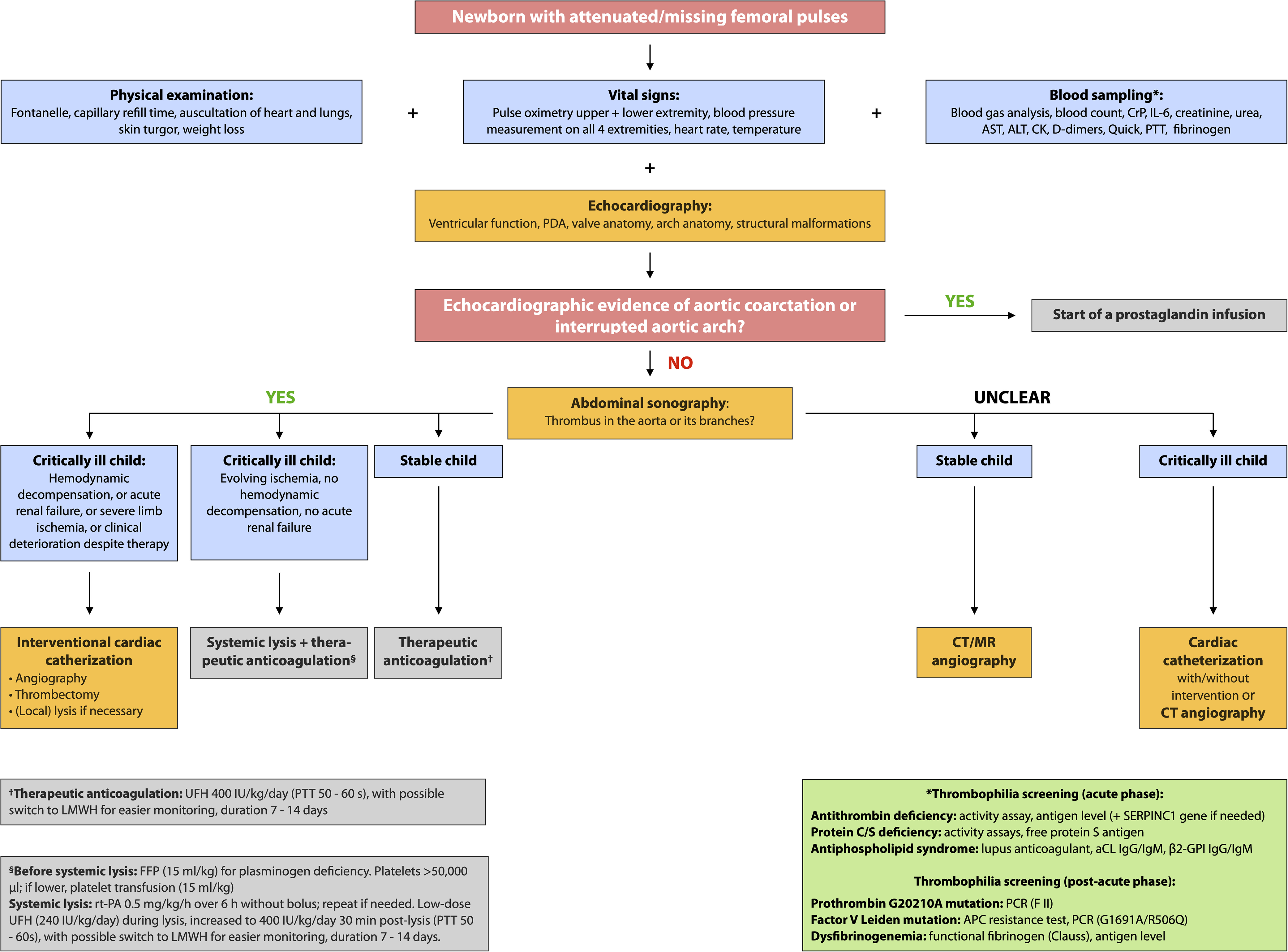
Figure 2. Proposed diagnostic approach for newborns with diminished or absent femoral pulses. Treatment data on medical therapies are scarce; the following protocols for therapeutic anticoagulation and systemic thrombolysis represent one of several possible approaches currently used in clinical practice. Reference Blanchette, Brandão and Breakey36 The green box summarises recommended acute and post-acute thrombophilia screening. Given the potential for rapid deterioration in neonates, whether due to aortic thrombosis or aortic coarctation, and the need for specialised diagnosis and treatment, we recommend immediate contact or referral to the nearest facility with a neonatal intensive care unit (NICU), paediatric cardiology department, and paediatric cardiac catheterisation laboratory. aCL: anticardiolipin antibodies; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CK: creatine kinase; CrP: C-reactive protein; FFP: fresh frozen plasma; β2-GPI: beta-2-glycoprotein I; IL-6: interleukin-6; LMWH: low molecular weight heparin; PDA: patent ductus arteriosus; PTT: partial thromboplastin time; PCR: polymerase chain reaction; rt-PA: recombinant tissue plasminogen activator; UFH: unfractionated heparin.
Cases 1 and 3 were associated with dehydration during the first week of life, potentially contributing to thrombus formation. In case 2, reduced blood flow to the lower extremities was apparent immediately after birth, suggesting the thrombus may have formed pre- or perinatally. This neonate also had moderate perinatal acidosis (umbilical artery pH 7.01, BE -10.4 mmol/L), which could have been an independent risk factor for arterial thrombosis. However, it is important to note that dehydration and perinatal acidosis/asphyxia could be consequences, rather than causes, of the aortic thrombus.
Cases 1 and 3 presented with significant clinical deterioration characterised by nonspecific symptoms such as lethargy, feeding difficulties, weight loss, and decreased urine output. Such signs can occur in a variety of neonatal conditions (e.g., infection, metabolic disorders, congenital heart defects), necessitating thorough clinical evaluation, including vital signs, blood pressure measurement in all four limbs, and pulse oximetry of upper and lower extremities. Importantly, careful palpation of femoral pulses is essential, as abdominal aortic thrombosis typically manifests with diminished or absent femoral pulses. The differential diagnosis for absent femoral pulses in a critically ill neonate includes coarctation or interrupted aortic arch. Reference Wieland, Jack and Seidemann14 Clinical differentiation of aortic thrombosis from coarctation can be challenging; however, symptoms like pallor, pain, and poikilothermia are more indicative of thrombosis. Rapid echocardiography by an experienced paediatric cardiologist is crucial to assess cardiac function, aortic arch anatomy, and exclude structural defects. When coarctation or interrupted arch is excluded, abdominal Doppler sonography should be performed to confirm or exclude thrombosis. Sonography is the most valuable diagnostic tool for detecting aortic thrombosis because it is readily available and non-invasive. Approximately 80% of aortic thromboses are diagnosed by sonography. Reference Nagel, Tuckuviene and Paes7 Typical sonographic findings include visualisation of intraluminal thrombus, echogenic in organised thrombi and hypoechoic in acute stages, as well as abnormal Doppler flow patterns such as absent or turbulent flow. Reference Tsung, Nickels and De Portu21 Visualisation of the abdominal aorta can be challenging due to intestinal gas, but imaging from the right or left flank, near the kidneys, can help avoid gas interference (Figure 1D). Flow in the celiac trunk, superior mesenteric artery, both renal arteries, and the iliac vessels should be documented.
In the critically ill Patient 3, echocardiography showed no evidence of coarctation, but sonographic suspicion of thrombosis was strong. Due to metabolic acidosis, renal failure, and the need for haemodynamic support, the team proceeded with cardiac catheterisation. For more stable patients, contrast-enhanced CT or MR angiography may be alternatives, balancing diagnostic accuracy against radiation exposure and sedation risks. Selection of imaging modality should consider clinical urgency, local resources, and patient risk factors. Reference Labruto, Blomqvist and Swedenborg22,Reference Callahan and Cravero23
Treatment strategies and therapeutic challenges
Treatment of neonatal aortic thrombosis depends largely on disease severity. Minor thrombosis is typically associated with decreased femoral pulses and systemic hypertension, while moderate thrombosis includes absent pulses, peripheral ischaemia, and evolving heart failure. Major thrombosis presents with severe limb ischaemia, paralysis, renal failure, visceral involvement, and acidosis. Reference Nagel, Tuckuviene and Paes7 Heparinisation is recommended for mild to moderate cases, while thrombolytic therapy is reserved for severe cases. Surgical or interventional thrombectomy is indicated for cases where thrombolysis is contraindicated or unsuccessful. However, treatment decisions are largely based on clinical experience and the analysis of historical case series, with individualised approaches due to the limited available evidence.
Systemic thrombolysis combined with anticoagulation remains the most commonly used treatment for neonatal spontaneous aortic thrombosis. Reference Mulcaire-Jones, Bailly and Frank8 Neonates present unique challenges for thrombolytic therapy due to their developing haemostatic system, which includes physiologically low plasminogen levels. Reference Monagle, Chan and Goldenberg24 Streptokinase, urokinase and rt-PA work by converting endogenous plasminogen to plasmin, but in neonates, plasma plasminogen concentrations are about half those of adults, potentially reducing the efficacy of these agents. Reference Herron, Covi and Pappas17 Fresh frozen plasma can be administered to increase plasminogen levels and enhance thrombolysis. A meta-analysis of 18 studies reported complete or partial thrombus resolution in 87.9% of neonatal cases treated with various thrombolytics, though treatment duration varied widely. Reference Leong, Patel and Samji25 However, Nowak-Göttl et al. reported overall patency rates for thrombolytic therapy, with rates as low as 39% in children with aortic thrombosis. Reference Newall, Browne and Savoia26 The variety of effective lysis strategies and thrombolytic agents results in the availability of multiple treatment protocols within our institution for comparable patient populations. Although not utilised in the cases presented, bivalirudin has emerged as a promising alternative anticoagulant in neonatal and paediatric thrombosis, particularly in situations of heparin resistance or heparin-induced thrombocytopenia (HIT). Bivalirudin is a direct thrombin inhibitor that binds both circulating and clot-bound thrombin, offering more complete thrombin inhibition. In contrast, unfractionated heparin acts indirectly by enhancing antithrombin activity and is only effective against circulating thrombin, which may limit its efficacy in organised or established thrombus. Moreover, neonates have physiologically low and fluctuating levels of antithrombin, making adequate heparin dosing and therapeutic monitoring more challenging. Bivalirudin’s mechanism of action bypasses this limitation and may provide a more stable anticoagulant effect. Although off-label, increasing clinical experience supports the safety and efficacy of bivalirudin in this population. Reference Shammas27–Reference Taha, Rajgarhia and Alsaleem30
In neonates with cardiac dysfunction, rapid recanalisation of the obstructed aorta is critical, as left ventricular failure often results from increased afterload due to hypertension. Persistent obstruction typically leads to poor clinical outcomes and possible decompensation. In cases of severe cardiac compromise or impending organ failure, prompt mechanical thrombectomy may improve prognosis by quickly restoring blood flow.
A case series by John P. Mulcaire-Jones et al. demonstrated the non-inferiority of combined systemic thrombolysis followed by heparinisation, compared to alternative treatment approaches, in terms of mortality before hospital discharge for neonatal aortic thrombosis. This systematic review of 80 cases found that surgical or catheter-based thrombectomy was performed in 34% of cases. Notably, in 18% of these cases, thrombectomy was conducted without adjunctive thrombolysis or anticoagulation, which, unlike the combination of thrombectomy with pharmacologic therapy, was associated with higher mortality rates compared to standard treatment with systemic thrombolysis and heparinisation. Reference Mulcaire-Jones, Bailly and Frank8 Due to the small sample size, interventional and surgical thrombectomy were analysed together in this historical cohort. Additionally, the retrospective nature of the study did not account for disease severity, leaving a gap in understanding the efficacy of various treatment modalities in severely affected patients, particularly regarding interventional thrombectomy. However, recent case reports suggest that interventional thrombectomy may be effective as a first-line treatment in time-critical cases involving cardiac decompensation or imminent organ failure. Reference Gutierrez, Alten and Law15,Reference McGovern, Qureshi and Goldstein16,Reference Kenny and Tsai-Goodman31–Reference Patel, Takao and Badran33 A literature review using the terms 'neonatal aortic thrombosis intervention' and 'neonate interventional thrombectomy' identified seven published cases of aortic thrombectomy employing various devices. Reference Gutierrez, Alten and Law15,Reference McGovern, Qureshi and Goldstein16,Reference Gerardin, Anderson and Armstrong32,Reference Cleveland, Pezeshkmehr and Hernandez34 In contrast to a previously published case report, we found that in our case, the off-label use of the Amplatzer Vascular Plug II was more effective than the Amplatzer Piccolo PDA Occluder for managing an organised thrombus. Reference Herron, Covi and Pappas17 Initial attempts with the softer Amplatzer Piccolo PDA Occluder were unsuccessful due to conformational changes during mechanical manipulation, whereas the stiffer and larger Amplatzer Vascular Plug II allowed for more effective thrombus capture and removal. Other authors have documented the successful use of aspiration thrombectomy catheters, including the Penumbra and AngioJet systems with a carotid access. Reference Gerardin, Anderson and Armstrong32,Reference Cleveland, Pezeshkmehr and Hernandez34 However, thrombectomy should not be used as a stand-alone therapy but should be combined with thrombolysis and anticoagulation for optimal outcomes. Reference Mulcaire-Jones, Bailly and Frank8
Our experience, alongside existing literature, supports a treatment strategy guided by clinical severity, haemodynamic status, and organ function. In stable neonates with partial aortic obstruction and preserved end-organ perfusion, therapeutic anticoagulation with unfractionated heparin (UFH) may be sufficient. Systemic thrombolysis (e.g., with rt-PA, urokinase, or streptokinase) in combination with UFH should be considered in neonates with confirmed aortic thrombosis who present with evolving ischaemia and preserved haemodynamics, provided that contraindications such as active bleeding or severe thrombocytopenia are absent. In contrast, catheter-based thrombectomy may be warranted in critically ill neonates who deteriorate despite anticoagulation and/or thrombolysis, or in those presenting with life-threatening complications such as cardiogenic shock, severe organ ischaemia, or progressive clinical decline despite therapy. In our view, specific indicators for such intervention include (1) left ventricular failure and/or haemodynamic decompensation, (2) acute renal failure, (3) severe limb ischaemia (e.g., pregangrenous changes, paralysis and/or absent Doppler flow >24 h distal to obstruction), and (4) rapid clinical decline under medical treatment. This structured, severity-based approach may help guide individualised treatment decisions in this rare but life-threatening condition. Figure 2 summarises our proposed diagnostic and therapeutic algorithm, intended to aid timely diagnosis and treatment escalation, when needed.
Case series outcomes and safety considerations
In our case series, patient 1 tragically died due to pulmonary haemorrhage and subsequent cardiopulmonary deterioration following interventional thrombectomy. This complication occurred 24 hours post-procedure during ongoing therapeutic heparinisation. The potential for catheter-based interventions to increase bleeding risk in such complex scenarios warrants further study. Patient 2, who showed no signs of cardiac impairment or impending renal failure, responded successfully to pharmacologic therapy alone. Patient 3, more severely affected, underwent successful interventional recanalisation with a favourable outcome, experiencing only minor puncture site bleeding following combined thrombectomy, thrombolysis, and anticoagulation. Overall, interventional thrombectomy appears to be an effective, rapid option for reestablishing blood flow in major spontaneous aortic thrombosis. However, the risk of severe haemorrhagic complications, such as pulmonary or cerebral bleeding, remains elevated in all patients receiving systemic lysis and anticoagulation. Consequently, regular cranial ultrasound monitoring is essential regardless of the chosen treatment strategy. It is important to note that, unlike with heparin anticoagulation, systemic lysis offers no laboratory monitoring to assess efficacy or risk of overdose. Reference Will35
Conclusion
Spontaneous aortic thrombosis in newborns is an extremely rare but life-threatening condition often complicated by delayed diagnosis due to limited clinical experience. The severity of the thrombosis and timely initiation of appropriate treatment critically influence outcomes. Our single-centre experience supports that in urgent cases with compromised left ventricular function and imminent organ failure, interventional thrombectomy can be considered a viable first-line therapy, alongside pharmacologic treatment. While promising, current evidence remains limited, highlighting the need for further research to evaluate the safety and efficacy of catheter-based interventions in this population. To minimise diagnostic and therapeutic delays, we recommend the use of our proposed diagnostic and treatment algorithm for neonates presenting with diminished or absent femoral pulses. Early referral to specialised centres equipped with a NICU, paediatric cardiology, and cardiac catheterisation facilities is essential for optimal management.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951125109724
Acknowledgements
None.
Financial support
Svea Kleiner is partially funded by the German Research Foundation (DFG), through the PRACTIS-Clinician Scientist Program of Hannover Medical School.
Competing interests
None.




