Introduction
Brain metastases develop in 10–40% of cancer patients. Reference Saria, Piccioni, Carter, Orosco, Turpin and Kesari1 The treatment modalities available include stereotactic radiosurgery (SRS), surgical resection and whole-brain radiotherapy. Reference Gondi, Bauman and Bradfield2–Reference Khaledi, Khan and Gräfe4 Among these, stereotactic radiosurgery has been found to be very effective with good prognosis. Reference Knisely, Sahgal, Lo, Ma and Chang5 SRS relies on highly accurate positioning, immobilization and treatment planning to deliver high doses of radiation to brain targets. Reference Chao, De Salles and Hayashi6 The primary goal of any treatment planning in radiotherapy is to deliver the highest achievable dose of radiation to the target volume while respecting the critical structures surrounding the target. Reference Abdel-Wahab, Fidarova and Polo7 It is common practice in conventional radiotherapy to place a margin around the clinical target volume (CTV) to form a planning target volume (PTV). The PTV compensates for the inaccuracies intrinsic to the process of radiotherapy, including patient alignment, geometrical setup uncertainties and patient intra-treatment motion. 8 Defining an excessive amount of volume as part of PTV can lead to higher chances of injury to the surrounding critical organs receiving collateral high doses. Reference Kron9–11
A Gamma Knife (GK) radiosurgery system is manufactured by Elekta AB, a Swedish company that specializes in advanced radiation oncology and neurosurgery systems. The Gamma Knife system uses small arcs of cobalt-60 sources to deliver high-dose precision local radiation. It is equipped with a treatment planning system (TPS). Reference Skourou, Hickey and Rock3,Reference Schmitt, Blanck and Gauer12 It is an uncommon practice in planning Gamma Knife radiosurgery to place additional planning margin around the target volume to compensate for treatment uncertainties from patient head motion during treatment, beam collimation uncertainties or both. Some studies suggest that planning target volume (PTV) margins can vary significantly for small targets and fractionated treatment compared to conventional fractionated treatments. Reference Duggar, Morris, He and Yang13,Reference Duggar, Morris, He and Yang14 The Leksell stereotactic coordinate system, which revolutionized radiosurgery delivery capabilities through multiple platforms, has raised the need to redefine margin addition. Reference Grishchuk, Dimitriadis and Sahgal15
PTV margins are important components in establishing acceptance and delivery appropriateness of external beam therapies including Gamma Knife radiosurgery (GKRS). They are added to the CTV to provide for uncertainties in the localization, contouring of the target volume and for uncertainties in the planning and delivery of the treatment. Reference Fiagbedzi, Hasford and Tagoe16 For intracranial targets including brain metastases (BM), the magnitude of the added PTV margin varies significantly across different GK models and treatment settings such as single session versus hypofractionation, based on the GK radiation delivery geometry and commissioning methods. Reference Agazaryan, Tenn and Lee17,Reference Mesko, Wang and Tung18 Most importantly, choosing appropriate PTV margins is essential in preventing marginal recurrence. The choice is based on the understanding that a CTV is delineating radioresistant tumour during brain imaging examinations. However, advancing technologies offer new detailed insights to allow for better approaches in radionecrotic risk estimation. Reference Duggar, Morris, He and Yang13,Reference Bernstein, Taylor, Nill and Oelfke19,20 The traditional method of rigid immobilization fixation for a single fraction GK SRS utilizes the convention of a 0 mm PTV, analogous to surgical excision of brain targets. PTV margins are important when performing Gamma Knife radiosurgery. Reference Kutuk, Kotecha and Tolakanahalli21 The prescription isodose which refers to the maximum dose in percentage that is selected to conform to the periphery of the target can also impact the PTV margins and ultimately the effectiveness and safety of the treatment. Reference Torrens, Chung and Chung22
When planning with PTV margins, there are several factors that should be taken into consideration. These include the size, location and shape of the brain metastasis, the patient’s overall health and medical history. Reference Gondi, Bauman and Bradfield2,Reference Stewart, Sahgal and Zadeh23 Depending on these factors, a suitable prescription of isodose may be used to optimize the balance between tumour control and preservation of healthy brain tissue.
With these concepts in mind, this study sought to evaluate the influence of PTV margins using various prescription isodoses on dosimetric parameters for the treatment of single brain metastasis on the Gamma Knife System.
Materials and Methods
Phantom
The Stereotactic End-to-End Verification (STEEV) head anthropomorphic phantom, manufactured by CIRS in Norfolk, VI, USA, was used for this study. The phantom was modified to accommodate a solitary, asymmetrical target positioned 10 mm in front of the brainstem. The design of the phantom facilitates the use of replaceable cuboid inserts for imaging and irradiating the brain’s central region. Additionally, it allows for the insertion of radiation detectors via two parallel cylindrical access cavities, as seen in Figure 1. The phantom underwent a treatment planning computed tomography (CT) scan, using a Philips Brilliance CT scanner (Philips HealthCare, Best, Netherlands) with a large bore size of 85 cm and slice thickness of 2 mm.

Figure 1. Image of STEEV phantom with dosimetry inserts.Reference Dimitriadis, Palmer, Thomas, Nisbet and Clark24
Treatment planning system
The CT images were imported into the Gamma Plan Treatment Planning System, version 11.3.1. As shown in Figure 2, the centre of the internal rectangular part which was the target insert in the phantom was delineated as the gross tumour volume (GTV) with the help of an experienced radiation oncologist and a medical physicist. A target volume measuring 4·9cc was centrally manually contoured. Clinically acceptable plans with 0 mm margin at five prescription isodose levels from 50% to 70% at 5% increments with the same tumour coverage were created. PTVs were then regenerated by GTV external expansion of 0·5 mm, 1 mm, 1·5 mm and 2 mm isotropically, resulting in volumes of 5·6cc, 6·4cc, 7·8cc and 8·7cc, respectively. These were recalculated at five different prescription isodose levels (50%, 55%, 60%, 65%, 70%). Manual adjustment of plans with shots was made when necessary to achieve the same coverage. A dose of 18Gy in single fraction was used. The dosimetric parameter values were generated at the end of the dose calculation for each plan. PCI, GI and S values were based on Equation 1, 2 and 3, respectively. Obtained values of PCI, GI and S were compared to ideal values set out by the International Leksell Gamma Knife Society Standardization Committee.
where PIV is prescribed isodose volume, TV is the Target Volume, and TVPIV is the TV receiving the prescription dose. Paddick’s conformity index (PCI) considers the spatial correlation between the prescribed volume and the TV. An ideal value for the PCI conformity should be < 1·18. Reference Torrens, Chung and Chung22,Reference Tham, Aleman, Nordström, Nygren and Coolens24 The TV, TVPIV and PIV, for each treatment plan, were obtained from the Dose Volume Histogram (DVH) in Figure 3.
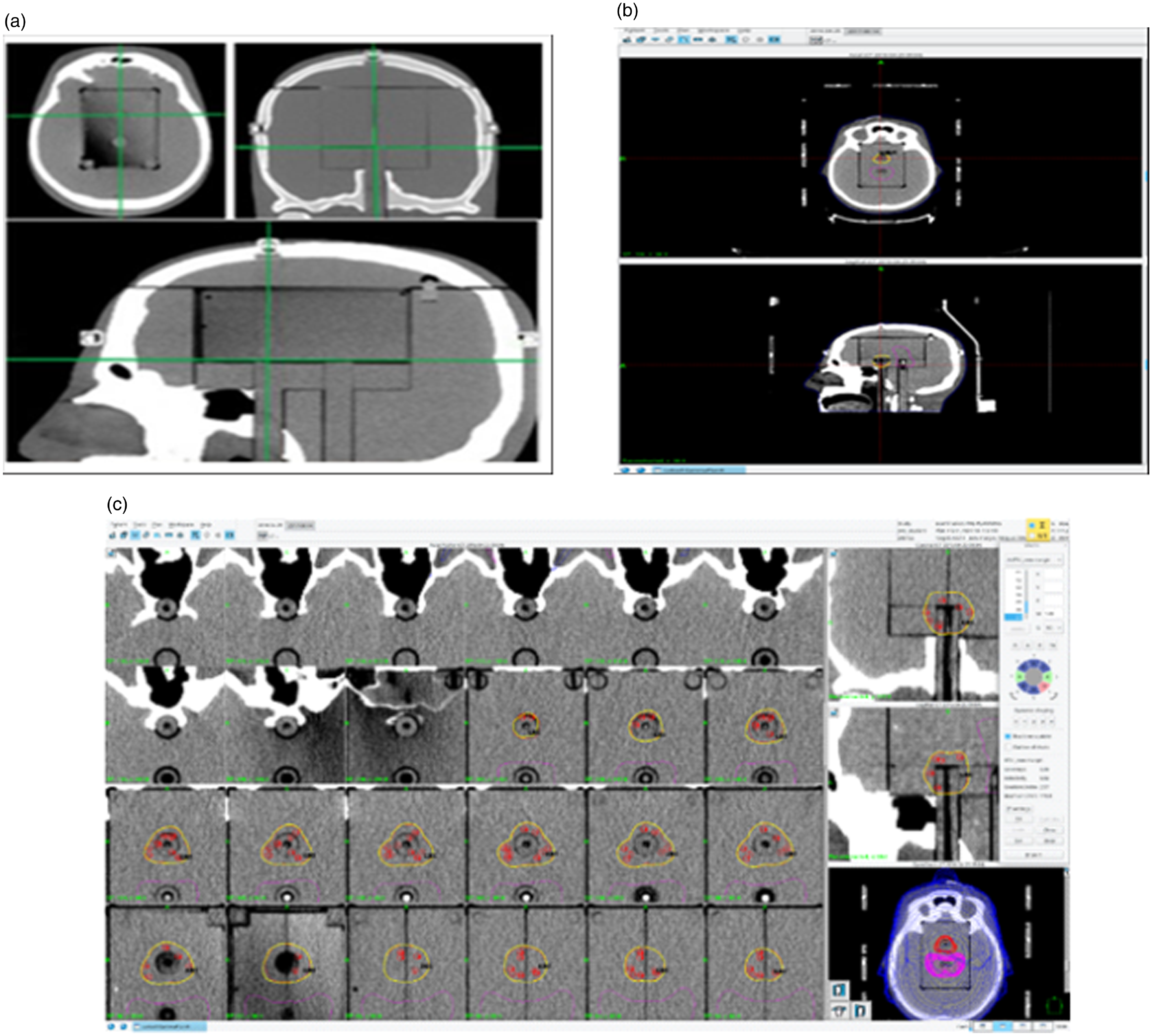
Figure 2. The phantom through sagittal, coronal and axial planes with the target insert (2a), inside the cavityReference Dimitriadis, Palmer, Thomas, Nisbet and Clark24 (2b), target delineation in gamma plan TPS (2c), dose distribution in the target in the treatment planning system.
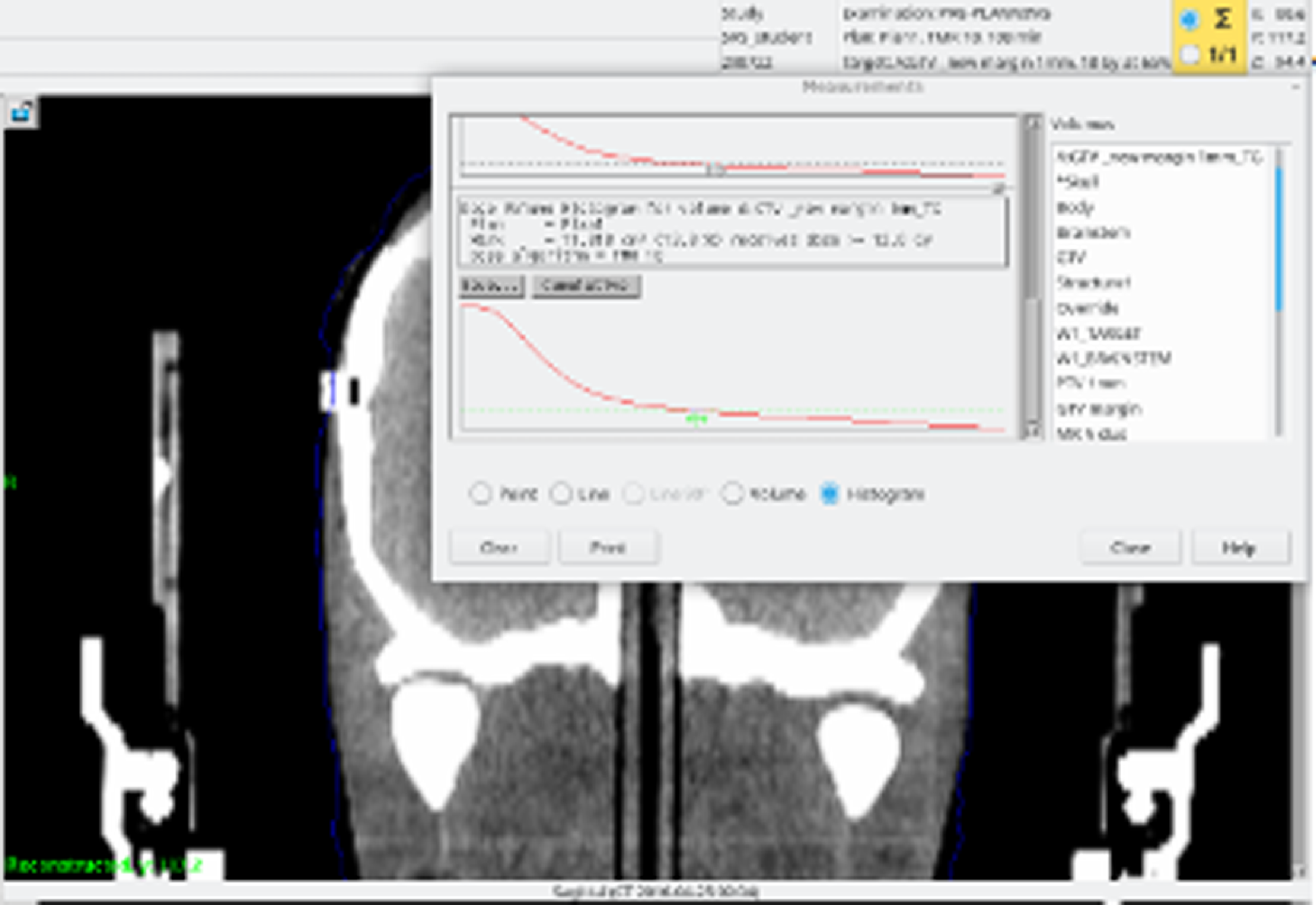
Figure 3. DVH and the volume analysis tools from the planning system.
The Gradient Index (GI) is calculated as the ratio of the volume contained by half of the prescribed isodose to the volume of the prescribed isodose itself. This is sometimes referred to as the 25/50 proportion. An ideal value for the Gradient Index might be less than 3·0. Reference Torrens, Chung and Chung22
Selectivity Index refers to the extent to which normal tissue around the target is preserved. This is calculated by dividing the target volume covered by the prescribed isodose by the total volume of the prescribed isodose. A measure of the selectivity may be needed if a compound conformity index is not applied. An ideal value for selectivity may be taken as > 0·9. Reference Torrens, Chung and Chung22,Reference Fallows, Wright and Bownes25
One-way ANOVA test was used to analyse the influence of planning target volume margins with the various prescription isodoses used on the parameters Selectivity [S], Gradient index [GI], V12, PCI and Treatment time [TI].
Results
Twenty-five plans were derived with the PTV margins sizes 0·0 mm, 0·5 mm, 1·0 mm, 1·5 mm and 2·0 mm used and the five prescription isodose levels 50%, 55%, 60%, 65% and 70%.
Values for selectivity, Gradient and PCL for all margin sizes and prescription isodoses all met the ideal values set out by the International Leksell Gamma Knife Society Standardization Committee (Table 1).
Table 1. One-way ANOVA test for planning dosimetric parameters and PTV margins in the study
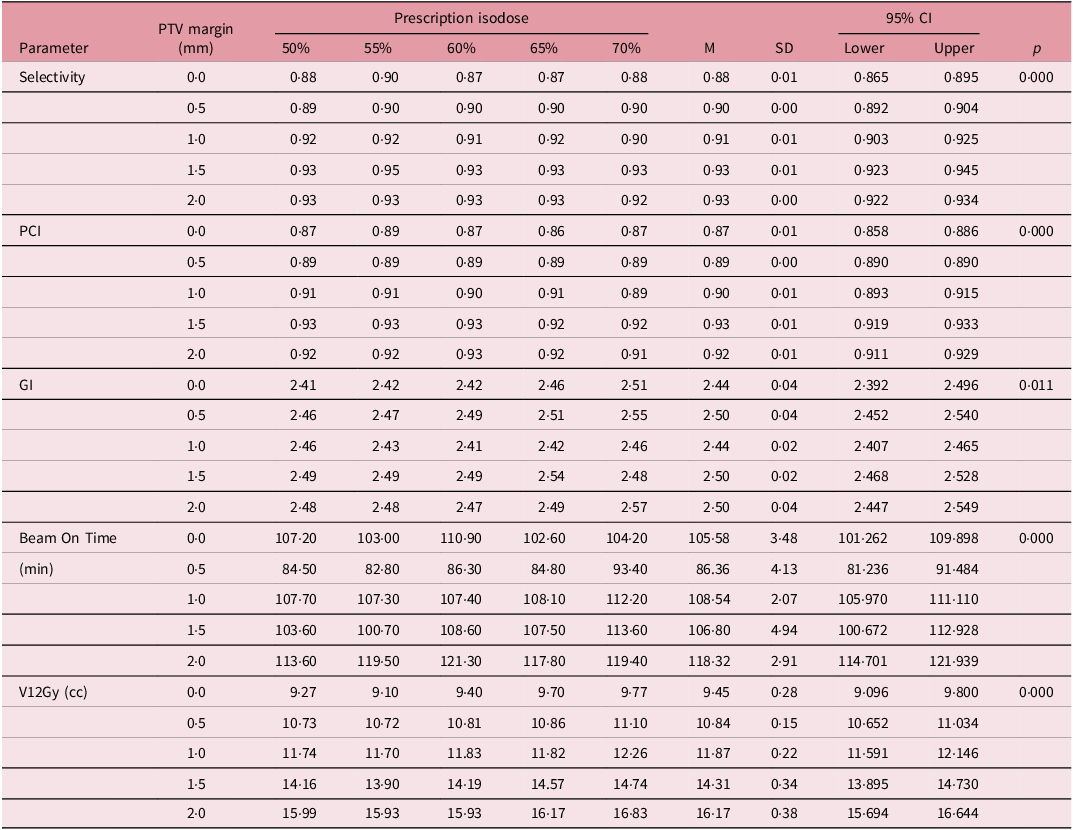
Table 1 shows the ANOVA test of the distribution of planning dosimetric parameter values across different PTV margins with the prescription isodoses levels in the study.
The overall means for selectivity for the 0·0 mm, 0·5 mm, 1·0 mm, 1·5 mm and 2·0 mm were 0·88, 0·90, 0·91, 0·93 and 0·93 respectively. This increases monotonically with increase in margin size but remain fairly steady as the prescription isodose levels increase from within the same margin size, with a statistically significant p-value of < 0·001. This trend suggests that larger PTV margins enhance the confinement of the radiation dose within the target volume, potentially increasing treatment effectiveness.
Similarly, the Planning Conformity Index (PCI) shows a positive correlation with PTV margin, also significant at p = < 0·001. The PCI increases from 0·87 at a 0·0 mm margin to 0·92 at a 2·0 mm margin. The PCI remain fairly steady for margins 0 mm and 0·5 mm but decreases marginally with margins 1·0 mm, 1·5 mm and 2·0 mm as prescription isodose levels increase. Higher PCI was seen in lower PILs. A higher PCI indicates improved conformity of the dose distribution to the target volume, which is critical for minimizing radiation exposure to surrounding healthy tissues.
However, the Gradient Index (GI) displays a less consistent pattern across the PTV margins, despite remaining statistically significant (p = < 0·001). Although there is a slight overall increase, as the prescription isodose increases, the GI fluctuates between 2·44 and 2·50, without a clear trend, indicating that the sharpness of dose fall-off does not uniformly improve with larger PTV margins. The 2·0 mm, 1·5 mm and 0·5 mm PTV margins had the highest and identical GI planning value of 2·50. The PTV margins of 1.0 mm and 0·0 mm had GI planning values of 2·44.
Beam-on time which refers to the treatment time also varies significantly across different PTV margins, with a p-value of < 0.001. It starts at 105.58 minutes for a 0.0 mm margin, decreases to 86.36 min at 0.5 mm and then increases to 118.32 min at 2.0 mm. The increase in beam-on time with larger PTV margins may result from the need for more extensive radiation delivery to cover the larger volume, potentially affecting treatment efficiency.
Finally, the V12Gy (cc) which is an important parameter in SRS shows the volume (V12) receiving 12Gy and is a prognosticator for radiation necrosis. Reference Gondi, Bauman and Bradfield2 The V12Gy increases steadily with the PTV margin, also with statistical significance at p = < 0·001. V12Gy rises from 9·45 cc at 0·0 mm to 16·17 cc at 2·0 mm. This trend suggests that while larger margins may enhance selectivity and PCI, they also lead to a greater volume of surrounding tissue being exposed to potentially harmful radiation doses. Across all the margin sizes, the highest V12 values were recorded with the 70% prescription isodose.
Discussion
In this study, the impact of planning target volume margins using various prescription isodose levels on plan parameters Selectivity [S], Gradient index [GI], V12, PCI and treatment time [TI] is explored in the Gamma Knife Treatment Planning System. Values for selectivity, Gradient and PCL for all margin sizes with the various prescription isodoses were in agreement with ideal values set out by the International Leksell Gamma Knife Society Standardization Committee. In the clinical setting, manual modification of the plans may be required if the ideal values are not met. The addition of margins to the GTV with the various prescription isodose levels significantly influences all parameters. The treatment time and V12 were much higher in the 2 mm margin than 0 mm margin. When a margin is added to the GTV, the treated volume increases. Reference Xu, Kubicek and Mulvihill26 The relationship of PTV margin addition on volume can be quantified in a linear relationship. The size increase may have detrimental consequences on surrounding healthy brain tissues and organs at risk. Reference ElBeltagi, Wall and Marignol27–Reference Ma, Sahgal and Larson29 Empirical effects of PTV margins used in SRS have been observed in the literature: Noel et al. selected a PTV margin of 1 mm for SRS and this improved local control, but toxicity rates were not influenced. Reference Noël, Simon and Valery30 Nataf et al. found adding a PTV margin of 2 mm to single fraction SRS resulted in an increase of 12·5% in complications; Reference Nataf, Schlienger and Liu31 Choi et al. found adding a 2 mm margin around the post-surgical cavity of brain metastasis for SRS improved local control without increasing toxicity when compared to using no margin. Reference Choi, Chang and Gibbs32 Increasing margins may be undesirable from the irradiation of healthy tissue perspective since they increase the volume of healthy tissue exposed to radiation, raising the risk of adverse effects and complications, such as tissue damage or necrosis. Reference Fiagbedzi, Hasford and Tagoe16 Should higher margin be used, strategies such as fractionation should be considered to reduce treatment-related toxicities to the patient. This enables adequate dose to be prescribed to the target, while limiting late side effects. Reference Xu, Kubicek and Mulvihill26
For each PTV margin, prescribing to the 70% isodose line increased treatment delivery time and V12, with minimum impact on selectivity, gradient and PCI although they increase as the prescription isodose increases from the 50% to 70% (Figures 4-8). The increase in gradient index has been demonstrated by Paddick et al. Reference Paddick and Lippitz33 Lower GIs (< 3·0) show an appropriately positioned isocentre and a steep dose gradient. GI is reliant on the size and form of the target and affects the dose that healthy brain tissue receives. Reference Jayaprakash, Pendse and Deshpande34 Lowering the isodose prescription to 50% would decrease dose to normal tissues immediately outside the target volume. This was in contrast to Brown et al who found out that toxicity correlated with tumour size but not prescription isodose line. Reference Brown, Marcrom and Patel35 This should be evaluated and considered in the clinical setting, especially when targets are close to sensitive organs at risk. Treatment time is one other important factor to consider for GK Inverse planning. Prolonged treatment led to patient inconvenience, distress and more treatment uncertainties. Reference Fallows, Wright and Bownes25 We also found significant increase in treatment time when PIL and margin increased except for the mean treatment time of the 0.5 mm margin which was found to be higher than the 0 mm. This is likely due to lower throughput from the smaller collimation (Figure 8). This was in agreement with a study carried by Xu et al, who reported that at 30% and 70% PIL plans, the treatment time was 21.9% (p = 0·001) and 11.7% (p = 0·009) longer than at 50% PIL plans, respectively, for larger tumours. Reference Xu, Kubicek and Mulvihill26 In all, largest impact of changing the isodose prescription was in time savings. Such decrease in treatment times also decreases the peripheral scatter dose. Decisions on various parameters should be considered based on all facets that impact on coverage and peripheral dose.
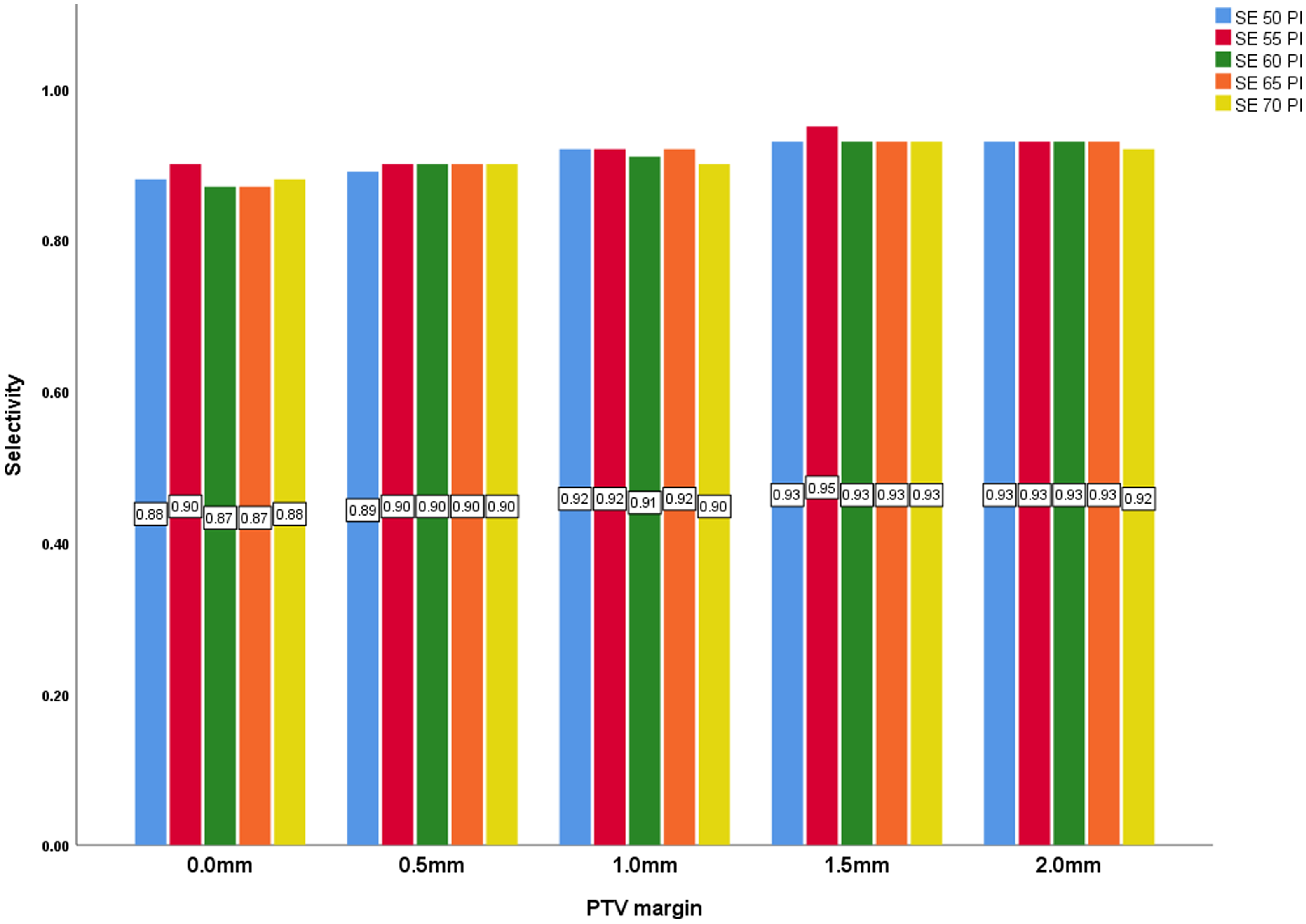
Figure 4. PTV margin and selectivity with all prescription isodose.
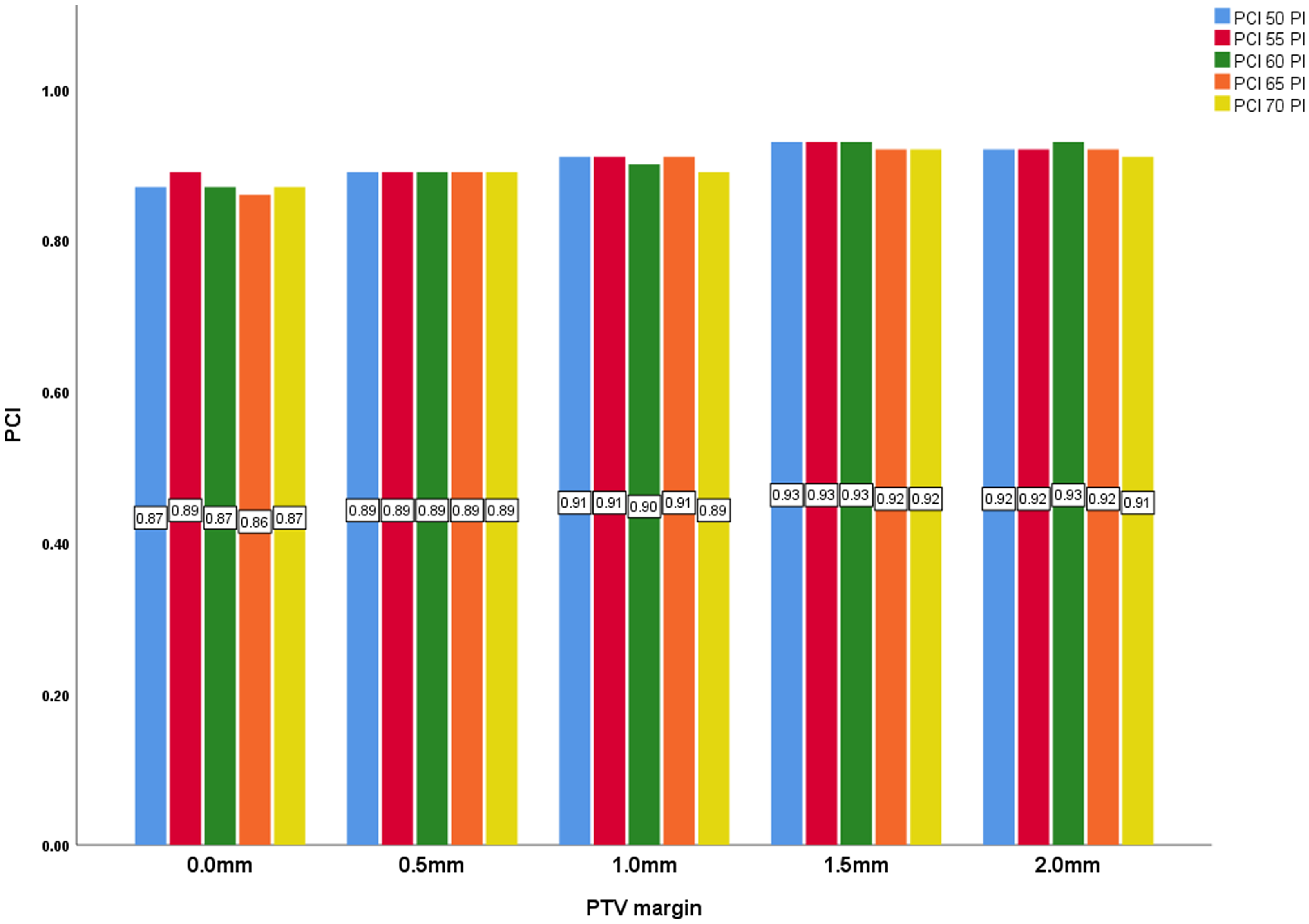
Figure 5. PTV margin and PCI with all prescription isodose.
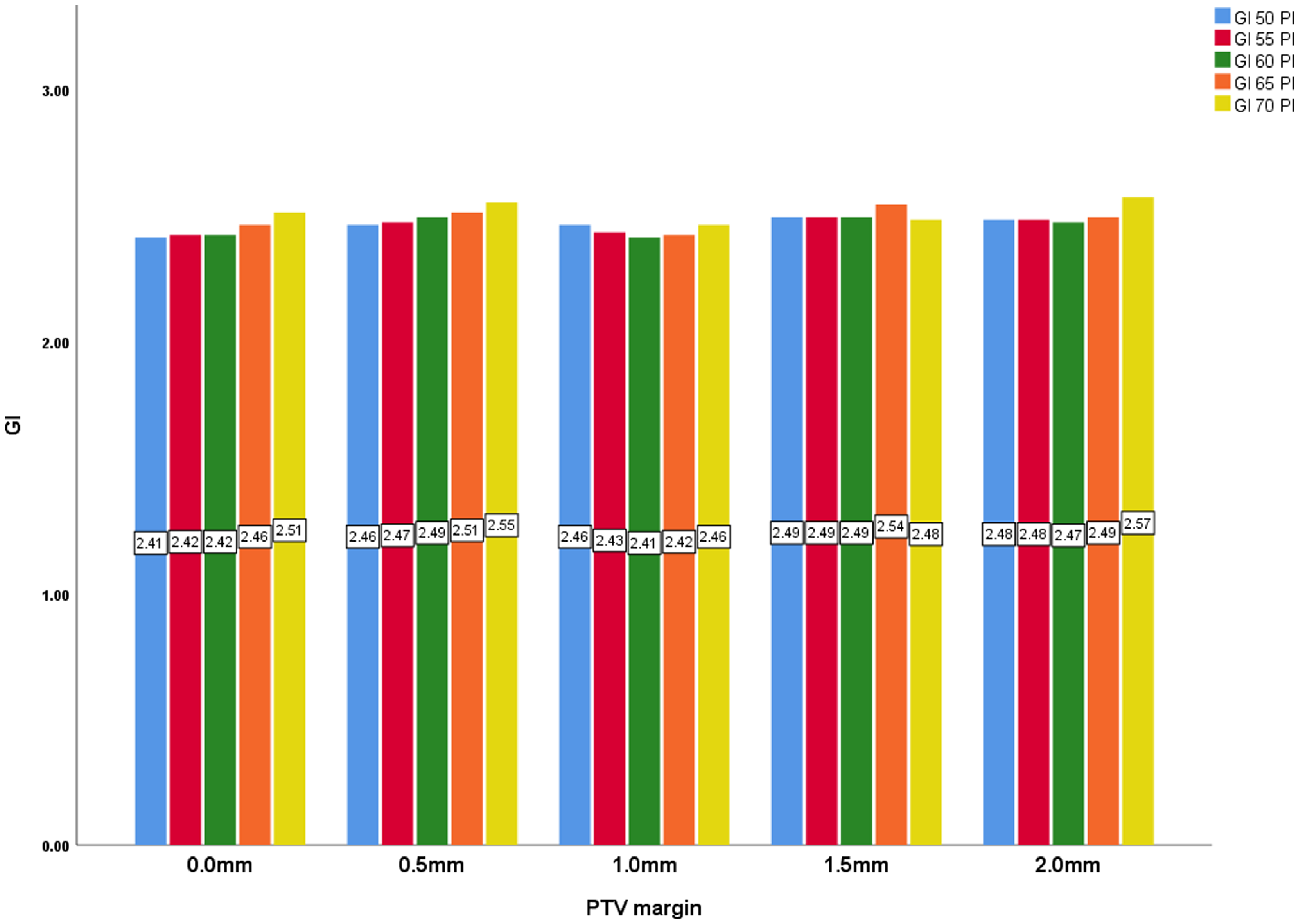
Figure 6. PTV margin and GI with all prescription isodose.
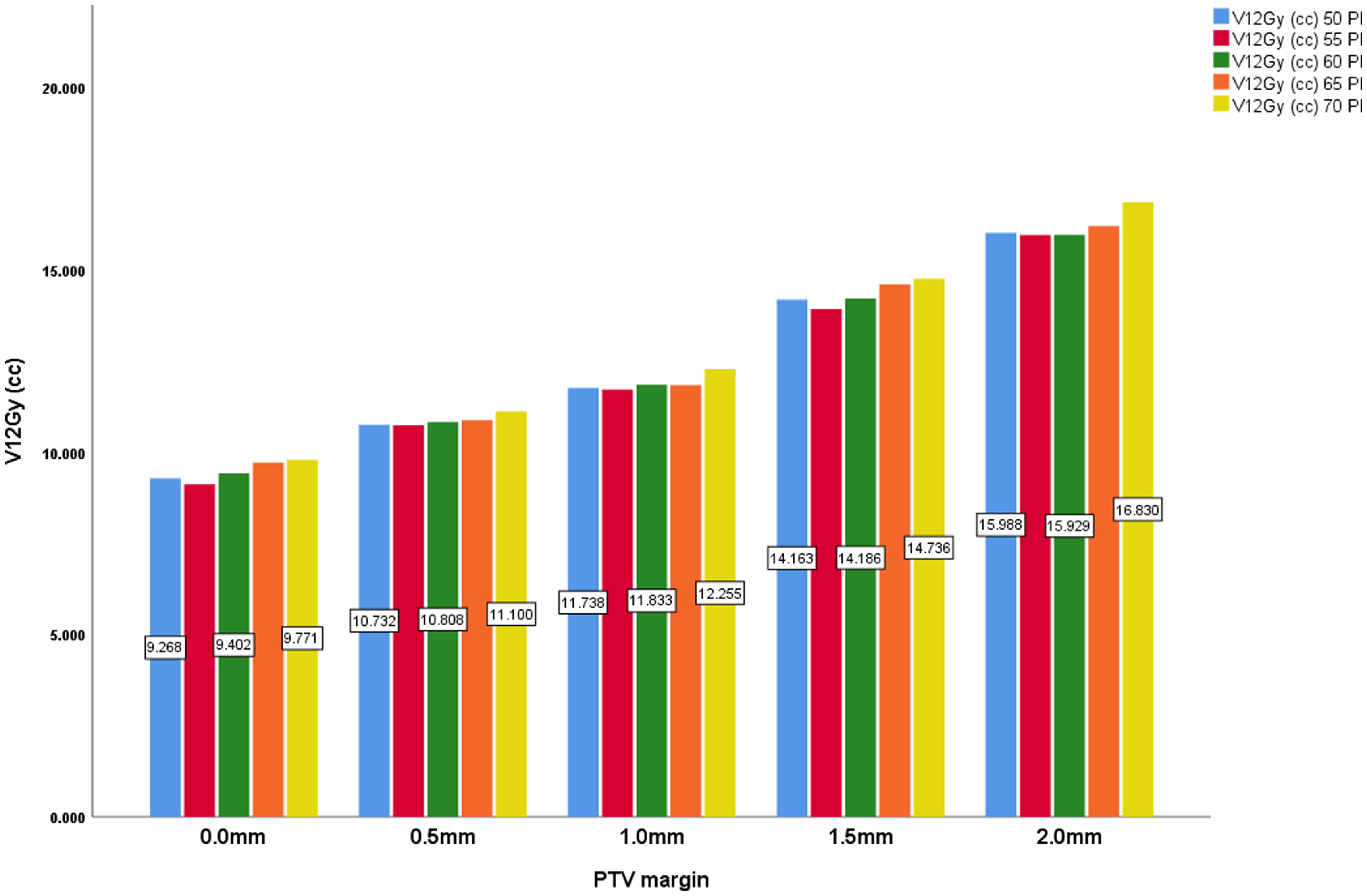
Figure 7. PTV margin and V12Gy (cc) with all prescription isodose.
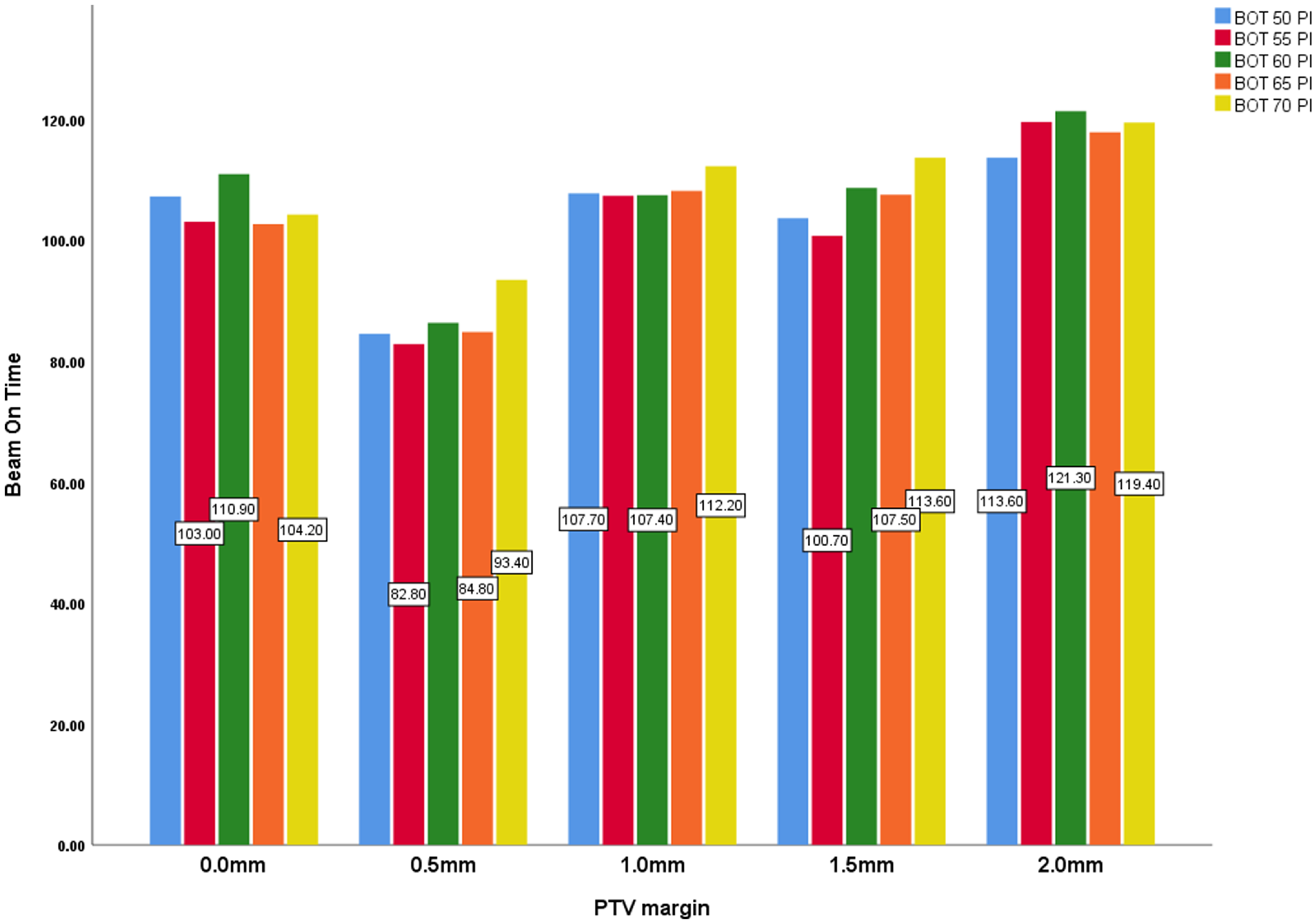
Figure 8. PTV margin and beam-on-time (min) with all prescription isodose.
Conclusion
PTV margin is an important consideration in Gamma Knife radiosurgery for single brain metastasis, and the expansion of this influences the dosimetric parameters, Selectivity [S], Gradient index [GI], V12, PCI and treatment time [TI]. From a clinical perspective, the decision of what PTV margin and prescription isodose to use for SRS treatments for brain metastasis depends on the clinical goals established for the treatment type. Incremental increases in PTV margins for GK SRS though a relatively controversial concept pose potential detrimental effects, and this needs to be carefully evaluated.
Acknowledgements
We would like to express our sincere gratitude to all staff especially Diana Grishchuk at the Queen Square Radiosurgery Centre, London, who supported in data collection and made my Commonwealth Scholarship research visit fruitful.
Financial support
Commonwealth Scholarship.
Competing interests
None.











