Introduction
The intestinal tract functions as the main location for digestion and nutrient absorption in broilers, highlighting the importance of maintaining intestinal health to optimize production performance and economic returns. Previous studies have shown that a diverse microbial community colonizes the intestinal mucosa of broilers, and the intestinal microbiota is crucial for maintaining the function of the intestinal barrier, enhancing mucosal immunity and sustaining host health by producing beneficial metabolites (Bozomitu et al., Reference Bozomitu, Miron and Adam2022; Fathima et al., Reference Fathima, Shanmugasundaram and Adams2022). The intricate relationship between gut microbiota and intestinal metabolites is significant. Gut microbiota ferments dietary fiber, proteins and other food components to produce gut metabolites. For example, gut microbiota fermentation of dietary fibers generates volatile fatty acids (VFAs), which play a crucial role in preserving gastrointestinal well-being, regulating immune system activity and providing energy to enterocytes (Lan et al., Reference Lan, Chen and Cao2021). Moreover, the composition and function of gut microbiota can be modulated by intestinal metabolites. Different metabolites exert diverse effects on the growth and proliferation of gut bacteria. For instance, specific metabolites promote the growth of beneficial bacteria, whereas others inhibit the proliferation of harmful bacteria, thereby maintaining a balanced gut microbial community (Ghosh et al., Reference Ghosh, Whitley and Haribabu2021). Specifically, VFAs serve as an energy source for beneficial bacteria, whereas particular bile acid metabolites exhibit inhibitory effects on the growth of harmful bacteria. Modulating gut microbiota has emerged as a novel strategy for enhancing gut health. The intricate interplay between intestinal microorganisms and their metabolites collectively constitutes a complex intestinal microecosystem. This balance is essential for the intestinal health of livestock and poultry, and any imbalance may lead to various diseases induced by inflammatory responses. In modern intensive farming systems, various factors can disrupt this balance and cause intestinal damage, including inadequacies in dietary processing technology, exposure to exogenous pathogenic bacteria within the environment, overcrowding conditions, and compromised immune function among animals (Abdollahi et al., Reference Abdollahi, Zaefarian and Ravindran2018; Yang et al., Reference Yang, Liang and Guo2020). Historically, antibiotics, recognized for their antibacterial and anti-inflammatory properties, have been widely used to address gastrointestinal disorders and reduce disease incidence and mortality rates in livestock and poultry. However, as the adverse effects of their use have become increasingly evident, the application of these substances as feed additives has been prohibited. In contemporary production practices, alternatives such as acidifying agents, plant extracts, bioactive peptides, immunomodulators and enzymes replace diet-based antibiotics. These alternatives help regulate the intestinal pH level, improve intestinal microbiota and metabolites, and enhance the intestinal barrier function of the body. These strategies ultimately contribute to improved animal health status and generate positive economic benefits (Sun et al., Reference Sun, Guo and Xu2022; Zou et al., Reference Zou, Ji and Qu2019).
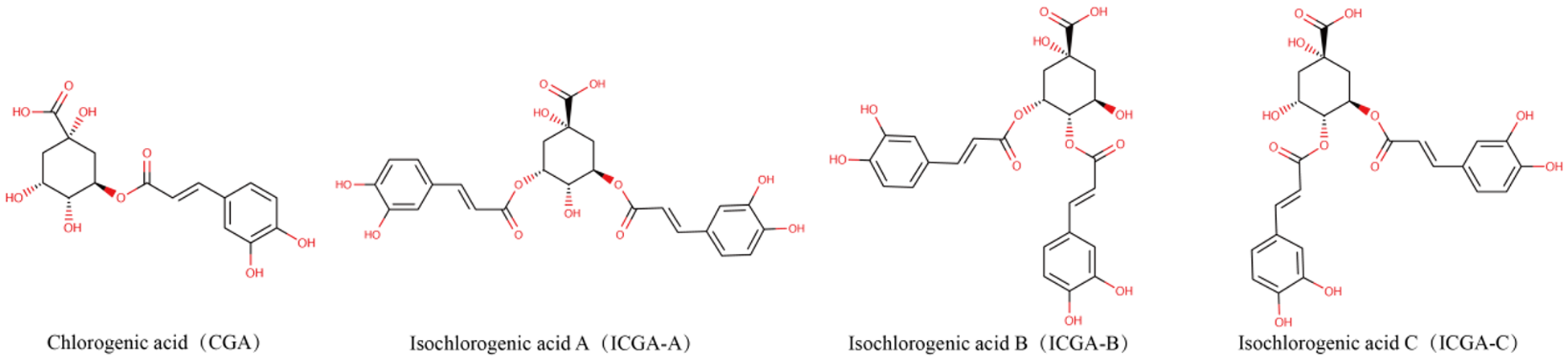
Stevia is indigenous to the subtropical regions of South America (Hossain et al., Reference Hossain, Islam and Islam2017). Following its successful introduction and cultivation in China in 1977, it has been extensively cultivated (Angelini et al., Reference Angelini, Martini and Passera2018). However, the widespread planting and extraction of stevia result in significant waste residue, and methods such as simple accumulation, incineration, or landfill disposal can lead to severe environmental pollution. Recent studies have indicated that stevia extracts contain various phenolic acids, including chlorogenic acid (CA) and isochlorogenic acid (ICA), which demonstrate biological functions including the elimination of free radicals, antibacterial characteristics, anti-inflammatory actions and tumor suppression (Pan et al., Reference Pan, Lin and Jiao2023; Zha et al., Reference Zha, Liu and Zhou2024). Previous research has demonstrated that CA is a phenolic compound derived from quinic acid and caffeic acid; its phenolic ring structure and hydroxyl groups confer diverse biological activities beneficial for animal gastrointestinal health (Chen et al., Reference Chen, Chen and Yu2022). CA enhances VFA production, promotes intestinal probiotic growth while inhibiting pathogens, and optimizes the gut microbiota composition, improving overall animal intestinal health (Liu et al., Reference Liu, Zhang and Bai2023b; Ye et al., Reference Ye, Liu and Hu2021). Additionally, CA exhibits notable anti-inflammatory and antioxidant properties and immune-enhancing capabilities (Feng et al., Reference Feng, Zhang and Fu2023; Zhao et al., Reference Zhao, Deng and Liu2019). However, the chemical properties of CA are unstable; its solubility in fat is limited, its bioavailability is poor, and esterases quickly degrade it. The ICA compound possesses one more caffeoyl moiety compared to CA, thereby conferring enhanced structural stability (as shown in Fig. 1). A previous study has shown that ICA has more excellent absorption and exhibits more significant effects in the ileum (Cheng et al., Reference Cheng, Wang and Zeng2014). Studies have shown that the antioxidant and antitumor effects of ICA increase in direct proportion to the number of caffeoyl (Mishima et al., Reference Mishima, Inoh and Narita2005; Xu et al., Reference Xu, Hu and Liu2012). ICA has a stronger ability to destroy bacterial cell membranes (Nie et al., Reference Nie, Dang and Ge2021), and its anti-inflammatory, antibacterial, and antioxidant effects are more pronounced (Wang et al., Reference Wang, Shen and Liu2020). As illustrated in Fig. 2, ICA exhibited multiple sources, diverse biological activities, and numerous remarkable pharmacological effects. Consequently, ICA was regarded as a key chemical indicator for ensuring the quality control of traditional Chinese medicine preparations that include this compound (Chang et al., Reference Chang, Liu and Bai2014). However, the literature has primarily focused on specific biological and pharmacokinetic studies of ICA (Mamat et al., Reference Mamat, Dou and Lu2017), with animal experiments focused mainly on mice models (Nie et al., Reference Nie, Dang and Ge2021), but it lacked studies on poultry. In previous research, lipopolysaccharide (LPS) was frequently employed to establish acute stress models in animals and serves as a biomarker indicative of the inflammatory response triggered by oxidative stress within the body (Jiang et al., Reference Jiang, Bai and Wang2024; Tong et al., Reference Tong, Yu and Chen2023). Therefore, in this study, we induced immune stress in broilers using LPS to investigate the alleviation mechanism of ICA on intestinal injury in the ileum of broilers challenged by LPS. These research findings can serve as references and provide a foundation for the rational application of ICA in broiler feed formulations and for extending the stevia industrial chain in the future.

Figure 1. Chemical structure formula of chlorogenic acid and isochlorogenic acid.
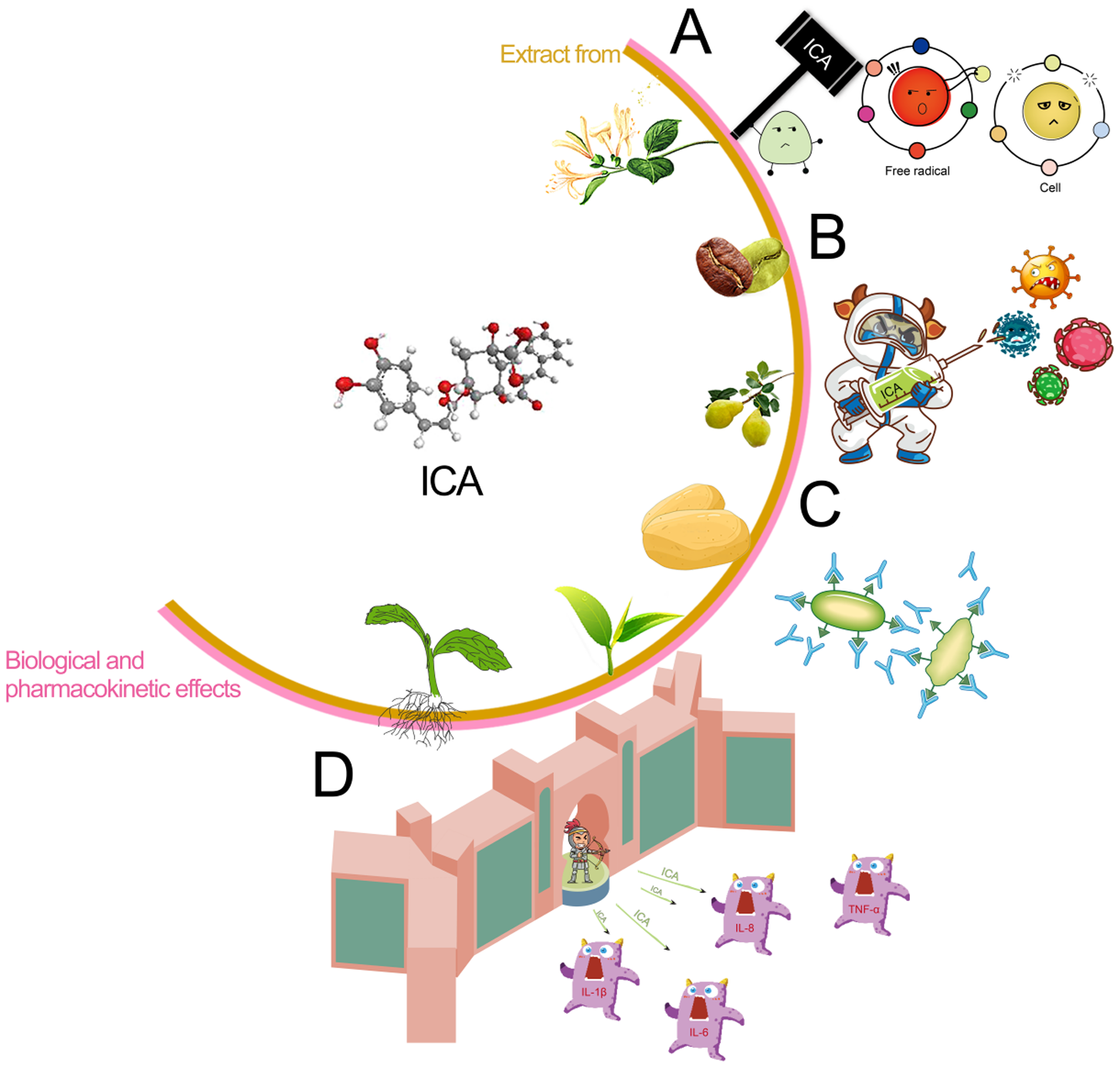
Figure 2. Biological and pharmacological effects of isochlorogenic acid. A: antioxidant effect; B: antibacterial and antiviral effect; C: immunologic effect; D: anti-inflammatory effect.
Materials and methods
Experimental design, diet and management
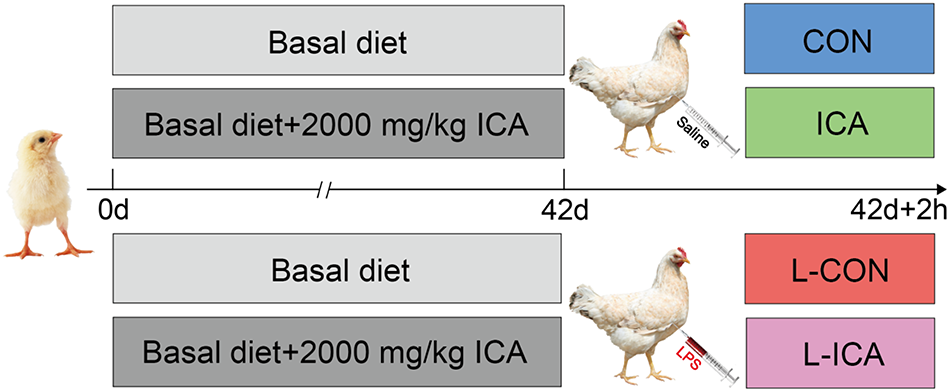
This study was designed as a 2 (with or without ICA) × 2 (with or without LPS) two-factor randomized group design. In this study, 320 1-day-old healthy Cobb broilers with similar initial body weight (44.93 ± 0.09 g) were selected and randomly divided into 4 groups with 8 replicates in each group and 10 broilers in each replicate, including half cocks and half hens. As shown in Fig. 3, the groups included the CON, L-CON, ICA, and L-ICA groups; the broilers in the CON and L-CON groups were fed a basal diet, and the ICA and L-ICA groups were fed a basal diet supplemented with 2000 mg/kg ICA. The optimal dosage of ICA was determined based on prior experiment conducted by our research group (Zhou et al., Reference Zhou, Jiang and Wang2024). The basal diet was formulated in accordance with the NRC standards (NRC, 1994), with detailed composition and nutrient levels presented in Table 1. The dietary nutritional level was determined following the AOAC (2019) method. The metabolizable energy (ME) value was calculated according to an established methodology (Xiong et al., Reference Xiong, Luo and Zheng2020). The amino acid content of the diet was computed as the sum of each ingredient’s proportion (%) multiplied by its respective amino acid content (%).

Figure 3. Experimental design.
Table 1. Composition and nutrient levels of basal diets (air-dry basis) a

a ME, metabolizable energy; CP, crude protein; Ca, calcium; P, phosphorus; GE, gross energy.
b Provided per kilogram of diet: VA, 7500 IU; VD3, 3 kIU; VE, 20 IU; VK3, 2 mg; VB1, 2 mg; VB2, 6 mg; VB6, 3 mg, VB12, 0.02 mg; calcium pantothenate, 10 mg; nicotinamide, 40 mg; Biotin, 0.12 mg; Cu, 10 mg; Fe, 20 mg; Zn, 80 mg; Mn, 90 mg; Se, 0.2 mg; I, 1 mg.
c The 5% premix contains 1.5% stone powder, 1.5% calcium hydrogen phosphate, 0.5% salt and 1.5% multivitamins and amino acids.
d ME, lysine, methionine and threonine were calculated, and other nutrient levels were measured values.
The ICA additive utilized in the present experiment was developed and provided by Chenguang Biotechnology Co., Ltd. (Handan, China). This additive is obtained from stevia rebaudiana after the extraction of flavonoids and CA, and it contains 55% ICA, 5% CA derivatives, 5% flavonoids, 10% glycosides, 10% moisture and 15% ash. At 42 d of age, after a 12 h fast, the broilers in the L-CON and L-ICA groups received an injection of 1 mL of LPS (200 µg/kg BW) into the pectoral muscle. The LPS (serotype O55:B5, L2880) was derived from Escherichia coli (E. coli) and purchased from Sigma (Merck KgaA, Darmstadt, Germany). The methodology and dosage of LPS used were detailed in earlier research (Liu et al., Reference Liu, Song and Yun2019; Wang et al., Reference Wang, Cui and Zhang2021). The broilers in the CON and ICA groups received injections of an equivalent volume of saline. After the 2 h injection period, one chicken was randomly selected from each replicate for blood collection, euthanasia and sampling.
Determination of growth performance
Feed intake and body weight of broilers in each cage were measured and documented at 42 d. Subsequently, the average daily gain (ADG), average daily feed intake (ADFI), and feed-to-weight ratio (F/G) of the broilers in each group were calculated. The calculation method refers to the method of Zhou et al. (Reference Zhou, Jiang and Wang2024).
Blood sampling and analysis
At 42 d of age, 10 mL of wing vein blood was sampled from the broilers 2 h after LPS or saline injection, then centrifuged at 850 × g for 10 min, and the resulting supernatant was collected and stored at −20 °C. According to the manufacturer’s instructions, all testing indexes were determined using respective enzyme-linked immunosorbent assay (ELISA) kits (Jiangsu Meimian Industrial Co., Ltd., Jiangsu, China). The measured indicators included the concentrations of immunoglobulin A (IgA), immunoglobulin G (IgG), immunoglobulin M (IgM), complement 3 (C3), complement 4 (C4), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), diamine oxidase (DAO) content, D-lactic acid (D-LA) and LPS.
Ileal sections sampling and analysis
After the broilers were euthanized, ileal tissues were collected from the middle segment of the ileum. The samples were fixed in 4% paraformaldehyde for 48 h, subsequently dehydrated and cleared, and then embedded in paraffin to form paraffin blocks. These blocks were sectioned into 5-μm-thick slices and mounted onto glass slides. The sections were then dewaxed, rehydrated and subjected to hematoxylin staining for nuclear visualization. Following differentiation with acid alcohol, eosin staining was applied for cytoplasmic contrast. Afterward, the sections were dehydrated and cleared once more before being mounted with a coverslip using mounting medium, thereby completing the hematoxylin and eosin staining process for histological observation. Pathological slice scanner (Pannoramic DESK; 3DHISTECH Co., Ltd., Budapest, Hungary) and observation software (Case Viewer 2.4; 3DHISTECH Co., Ltd., Budapest, Hungary) were used to observe the histological damage of the ileum. The villus height (VH), crypt depth (CD), mucosal thickness (MT), and goblet cells (GC) number of the ileum were measured; subsequently, the villus height/crypt depth (V/C) was calculated.
Ileum sampling and analysis
After the broilers were euthanized, the ileal tissues were dissected, collected, frozen in liquid nitrogen, and stored at −80 °C to determine ileal secretory IgA (sIgA) content and ileal gene expression.
The content of sIgA was measured via an ELISA kit (Jiangsu Meimian Industrial Co., Ltd., Jiangsu, China), and the measurement was performed according to the steps described in the manual.
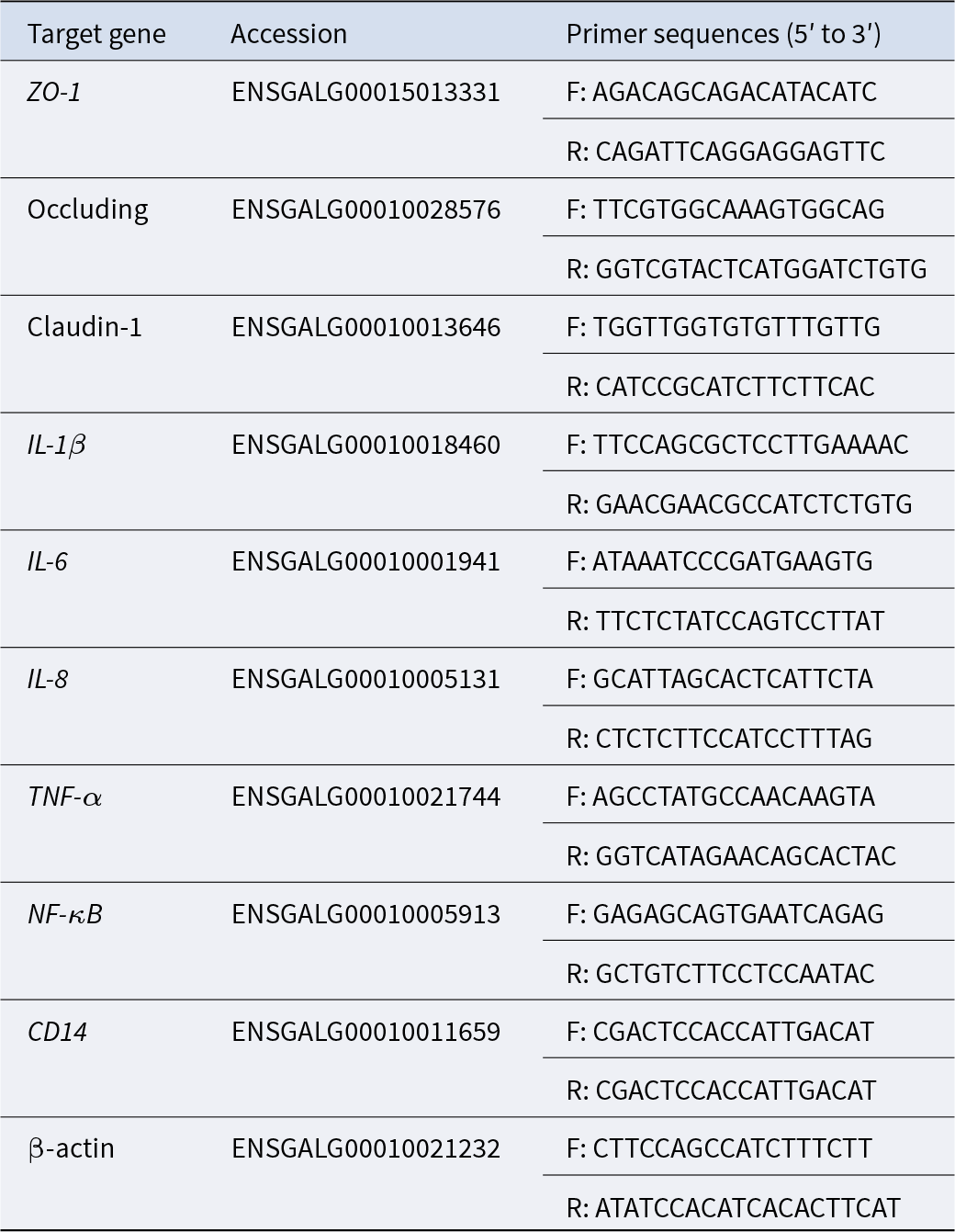
The relative mRNA expression levels of target genes in the ileal tissue samples were measured using quantitative real-time PCR (qRT-PCR). The target genes included Zonula occludens-1 (ZO-1), Occludin, Claudin-1, IL-1β, IL-6, IL-8, TNF-α, NF-κB and CD14. The primers used for qRT-PCR synthesis were designed and synthesized by Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China). The primer sequences utilized in this study are presented in Table 2. The mRNA sequences were retrieved from the National Center for Biotechnology Information.
Table 2. Primer sequences of qRT-PCR
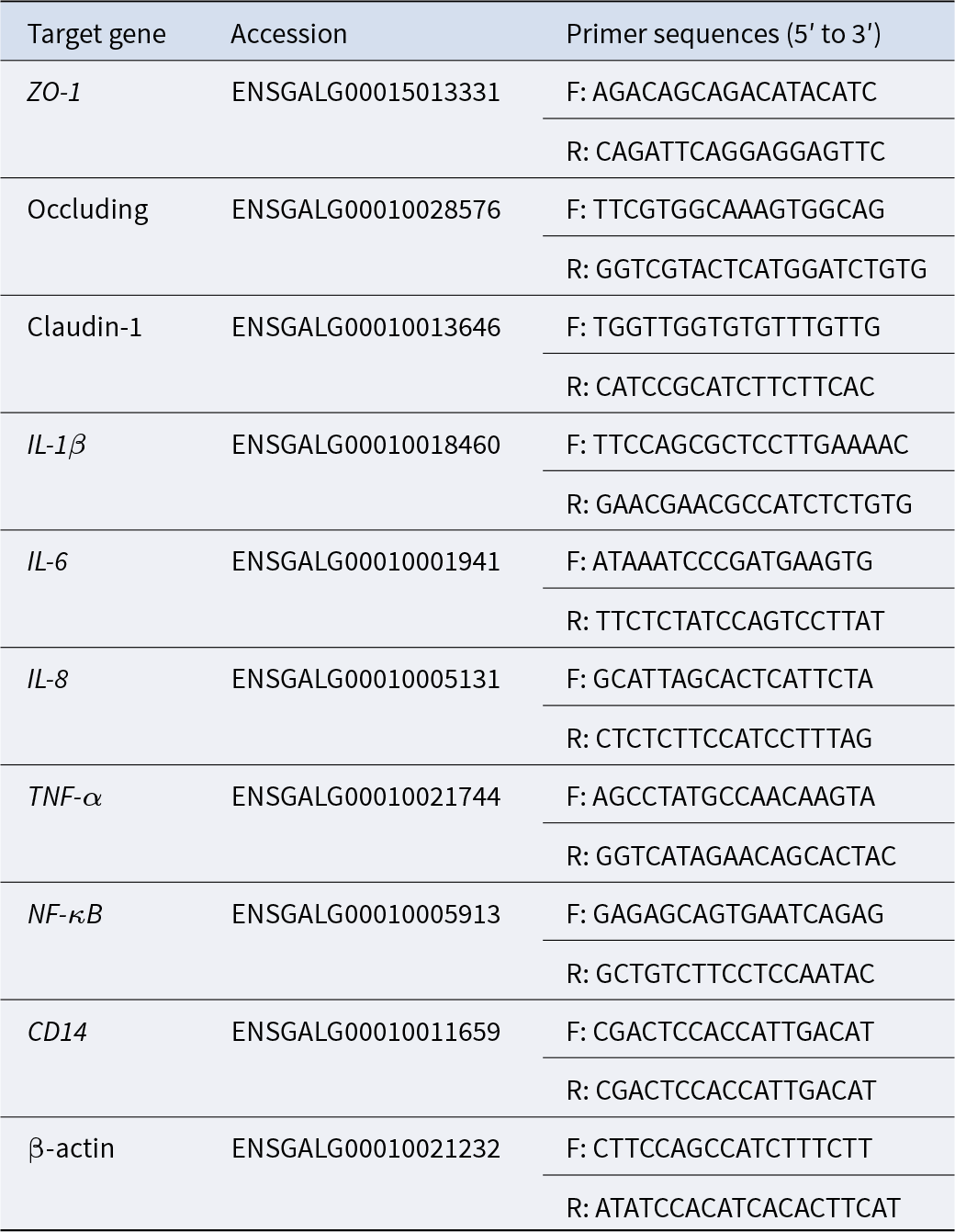
F, forward; R, reverse.
Ileal chyme sampling and analysis
After the broilers were euthanized, the pH of the ileal chyme was measured by a pH meter (Fisher Scientific, Sunnyvale, USA) according to the method of Wu et al. (Reference Wu, Yin and Wang2020). The remaining chyme in the ileum was divided into several frozen tubes, frozen in liquid nitrogen and stored at −80 °C for further analysis.
About 0.1 g of ileal chyme was transferred to a 2.5 mL centrifuge tube. Following the addition of an adequate volume of normal saline, the mixture was centrifuged at 850 × g for 10 min to collect the supernatant. The enzymatic activities of amylase, trypsin and pancreatic lipase were then measured using ELISA kits (Jiangsu Meimian Industrial Co., Ltd., Jiangsu, China) following the manufacturer’s manual.
Precise 0.5 g samples of ileal chyme were combined with 2.5 mL of prechilled ultrapure water and then centrifuged at 10,000 × g for 10 min at 4 °C. Following this, 25% metaphosphoric acid was added to the supernatant in a volume ratio of 1:5, and the mixture was filtered using a 0.45 μm filter prior to VFAs analysis. The VFAs were quantified via gas chromatography (GC-2014, Shimadzu, Japan) under the following conditions: a capillary column (30 m × 0.32 mm × 0.25 μm film thickness) was employed. The column temperature was kept constant at 110 °C, and both the injector and detector temperatures were set to 180 °C.
Total DNA was isolated from the aforementioned ileal chyme samples, and the V3-V4 region of the bacterial 16S rRNA gene was amplified using PCR. Sequencing was performed on the Illumina NovaSeq platform with the NovaSeq 6000 SP kit provided by Shanghai Person Biotechnology Co., Ltd. (Shanghai, China), employing double-end 2 × 250 bp sequencing. The resulting sequence data were processed and analyzed using QIIME2 and R packages (version 3.2.0). The relative abundance of the microbial community, ranging from phylum to species level, was determined through original sequence analysis, DADA2 analysis and Vsearch analysis within QIIME2. Principal component analysis (PCA) was based on the composition spectra at the genus level and analyzed in QIIME2 using multivariate analysis of variance, similarity analysis and permdisp. The taxonomic composition and abundance were visualized through MEGAN and GraPhlAn. Based on the incidence of ASV between samples or groups (without considering their relative abundance), a Venn diagram was created using the R package “VennDiagram” to visualize both the shared and unique ASV among samples or groups. In addition, the differential gut microbiota produced by ICA treatment and LPS-challenged was identified by linear discriminant analysis of effect size (LEfSe) with a threshold of 2.5.
The ultra-high performance liquid system (Thermo Fisher Scientific Inc., Waltham, USA) of Shanghai Person Biotechnology Co., Ltd., was used to extract and analyze the metabolites in the ileal chyme. In this experiment, quality control samples were utilized throughout the detection process to maintain the accuracy and precision of the results. The ACQUITY UPLC® HSS T3 chromatography column (2.1 × 150 mm, 1.8 µm; Waters Co., Milford, USA) was utilized for the analysis. The mobile phase flow rate was maintained at 0.25 mL/min, the column temperature was kept at 40 °C, and the injection volume was set to 2 μL. Throughout the procedure, samples were stored in an autosampler at 4 °C and separated using ultrahigh-performance liquid chromatography. Metabolite detection was carried out on a Q Exactive mass spectrometer (Thermo Fisher Scientific Inc., Waltham, USA) equipped with an electrospray ionization source. Data acquisition was performed in both MS1 and MS/MS modes (Full MS-ddMS2 mode, data-dependent MS/MS). In this setup, the top 10 ions from the acquired signals were selected for fragmentation, while redundant MS/MS information was eliminated through dynamic exclusion. Partial least squares discriminant analysis (PLS-DA) was conducted to investigate the differences in metabolite profiles among groups. PLS-DA enables constructing a predictive model that links omics data with sample categories, thereby assessing the overall distinctions between the datasets of each comparison group. Subsequently, analyses were conducted on the relative abundance of differentially abundant metabolites, clustering of differential metabolites and Kyoto Encyclopedia of Genes and Genomes (KEGG) metabolic pathways using criteria of variable importance in projection (VIP) > 1 and P < 0.1. The Z-score was utilized to assess the relative levels of metabolites within the same category. The differentially abundant metabolite data were uploaded to the Genes cloud platform (https://www.genescloud.cn/) to perform cluster analysis and generate heat maps. The KEGG Mapper visualization tool was utilized to illustrate the enriched pathways and corresponding pathway maps for the differential metabolites. Statistical tests were conducted to determine the significance of the data, where parameters such as P, VIP and Fold Change were calculated. These calculations helped quantify the fold difference of the components, assess the impact and explanatory power of each metabolic component’s concentration on sample differentiation, and support the selection of key metabolites.
Statistical analysis
SPSS software (version 26.0) was used for statistical analysis. An independent sample T-test was used to analyze growth performance data between groups. In addition to the microbial and metabolomic data, the remaining data were subjected to a two-way analysis of variance via a general linear model program. The primary effects of the model included LPS, ICA and their interaction. When the interaction effect was significant, Duncan’s method was used for multiple comparisons. Significant differences in the microbial flora were analyzed via non-parametric tests using GraphPad software. The significance of metabolite data was determined by calculating P, VIP and Fold Change to assess component differences. Spearman correlation analysis was conducted, and the data were imported into the Gene Cloud Platform (https://www.genescloud.cn/) for correlation analysis. Data were expressed as means and standard errors. P < 0.05 was considered significant, P > 0.05 was considered insignificant, and 0.05 < P < 0.10 indicated a changing trend. Metabolite molecules were considered statistically significant when P < 0.05 and VIP > 1.
Results
Effects of ICA on broiler growth performance
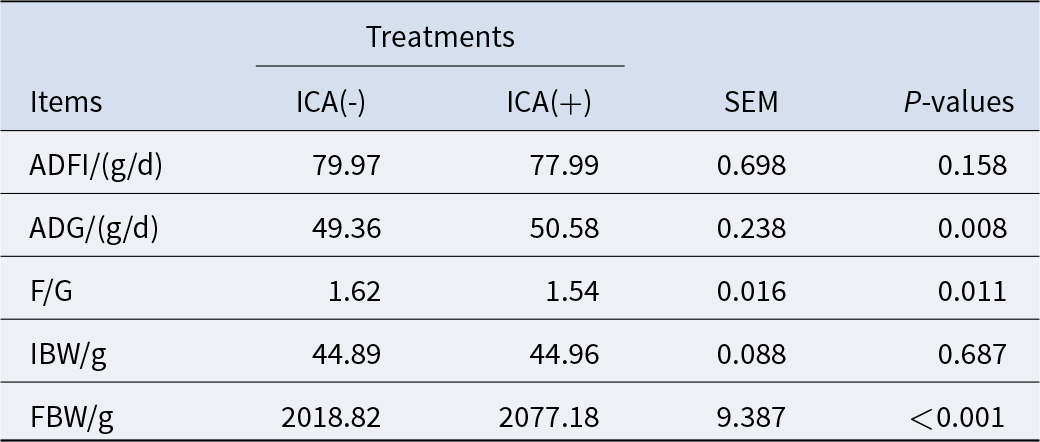
As presented in Table 3, the impact of ICA on ADFI was relatively minor, while ADG and final body weight (FBW) from 1 to 42 d exhibited significant increases (P < 0.05), and F/G demonstrated a significant decrease (P < 0.05). Additionally, effects of ICA on the ileum microbial barrier in LPS-challenged broilers were observed.
Table 3. Effects of dietary isochlorogenic acid supplementation on growth performance of broilers

ICA (+): broilers fed a basal diet +2000 mg/kg ICA; ICA (−: broilers fed a basal diet. ADFI, average daily feed intake; ADG, average daily gain; F/G, feed-to-gain ratio; IBW, initial body weight; FBW, final body weight.
Microbial species composition analysis
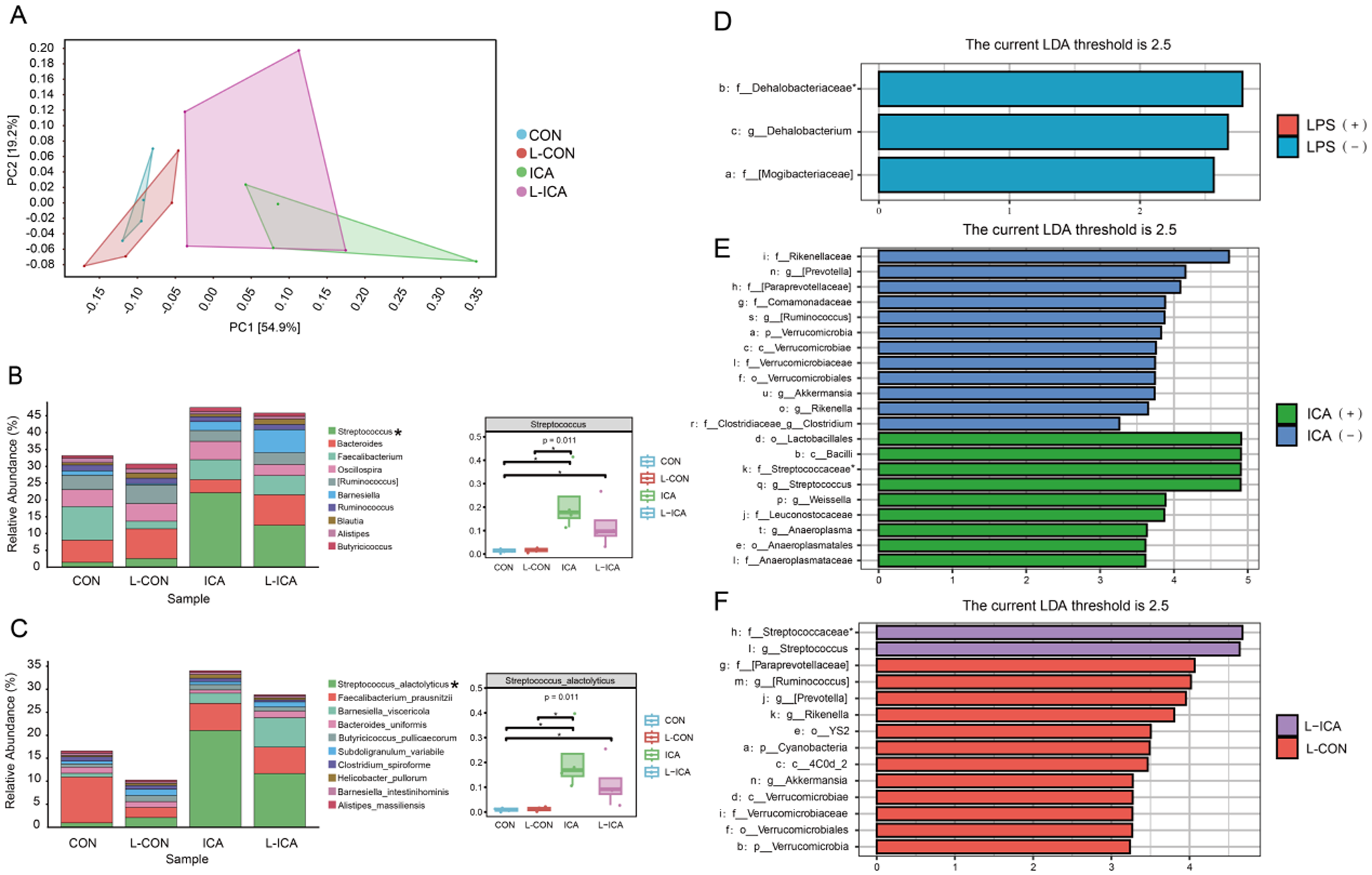
As presented in Fig. 4A, PCA1 could explain 54.9% of the total ileal chyme flora community, PCA2 could explain 19.2% of the total ileal chyme flora community, and both could explain 74.1% of the total flora. Figure 4A also indicated that adding ICA to the diet significantly affected the ileal chyme microbial communities of LPS-challenged broilers. Figure S2A illustrated the similarity and overlap between the groups: 4058 OTUs were unique to the CON group, 3174 OTUs were unique to the L-CON group, 3305 OTUs were unique to the ICA group, 3250 OTUs were unique to the L-ICA group, and 961 OTUs were shared among all four groups.
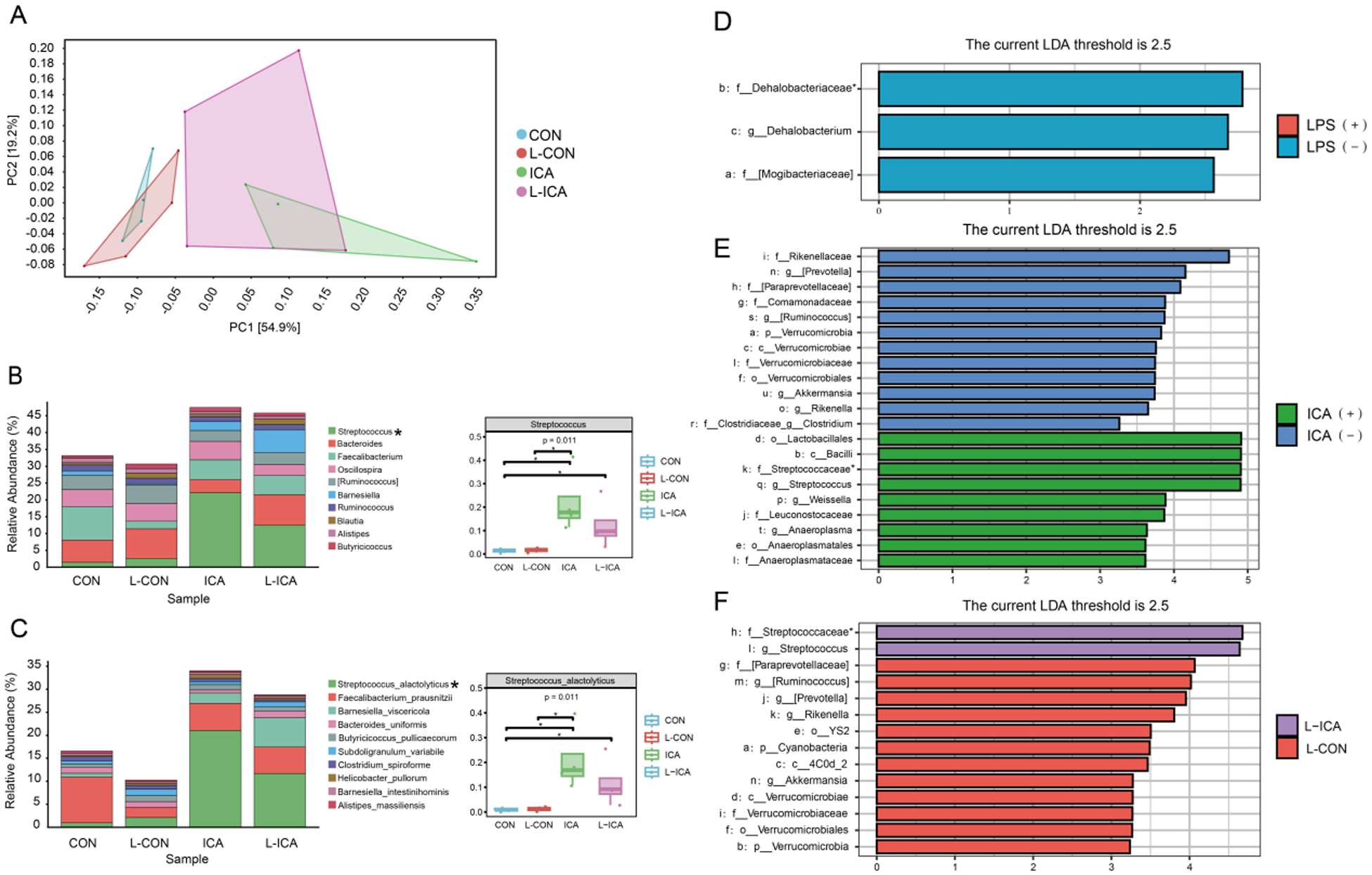
Figure 4. Effects of dietary isochlorogenic acid supplementation on the LPS-challenged ileal microbial barrier in broilers (n = 4).
Figure 4B and 4C showed the species abundances of the four groups of ileal chyme flora at the genus and species levels. Based on the sample species abundance table, the top 10 species with the highest abundance were chosen for the calculation of relative abundance. The results revealed that the top 10 bacteria in the ileal chyme of broilers were Streptococcus, Bacteroides, Faecalibacterium, Oscillospira, [Ruminococcus], Barnesiella, Ruminococcus, Blautia, Alistipes and Butyricicoccus. The relative abundance of Streptococcus was notably elevated in the ICA group compared to the CON and L-CON groups. Additionally, the L-ICA group exhibited a significantly greater relative abundance of Streptococcus than the CON group (P < 0.05). The top 10 bacteria in the ileal chyme of broilers were Streptococcus_alactolyticus, Faecalibacterium_prausnitzii, Barnesiella_viscericola, Bacteroides_uniformis, Butyricicoccus_pullicaecorum, Subdoligranulum_variabile, Clostridium_spiroforme, Helicobacter_pullorum, Barnesiella_intestinihominis and Alistipes_massiliensis. The relative abundance of Streptococcus_alactolyticus was notably elevated in the ICA group compared to the CON and L-CON groups. Additionally, the L-ICA group exhibited a significantly greater relative abundance of Streptococcus_alactolyticus than the CON group (P < 0.05).
Microbial LEfSe analysis
A comparative analysis was performed between treatment groups to gain a deeper understanding of the efficacy of ICA in mitigating LPS-challenged effects on the intestinal microbiota of broilers. Subsequently, linear discriminant analysis effect size (LEfSe) was applied to evaluate the differences further, using an LDA score threshold of 2.5. As shown in Fig. 4D–3F and Fig. S2B–S2D, the LPS challenge significantly reduced the relative abundance of f_Dehalobacteriaceae in the ileum of broilers (P < 0.05). Dietary supplementation with ICA significantly increased the relative abundance of f_Streptococcaceae in the ileum of broilers (P < 0.05). Specifically, compared to the L-CON group, the L-ICA group exhibited a significant increase in the relative abundance of f_Streptococcaceae (P < 0.05).
Metabolomics
PLS-DA analysis
To elucidate the differences in metabolites among groups, PLS-DA analysis was performed to examine how dietary ICA mitigates the effects of the LPS challenge on the ileal metabolites of broilers.
Z-score analysis
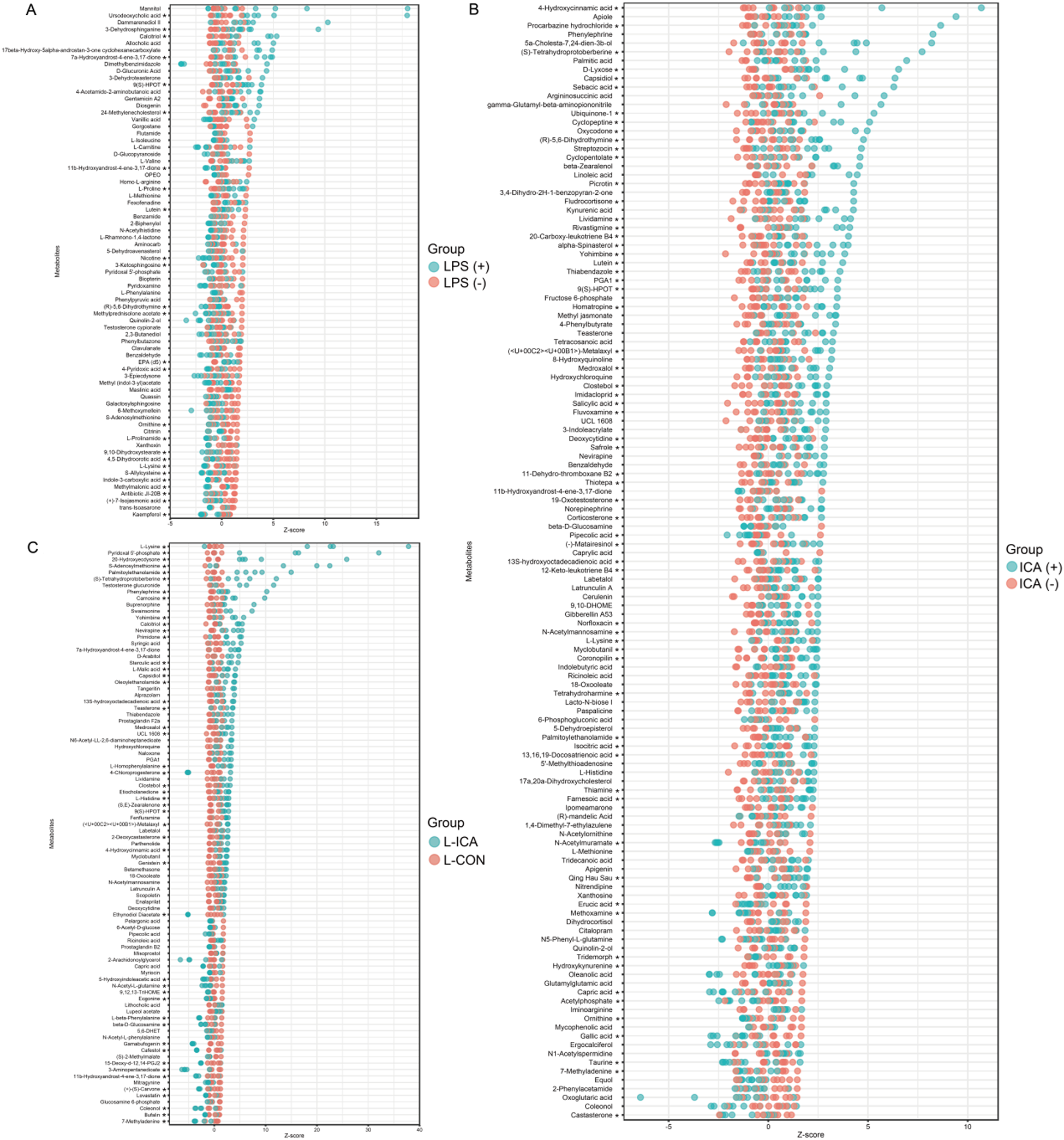
Differentially accumulated metabolites (DAMs) were screened using VIP > 1 and P < 0.1. Z-score calculations were performed to compare the content of DAMs on the same horizontal plane between groups. As shown in Fig. 5A, the LPS challenge significantly reduced the contents of DAMs such as ornithine, L-lysine and kaempferol (P < 0.05) and significantly increased the content of DAMs such as Ursodeoxycholic acid, 3-dehydrosphinganine and gentamicin A2 (P < 0.05). As shown in Fig. 5B, dietary ICA supplementation significantly decreased the contents of DAMs such as erucic acid, gallic acid and tridemorph (P < 0.05), and significantly increased the contents of DAMs such as 4-hydroxycinnamic acid, L-lysine and palmitoylethanolamide (P < 0.05). As shown in Fig. 5C, compared with the L-CON group, the L-ICA group could alleviate the stress response caused by the LPS challenge, increasing the contents of DAMs such as L-lysine, pyridoxal 5’-phosphate and S-adenosylmethionine (P < 0.05), while decreasing the contents of DAMs such as ecgonine and coleonol (P < 0.05).
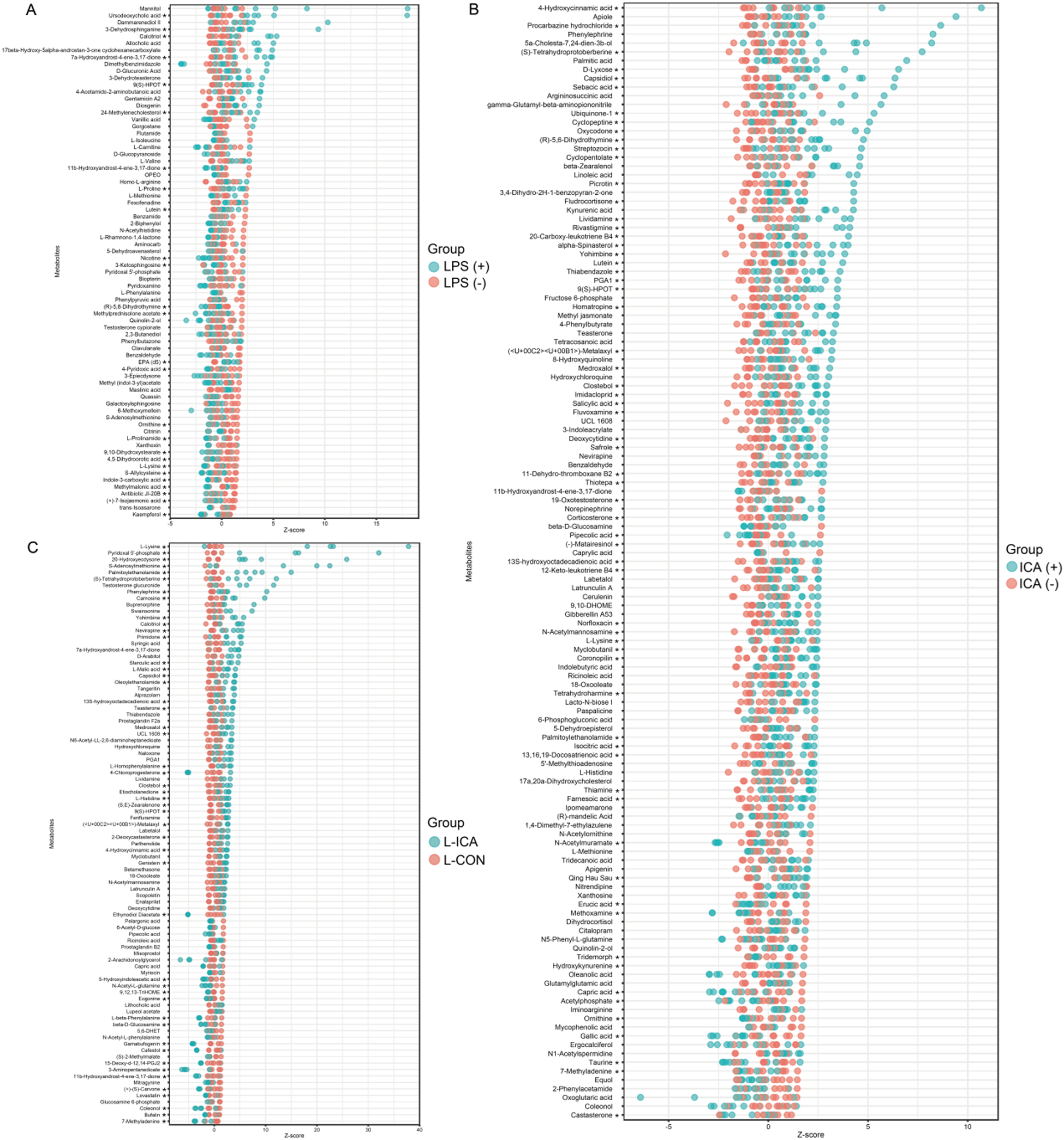
Figure 5. Effects of dietary isochlorogenic acid supplementation on LPS-challenged differentially abundant metabolites in the ileum of broilers (n = 5).
Analysis of differential metabolic pathways
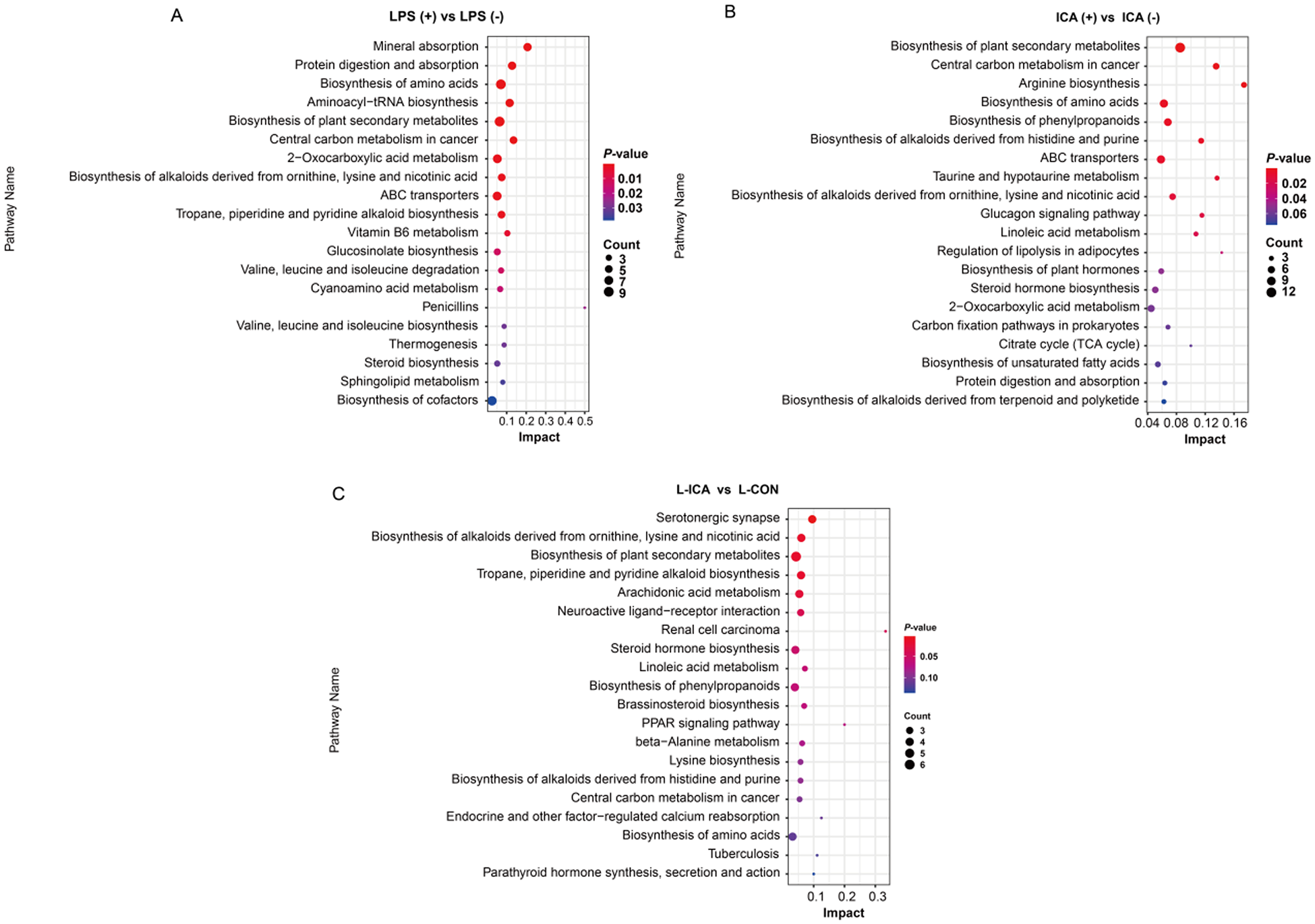
As presented in Fig. 6, KEGG enrichment analysis was performed on the differentially abundant metabolites between the comparison groups. The biosynthesis of alkaloids derived from ornithine, lysine and nicotinic acid, as well as the biosynthesis of amino acids, protein digestion and absorption, ABC transporters and other differential metabolic pathways, were enriched in the ileum (P < 0.05). The biosynthesis of alkaloids derived from ornithine, lysine and nicotinic acid showed significant enrichment across all three comparison groups (P < 0.05).
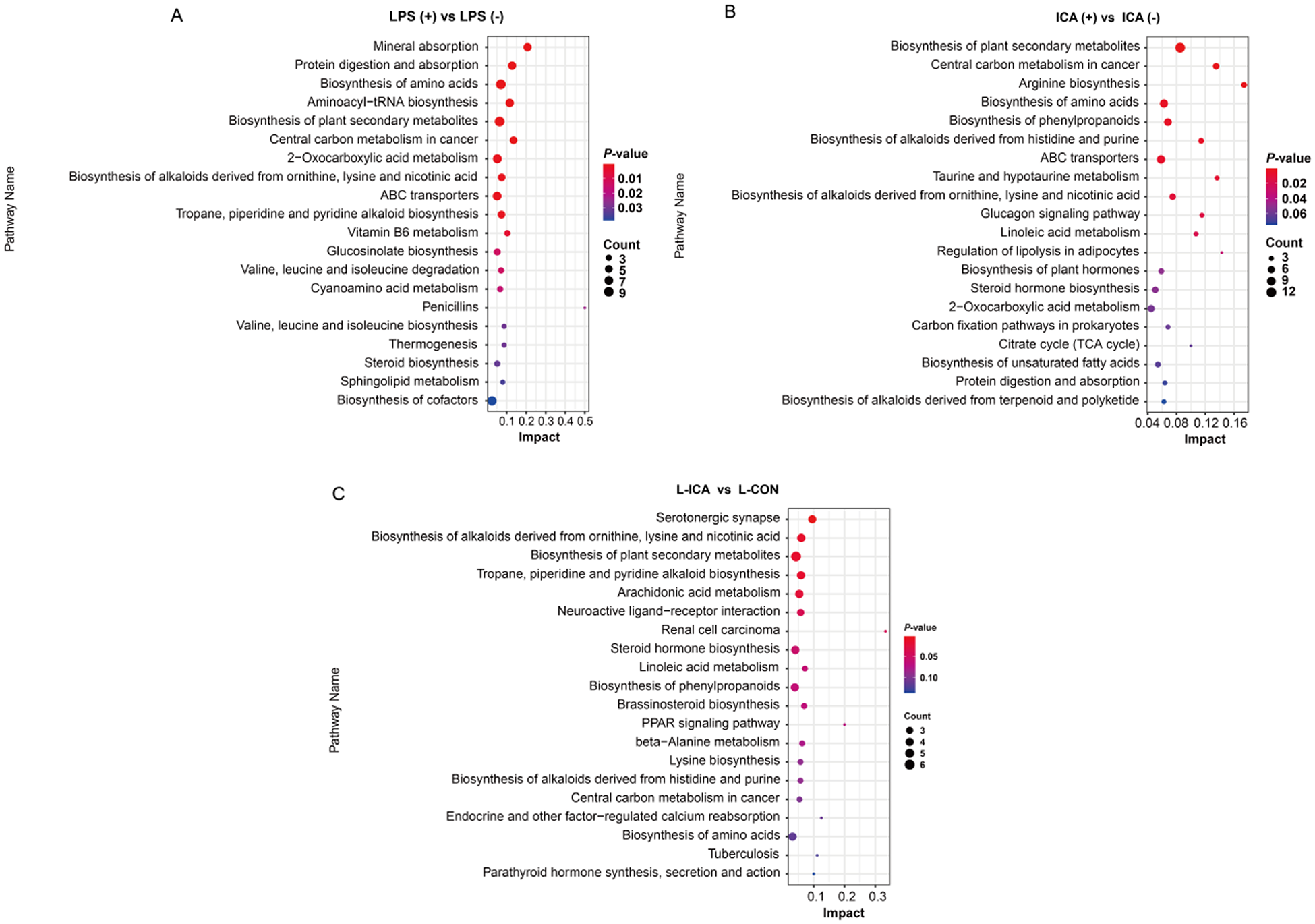
Figure 6. Effects of dietary isochlorogenic acid supplementation on the LPS-challenged metabolic pathways in the ileum of broilers (n = 5).
Effects of ICA on the ileum physical barrier in LPS-challenged broilers
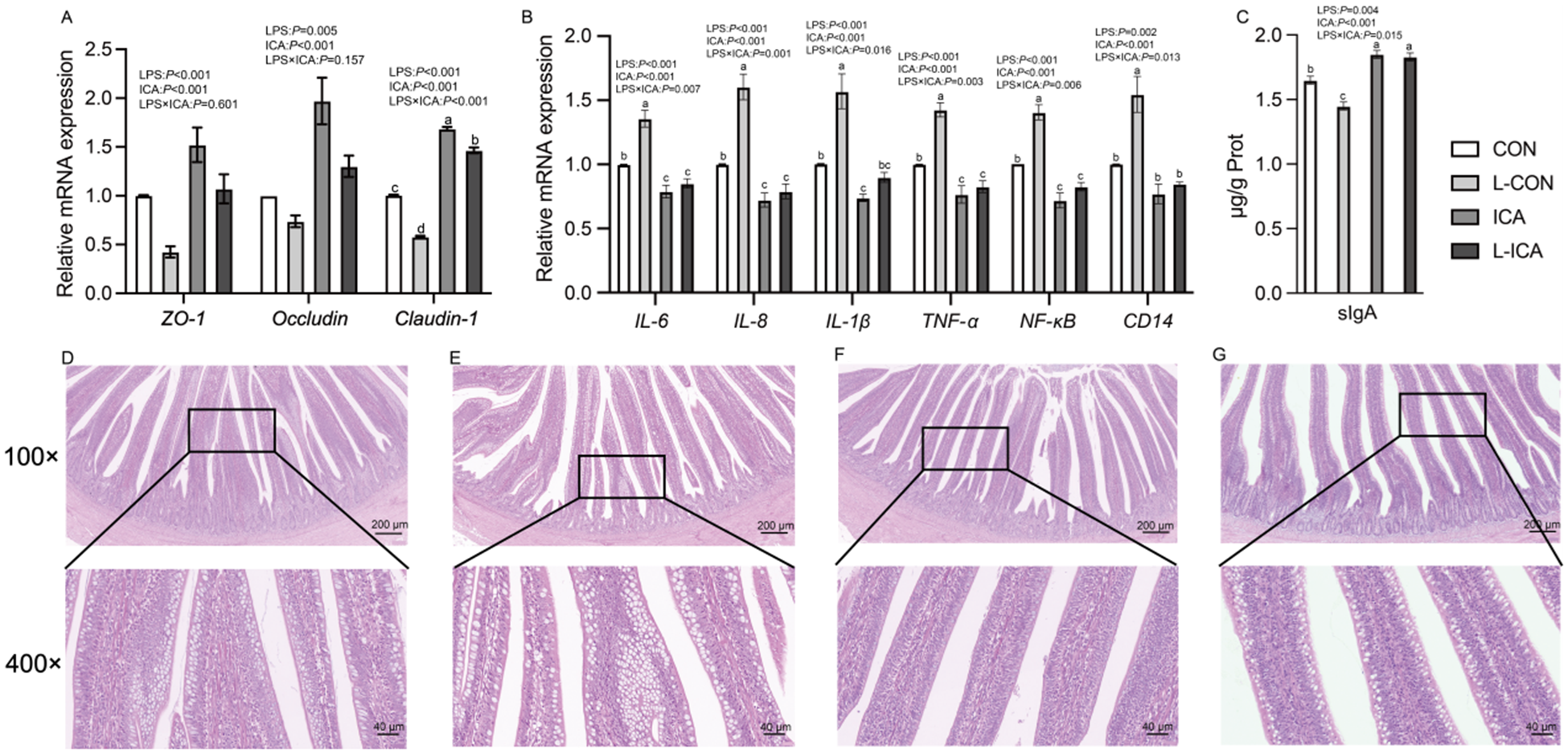
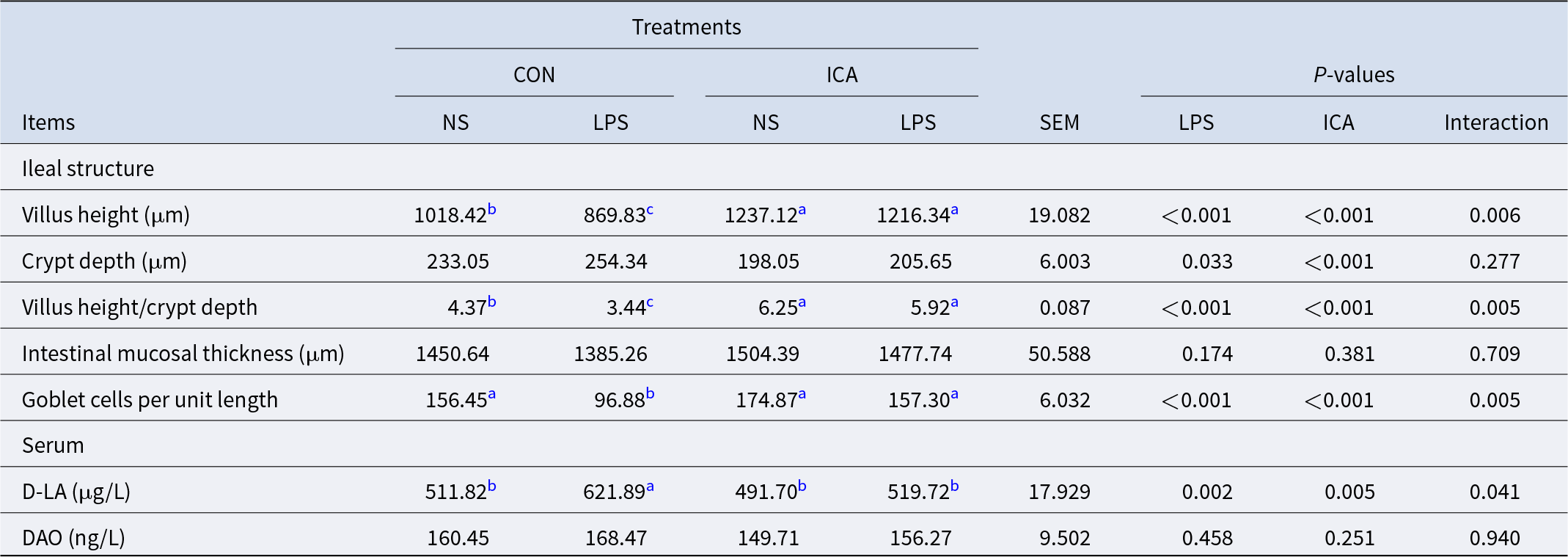
The effects of ICA on the physical barrier in the ileum of LPS-challenged broilers were summarized in Table 4 and Fig. 7A. A significant interaction was observed between ICA supplementation and LPS challenge on the VH, V/C, GC number, expression of Claudin-1 and serum D-LA concentration (P < 0.05). Specifically, the LPS challenge led to a substantial decrease in the GC number, VH, CD and V/C ratio, while also decreasing the expression of ZO-1, Occludin and Claudin-1 (P < 0.05). Additionally, the LPS challenge markedly elevated the serum D-LA concentration (P < 0.05). Supplementation with ICA in the diet markedly enhanced the GC number, VH and V/C, as well as the expression levels of ZO-1, Occludin, and Claudin-1 (P < 0.05). Conversely, it led to a significant reduction in the serum D-LA concentration (P < 0.05). Multiple comparisons revealed that the ileal VH and V/C in the ICA group were significantly increased compared with the CON group under non-stress conditions (P < 0.05). Under stress, the VH, V/C and GC number in the ileum of broilers in the L-ICA group showed a significant rise compared to those in the L-CON group (P < 0.05). Additionally, the serum D-LA level was notably reduced (P < 0.05). As shown in Fig. 7C–7F, under normal conditions, the villi of the ileum were tightly connected. However, the LPS challenge could damage the morphology of the ileum; some villi were broken and shed, and the cells were severely vacuolated. Under the influence of dietary ICA, the damage caused by LPS to the ileum of broilers was significantly alleviated.

Figure 7. Effects of dietary supplementation with isochlorogenic acid on the sIgA content, structure and mRNA expression of tight junction and inflammatory factors in the ileum of LPS-challenged broilers (n = 4).
Table 4. Effects of dietary isochlorogenic acid supplementation on the LPS-challenged ileal physical barrier in broilers

DAO, diamine oxidase; D-LA, D-lactic acid.
a,b,c Within a row, values with different superscripts indicate a significant difference (P < 0.05) (n = 4).
Effects of ICA on the ileum chemical barrier in LPS-challenged broilers
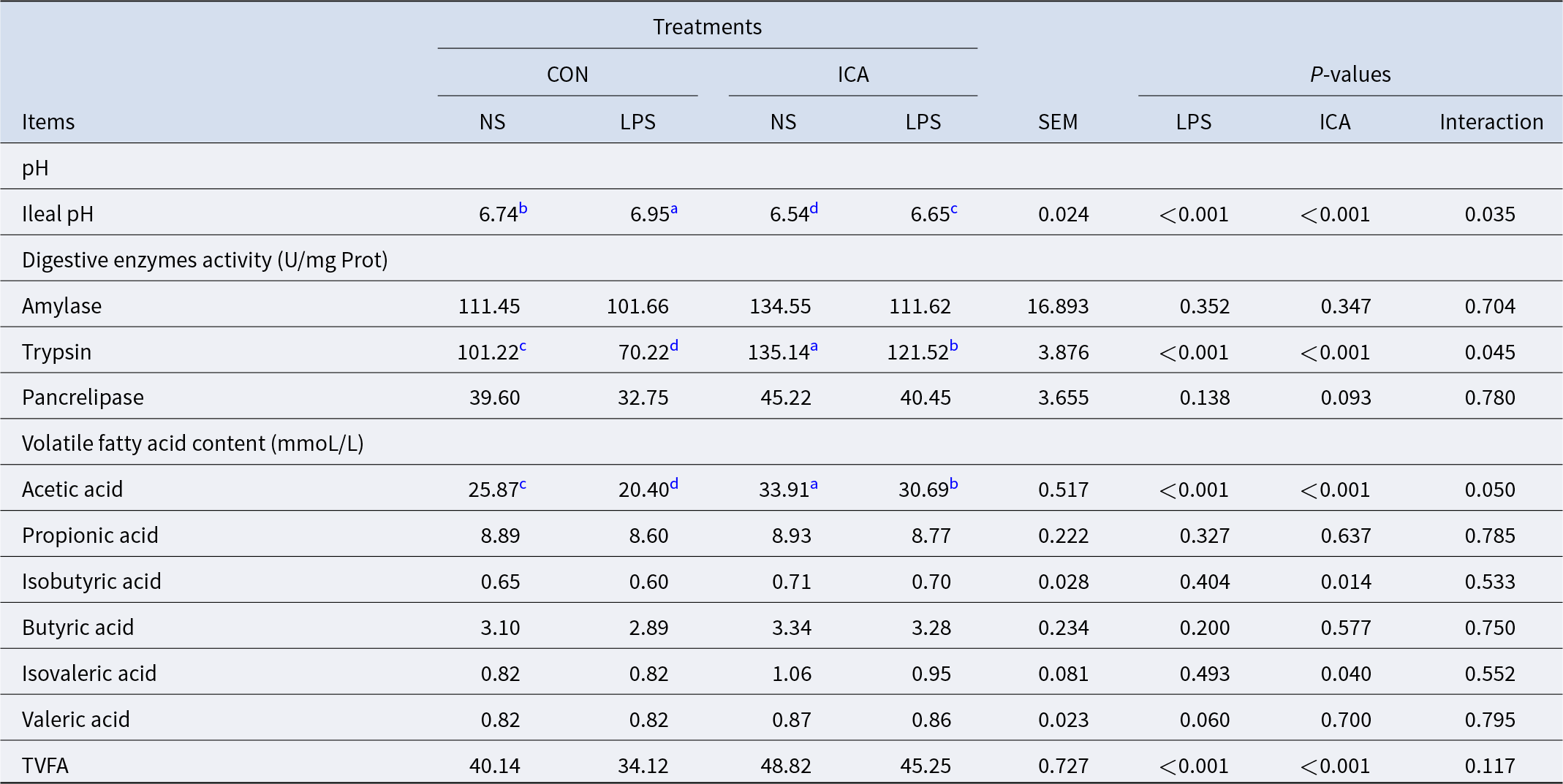
The effects of ICA on the chemical barrier of the ileum in LPS-challenged broilers were shown in Table 5. The results indicated a notable interaction effect between ICA supplementation and LPS challenge regarding pH, trypsin activity and acetic acid level of the ileum in broilers (P < 0.05). The LPS challenge led to a substantial rise in ileal pH while reducing trypsin activity, acetic acid content, and the total volatile fatty acids (TVFAs) content (P < 0.05). Adding ICA to the diet resulted in a notable reduction in the pH of the ileum, a considerable enhancement in trypsin activity within the ileum, and an increase in the contents of acetic acid, isobutyric acid, isovaleric acid and TVFAs (P < 0.05). The multiple comparison analysis indicated that, in contrast to the CON group, the ICA group exhibited a significantly lower ileal pH (P < 0.05), along with a significant increase in trypsin activity and acetic acid content (P < 0.05). Under stress, the ileal pH of the L-ICA group was significantly decreased compared with that of the L-CON group (P < 0.05). In contrast, the L-ICA group significantly increased the trypsin activity and acetic acid content (P < 0.05).
Table 5. Effects of dietary isochlorogenic acid supplementation on LPS-challenged ileal chemical barrier in broilers
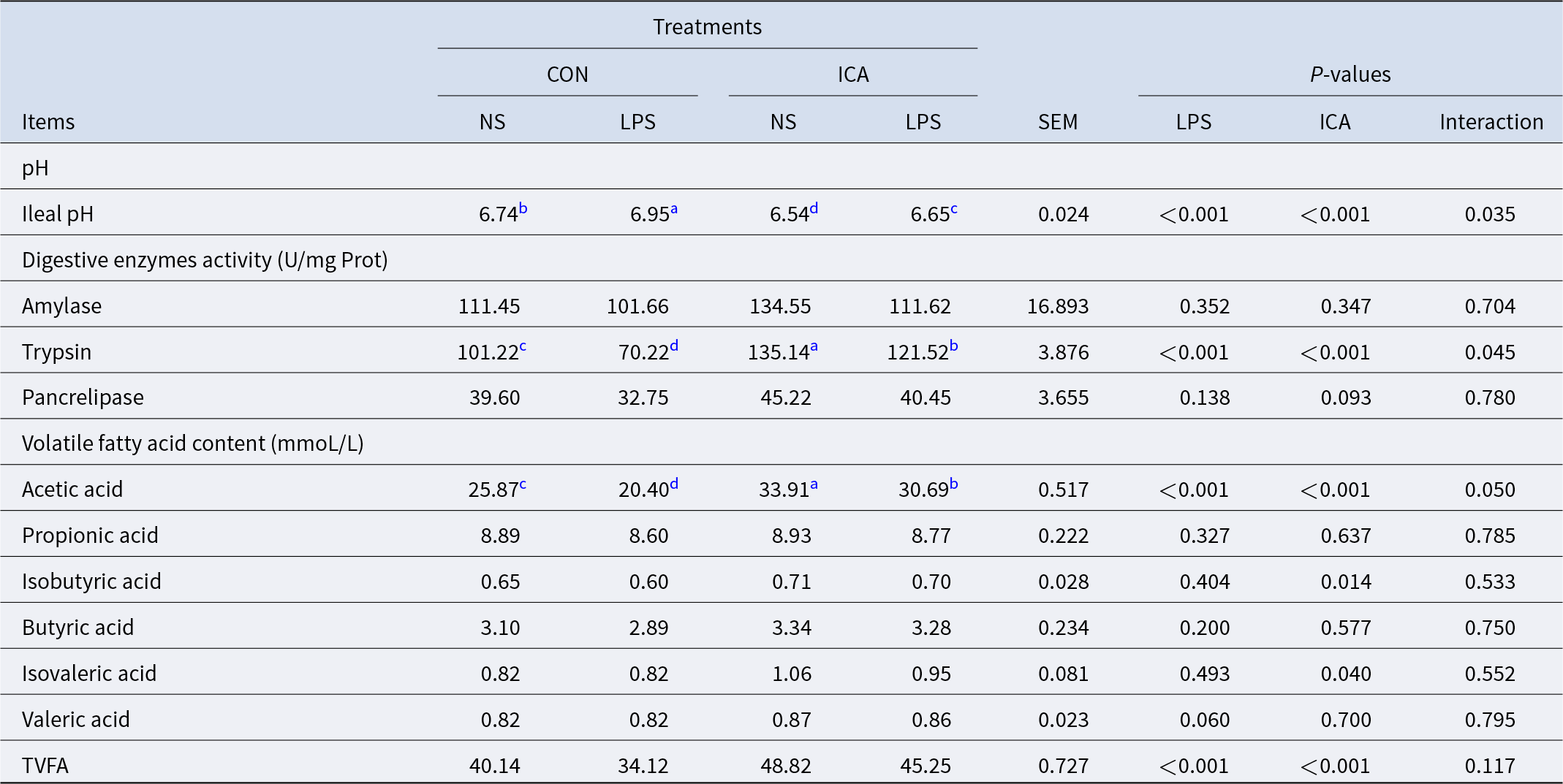
TVFA, total volatile fatty acid.
a,b,c,d Within a row, values with different superscripts indicate a significant difference (P < 0.05) (n = 4).
Effects of ICA on the serum immunity in LPS-challenged broilers
The effects of dietary ICA supplementation on the serum immunity in LPS-challenged broilers were shown in Table 6. There was a notable interaction between ICA supplementation and LPS challenge regarding serum IgG, IgM, IL-1β, IL-6, IL-8, TNF-α and LPS levels in broilers (P < 0.05). LPS challenge led to a substantial rise in the serum levels of IgA, IgM, IL-1β, IL-6, IL-8, TNF-α and LPS (P < 0.05), while significantly decreasing IgG levels (P < 0.05). Dietary supplementation with ICA led to a significant reduction in the levels of serum IgA, IgG, IL-1β, IL-6, IL-8, TNF-α and LPS (P < 0.05) and significantly increased the levels of IgG (P < 0.05). The multiple comparisons revealed that, under non-stress conditions, the levels of IL-1β and TNF-α in the ICA group were markedly lower than those in the CON group (P < 0.05). Under stress conditions, in comparison to the L-CON group, there was a significant increase in IgG levels in the L-ICGA group (P < 0.05). Conversely, the levels of IgM, IL-1β, IL-6, IL-8, TNF-α and LPS showed a marked decrease (P < 0.05).
Table 6. Effects of dietary isochlorogenic acid on serum immune index of broilers induced by LPS
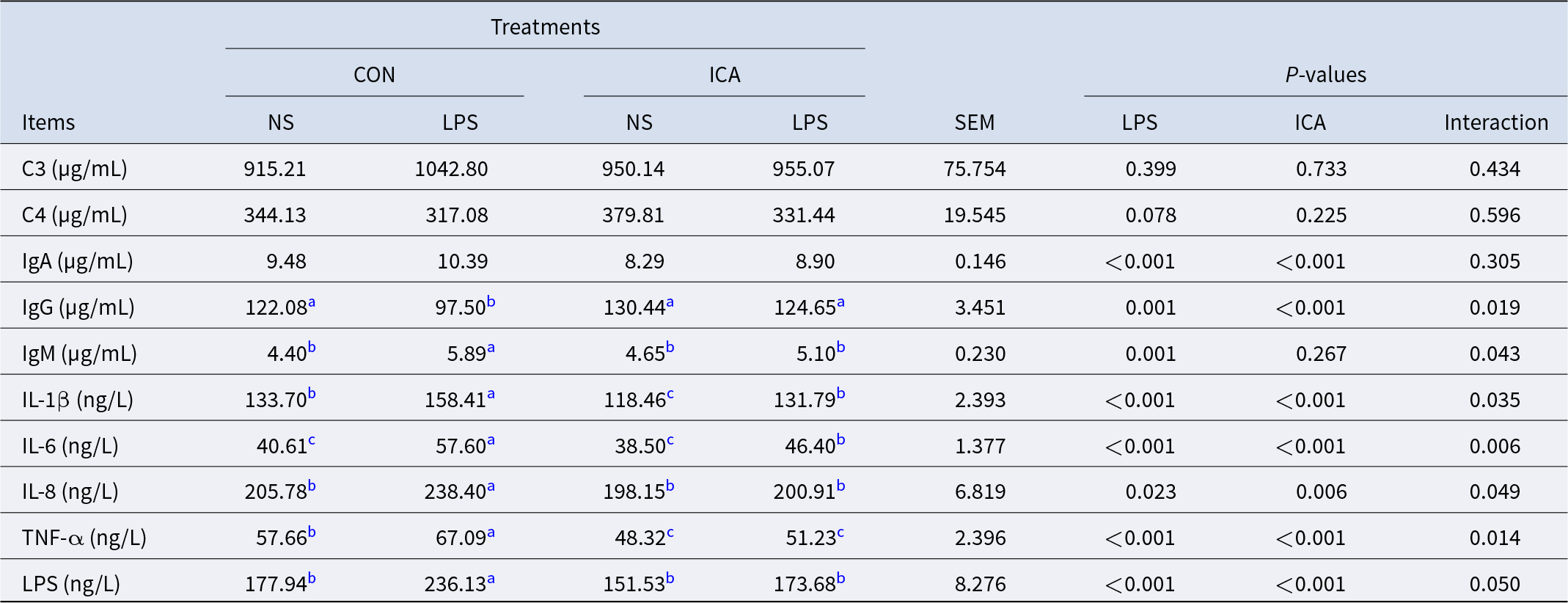
C3, complement 3; C4, complement 4; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; IL-1β, interleukin 1β; IL-6; interleukin 6. IL-8, interleukin 8; TNF-α, tumor necrosis factor α; LPS, lipopolysaccharide.
a,b,c Within a row, values with different superscripts indicate a significant difference (P < 0.05) (n = 4).
Effect of ICA on the ileum immune barrier in LPS-challenged broilers
The effects of ICA supplementation on the immune barrier of the ileum in LPS-challenged broilers were shown in Fig. 7B–C. The interaction between dietary ICA and LPS challenge significantly influenced the secretion of sIgA and the expression of IL-1β, IL-6, IL-8, TNF-α, NF-κB and CD14 in the ileum of broilers (P < 0.05). LPS challenge significantly reduced the secretion of sIgA in the ileum (P < 0.05) and significantly increased the expression of IL-1β, IL-6, IL-8, TNF-α, NF-κB and CD14 (P < 0.05). Dietary supplementation with ICA significantly increased the secretion of sIgA in the ileum (P < 0.05) and significantly decreased the expression levels of IL-1β, IL-6, IL-8, TNF-α, NF-κB and CD14 in the ileum (P < 0.05). Multiple comparisons indicated that, in contrast to the CON group, there was a significant increase in sIgA content in the ileum of the ICA group (P < 0.05). Additionally, the expression levels of IL-6, IL-8, IL-1β, TNF-α and NF-κB in the ileum were markedly reduced (P < 0.05). Under stress, the L-ICA group exhibited a significantly higher sIgA content in the ileum compared to the L-CON group (P < 0.05). Additionally, the levels of IL-6, IL-8, IL-1β, TNF-α, NF-κB and CD14 expression in the ileum were markedly reduced in the L-ICA group (P < 0.05).
Correlation analysis between the microbial barrier and dams
To demonstrate the correlation between DAMs, a heat map was created through association analysis of DAMs in different metabolic pathways. Subsequently, the correlations between these DAMs and the families f_Streptococcaceae and f_Dehalobacteriaceae, which exhibited significant changes in intestinal microbial indicators, were analyzed. This analysis created a network diagram to explore the correlation between microorganisms and DAMs. As depicted in Fig. 8A, the abundance of f_Dehalobacteriaceae, which showed a significant change in intestinal microorganisms after LPS stimulation, was significantly positively correlated with L-lysine in DAMs. Additionally, a significant positive correlation existed between DAMs such as 3-dehydrosphinganine, 24-methylenecholesterol and diosgenin. In Fig. 8B, the abundance of f_Streptococcaceae, which changed significantly after ICA supplementation, was positively correlated with L-lysine and norfloxacin. Additionally, a significant positive correlation existed between DAMs such as argininosuccinic acid, isocitric acid and thiamine. As shown in Fig. 8C, the f_Streptococcaceae showed a significant change in the L-ICA group when compared to the L-CON group and exhibited a strong positive correlation with L-lysine among differentially abundant microbes DAMs. Additionally, a significant positive correlation existed between DAMs such as 15-Deoxy-d-12,14-PGJ2 and Prostaglandin B2.
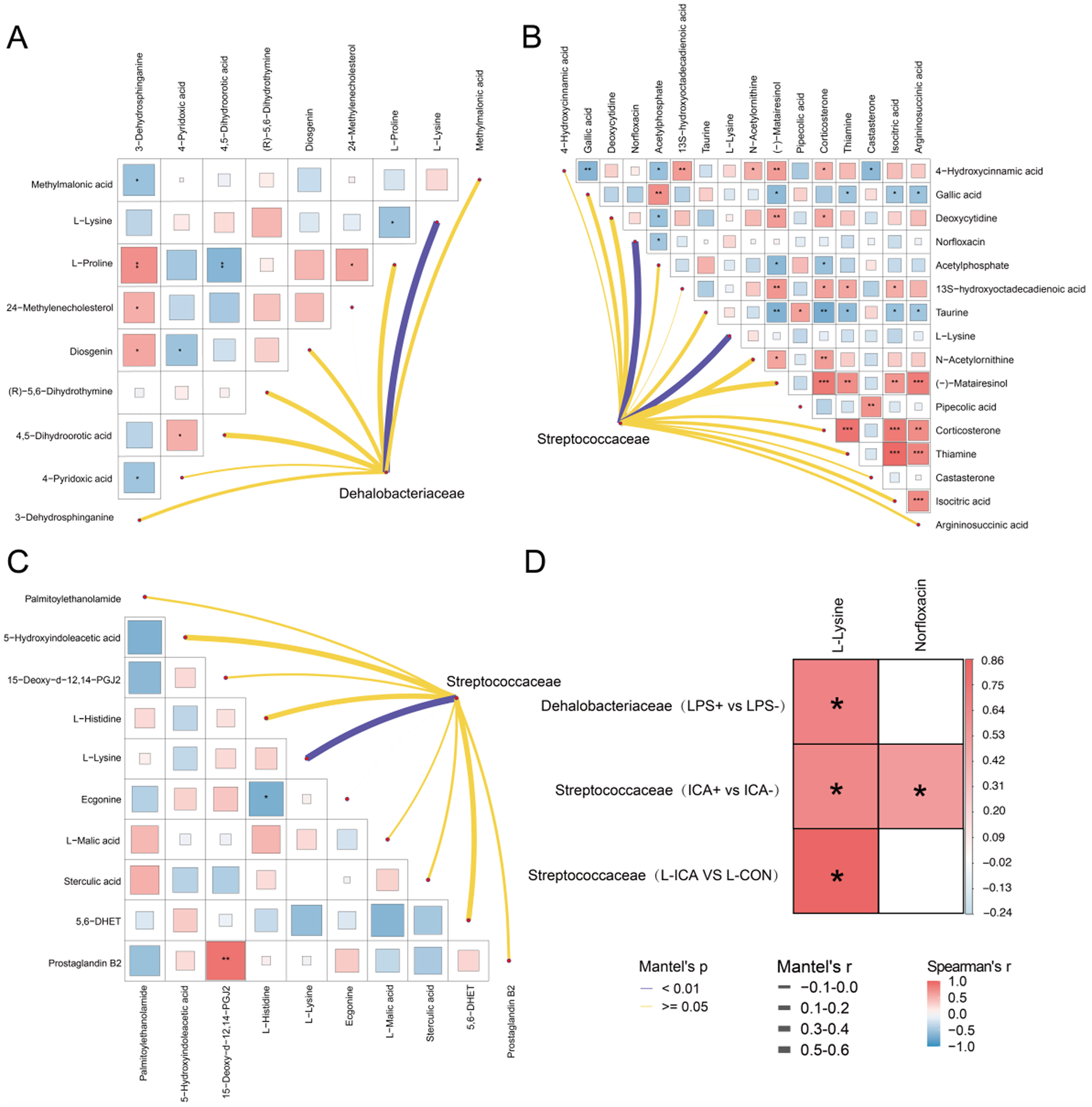
Figure 8. Correlation analysis between differential microorganisms and differentially abundant metabolites.
Correlation analysis between L-lysine and other barriers
As shown in Fig. 9, to investigate the correlation of L-lysine with other barriers, the correlation between L-Lysine and significant changes in physical, immunological and chemical barriers was analyzed. The results showed that L-lysine exhibits significant correlations with various indicators in the ileum’s physical, immunological and chemical barriers, including VH, trypsin activity, mRNA expression levels of NF-κB and others (P < 0.05).
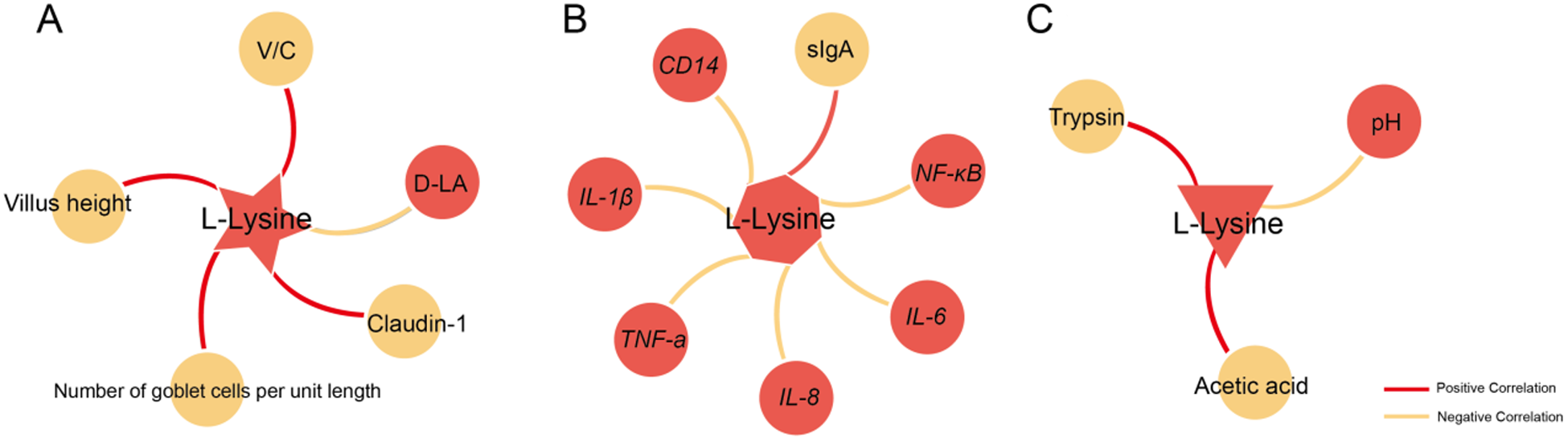
Figure 9. Correlation analysis of L-lysine with physical barrier, immune barrier and chemical barrier.
Discussions
Effects of dietary ICA supplementation on the LPS-challenged ileal microbial barrier and metabolomics in broilers
Gut microbiota is of vital importance to the health of the organism, and many human diseases are caused by microbial dysbiosis (Gong et al., Reference Gong, Zhang and Hu2024). Recent studies have highlighted the critical role of the gut microbiota in maintaining intestinal homeostasis and modulating the host’s response to LPS-challenged stress (Hu et al., Reference Hu, Wu and Li2022; Wang et al., Reference Wang, Zhen and Zhang2025). These microorganisms play a crucial role in modulating the immune response by regulating the immune barrier to counteract pathogen invasion. Moreover, they compete for limited nutrients and adhesion sites on epithelial cells to prevent the excessive growth of pathogenic bacteria (Wang and Xiao Reference Wang and Xiao2019). Additionally, intestinal microorganisms produce metabolites such as lactic acid, bile acid and SCFAs from nutrient utilization, which are pivotal in preventing and combating pathogenic diseases (Tomasello et al., Reference Tomasello, Mazzola and Leone2016). Broilers can endogenously produce LPS to modulate the immune system. However, when exposed to exogenous environmental stimuli or toxins, an overabundance of LPS can lead to the growth of harmful microorganisms and the enrichment of detrimental metabolites, such as phenols and sulfur-containing compounds, which threaten intestinal epithelial cells (Hamer et al., Reference Hamer, De and Windey2012; Lucke et al., Reference Lucke, Böhm and Zebeli2018). In this experiment, following LPS induction, the abundance of f_Dehalobacteriaceae in the ileum showed a notable reduction. As f_Dehalobacteriaceae is considered beneficial due to its ability to eliminate human carcinogens (Hamer et al., Reference Hamer, De and Windey2012), this decline suggests that the body’s microbial community has experienced specific impairments.
After adding ICA to the feed, the ileum showed a notable rise in the abundance of f_Streptococcaceae. The comprehensive analysis of the microbial species composition revealed that Streptococcus_alactolyticus was the main change observed. A previous study has demonstrated that Streptococcus_alactolyticus does not exhibit adverse effects on hemolytic activity or antibiotic sensitivity. It also demonstrated exemplary performance regarding acid and bile salt tolerance, adhesion to Caco-2 cells, and inhibition of E. coli (Zhang et al., Reference Zhang, Zhang and Wang2021). Other experiments have indicated that lactic acid bacteria can perform site-specific hydrolysis with CA (Aguirre et al., Reference Aguirre, Schieber and Weber2018). The substantial enrichment of Streptococcus_alactolyticus observed in this study may be attributed to its ability to hydrolyze ICA within the intestine; however, further investigation is needed to elucidate the underlying mechanisms involved. The significant enrichment of Streptococcus_alactolyticus and its various probiotic properties establish it as a key player in maintaining intestinal microbial barrier function within this experimental context.
Metabolomics analysis can identify various metabolites produced by microorganisms, further elucidate the mechanism by which ICA influences the ileal microbiota of broilers after LPS stimulation, clarify how microorganisms utilize and transform feed nutrients, and investigate the mechanisms underlying microbial actions in alleviating immune stress and maintaining intestinal barrier function. The findings revealed significant alterations in L-lysine levels upon supplementation with ICA and exposure to LPS. Additionally, substantial enrichment of alkaloid biosynthesis pathways derived from ornithine, lysine and nicotinic acid metabolism was observed across all comparison groups. In broiler breeding, L-lysine plays an indispensable role as an essential amino acid (Konieczka et al., Reference Konieczka, Tykałowski and Ognik2022). Hong et al. (Reference Hong, Rhee and Pyo2017) demonstrated that Maillard reaction products formed by glucose-lysine interaction can down-regulate the content of inflammatory factors and the mRNA expression levels of key genes in the colonic tissues of rats. Ghosh et al. (Reference Ghosh, Harmouche and Bechinger2018) reported that aryl-alkyl-lysines compounds comprising naphthalene rings, L-lysine and alkyl chains interact with LPS to mitigate the induction of pro-inflammatory cytokines such as TNF-α and IL-6 while exerting anti-inflammatory effects.
We investigated the correlations between DAMs involved in enriched pathways and the significantly changed microorganisms in each comparison group, aiming to clarify the potential contributions of DAMs such as L-lysine. The results indicated a significant positive correlation between L-lysine and specific differential microorganisms. The association analysis with key indicators reflecting intestinal barrier function showed that L-lysine was also significantly associated with intestinal pH, acetic acid production, villus development and the generation of inflammatory factors. This suggests that f_Streptococcus (containing microorganisms that can hydrolyze ICA in the diet) and f_Dehalobacteriaceae (microorganisms significantly altered after LPS challenge) may exert protective or damaging effects on intestinal barrier function by altering L-lysine metabolism.
Effects of dietary ICA supplementation on the LPS-challenged ileal physical barrier in broilers
The intestine plays a vital role in the digestion and absorption of nutrients in animals and also serves as the largest immune organ in the body (Filipp et al., Reference Filipp, Brabec and Vobořil2019). The physical barrier of the intestine constitutes the foundation of its barrier function, primarily encompassing histomorphological structures such as villi, crypts and intestinal mucosa that reflect both the health status and functionality of the intestine alongside tight junctions that regulate intestinal permeability. Extensive research has shown that stress in livestock and poultry can lead to the degradation of the intestinal architecture, which is characterized primarily by villous rupture, exfoliation, atrophy, cellular vacuolization, crypt hyperplasia and thinning of the intestinal mucosa (Abdelqader et al., Reference Abdelqader, Abuajamieh and Abedal-Majed2023; Li et al., Reference Li, Zhang and Bai2023; Zhang et al., Reference Zhang, Zhong and Liu2022a). Concurrently, this stress may disrupt the tight junctions between epithelial cells, resulting in a reduction in epithelial cell numbers and an increase in intestinal permeability. Consequently, pathogens, toxins and other antigens may infiltrate the internal environment, adversely affecting human health (Tang et al., Reference Tang, Shu and Du2023). In this research, the LPS challenge resulted in substantial morphological damage to the ileal villi in broilers, particularly evident by a marked reduction in VH, V/C and GC numbers. The intestinal section scan revealed severe vacuolation of villus cells in the ileum. Additionally, there was a significant inhibition in the expression levels of the tight junction proteins ZO-1, Occludin and Claudin-1. Moreover, serum analysis indicated a significant increase in D-LA content. These findings indicate that the intestinal physical barrier of broilers was significantly compromised following LPS induction.
Previous studies have demonstrated that incorporating CA into the diet can effectively mitigate the damage to villi induced by LPS stimulation in the broilers’ LPS challenge model, thereby enhancing intestinal digestive and absorptive functions while upregulating the expression of the tight junction protein to increase resistance to LPS-challenged intestinal damage (Liu et al., Reference Liu, Meng and Du2024; Tan et al., Reference Tan, Zhen and Bai2023). In this research, ICA had one more caffeoyl group than CA, which increased the expression of the tight junction protein Claudin-1 in ileal epithelial cells, prevented the LPS-challenged increase in intestinal permeability, and thus slowed the increase in serum D-LA content after stress. Furthermore, research has indicated that the Claudin-1 tight junction protein overexpression inhibits stem cell differentiation into GC (Pope et al., Reference Pope, Bhat and Sharma2014). Interestingly, our research revealed that dietary supplementation with ICA increased the number of GC, suggesting that Claudin-1 was not overexpressed. The increased number of GC promoted mucus secretion within the intestinal wall, facilitating numerous microorganisms’ attachment. This attachment led to increased production of VFAs, providing energy for regeneration and repair processes in intestinal epithelial cells as well as promoting villus development and reinforcing barrier function. In addition, as mentioned above, the improvement of the microbial barrier promotes the enrichment of L-lysine, and the findings of this study demonstrated that L-lysine had a significant correlation with the main indicators of the physical barrier. Yuan et al. (Reference Yuan, Liu and Nie2024) found that lysine compounds can significantly increase the mRNA expression of tight junction proteins ZO-1 and Claudin-1 in mice colon. Kirupananthan et al. (Reference Kirupananthan, Bertolo and Brunton2022) found that adding lysyl-lysine to the diet notably enhanced the VH and mucosal weight in piglets. Drawing on the findings from prior research, it is not difficult to conclude that the protective influence of ICA on intestinal epithelial cells may be through the enrichment of L-lysine. The enhancement of intestinal epithelial structure ultimately influences the growth and development of the organism, thereby promoting improved growth performance.
Effects of dietary ICA supplementation on the LPS-challenged ileal chemical barrier in broilers
The ileal chemical barrier, also known as the mucus barrier, serves as the primary habitat for digestive enzymes and commensal bacteria within the intestine, facilitating the breakdown of food into smaller molecules. Previous studies have demonstrated that intestinal damage can lead to microbial disorders, while microbial imbalances can also cause intestinal damage (Xue et al., Reference Xue, He and Gao2017). Following LPS stimulation, this study revealed a reduction in the amount of beneficial bacteria (such as Bifidobacterium and Dehalobacteriaceae) in the ileum of broilers, indicating disruption of the intestinal microbiota and potential damage to the intestines. Alterations in the composition of intestinal microorganisms can result in VFAs content alterations. VFAs are produced through microbial fermentation of carbohydrates, proteins and other substances within the intestine; they play crucial physiological roles, such as regulating the intestinal pH, modulating the gut flora composition, providing energy to mucosal cells and reducing pro-inflammatory factor production (Che et al., Reference Che, Adams and Wei2019; Singh et al., Reference Singh, Gurav and Sivaprakasam2014). This research revealed that microbial disorders reduced VFAs production within the ileum, subsequently elevating intestinal pH levels. Increased pH levels negatively impact digestive enzyme activity. In conclusion, this study revealed significant impairment of the chemical barrier of the broiler ileum following the LPS challenge.
Gao et al. (Reference Gao, Shi and Xie2021) demonstrated that a decrease in pH within a specific range can increase pepsin activity and protein hydrolysate concentration, thereby promoting the secretion of digestive enzymes and increasing enzyme activity correspondingly. The acidic gastrointestinal tract inhibits pH-sensitive bacteria (such as E. coli, Salmonella, et al.), favoring the survival of acid-resistant bacteria (such as Lactobacillus, Bifidobacterium, et al.). Due to the presence of caffeic acid and quinic acid in the structure of ICA, it can acidify the intestinal tract. This experiment also confirmed a significant reduction in the ileal pH value in broilers treated with ICA, alleviating the decrease in trypsin activity induced by LPS. Furthermore, the addition of ICA significantly enhanced the abundance of Streptococcus_alactlyticus, thereby facilitating L-lysine metabolism. A positive relationship was also noted between L-lysine content and acetic acid levels. These findings suggested that ICA might have stimulated acetic acid production. On the one hand, elevated acetic acid levels contributed to a reduced pH in the ileum and increased activity of digestive enzymes; on the other hand, acetic acid, as a byproduct of bacterial fermentation, provided energy for intestinal epithelial cells and expedited intestinal barrier formation, which was beneficial for broilers to resist bacterial invasion and guaranteed the rapid growth of the body.
Effects of dietary ICA supplementation on the LPS-challenged serum immune and ileal immune barrier in broilers
Serum immunoglobulins and complement proteins play a crucial role in the serum immune system of broilers, and the serum inflammatory factors are key indicators of the body’s inflammatory response. Their content in the blood can indicate the serum immune function of the body. In the previous broilers LPS challenge model study, LPS induction disrupted serum immune balance, significantly increasing pro-inflammatory factor levels while inhibiting immunoglobulin production (Zhang et al., Reference Zhang, Liu and Liu2022b). The findings from this experiment aligned with the results of prior research, suggesting that the LPS challenge had a substantial effect on serum immunity. After LPS stress in this experiment, IgM, as the earliest immunoglobulin involved in humoral immunity (Chen et al., Reference Chen, Menon and Masino2023), bound to the antigen first, resulting in a significant increase in its secretion, but at this time, no new IgG antibodies may be produced in the body. The body used existing serum IgG for the immune response, leading to a significant decrease in the IgG content after LPS stimulation. This finding was consistent with Ramadan et al. (Reference Ramadan, Fouda and Ellamie2020). ICA may have an anti-inflammatory role by regulating serum immunoglobulin secretion, although the mechanism of action is still unclear.
To gain a deeper understanding of how ICA enhances immune function in broilers, the secretion of sIgA in the ileum, the expression of inflammatory factors in the ileum, and the expression of key genes in the inflammatory pathway were detected. Among them, sIgA acts as the primary defense mechanism, safeguarding the epithelium against intestinal pathogens and toxins. It primarily clears the harmful substances on the intestinal mucosa through receptor blocking, steric hindrance and immune exclusion (Mantis et al., Reference Mantis, Rol and Corthésy2011). Under normal conditions, the stable binding of the inhibitor of κB (IκB) to NF-κB maintains NF-κB in a steady state. When LPS stimulates the body, LPS binds to the CD14 receptor protein and subsequently phosphorylates IκB through cascade reactions, disrupting NF-κB homeostasis. Activated NF-κB is translocated from the cytoplasm to the nucleus, where it attaches to the DNA region to promote the expression of inflammatory factors, including IL-Iβ, IL-6, IL-8 and TNF-α (Hsieh et al., Reference Hsieh, Hsu and Lin2021; Ness et al., Reference Ness, Abdallah and Adams2017). The results of this research revealed that LPS challenge triggered the activation of the NF-κB pathway, increased the expression of inflammatory factor genes, and decreased the secretion of sIgA, indicating that the intestinal immune barrier is compromised after LPS stimulation. However, ICA alleviated the decrease in sIgA content in the ileum caused by LPS stimulation and weakened the inflammatory response mediated by the NF-κB pathway by reducing the expression of CD14. Previous studies have shown phenolic acids have a good anti-inflammatory effect (Hu et al., Reference Hu, He and Liu2020; Wang et al., Reference Wang, Hu and Ma2024; Weremczuk-Jeżyna et al., Reference Weremczuk-Jeżyna, Gonciarz and Grzegorczyk-Karolak2023). Wang et al. (Wang and Xiao Reference Wang and Xiao2019) found that ICA inhibited the protein expression of the NF-κB/NLRP3 signaling pathway and the levels of inflammatory factors induced by LPS. Similarly, Liu et al. (Reference Liu, Li and Zhang2023a) showed that CA improved the immune function of broilers after dexamethasone stimulation by regulating microbial populations and the PPAR and MAPK pathways. In addition, L-lysine enriched in the gut was also confirmed to have anti-inflammatory activity in this test. Hu et al. (Reference Hu, Feng and Jiang2021) showed that lysine deficiency aggravated the inflammatory response within the immune organs of grass carp, promoted the production of TNF-α, IL-6, IL-8 and other proinflammatory factors, and down-regulated the content of anti-inflammatory factors IL-10, IL-11 and other cytokines. Cheng et al. (Reference Cheng, Tang and Pan2020) showed that L-lysine could achieve its inhibitory effect on proinflammatory cytokines through the miR-575 up-regulation/PTEN down-regulation signaling pathway. In conclusion, ICA might have attenuated the production of intestinal inflammatory factors, thereby suppressing the consumption of sIgA by inhibiting the inflammatory pathway. Additionally, it could enhance the secretion of sIgA and L-lysine to augment the intestinal immune response and then increase the ability of broilers to resist external stress and alleviate the effects of stress on the growth of broilers.
However, as mentioned above, this experiment exclusively investigated the impact of ICA on broilers under acute stress conditions. Whether ICA can effectively safeguard the intestinal barrier function and growth performance of broilers under chronic stress remains unclear. A comprehensive evaluation and confirmation of ICA’s effects under broiler stress conditions will require further validation through additional experimental data.
Conclusion
In conclusion, under normal growth conditions, the addition of ICA to the feed significantly enhanced the growth performance of broilers. Under acute LPS-challenged stress conditions, ICA effectively alleviated the negative impacts of LPS on the intestinal barrier function in broilers. This could be attributed to the creation of an acidic environment through ICA supplementation, which facilitated the abundant proliferation of Streptococcus_alactolyticus in the ileum – an acid-tolerant lactic acid bacterium with hydrolytic capability for ICA. The metabolic activity of this bacterium within the intestine led to a substantial increase in L-lysine production, thereby enhancing intestinal barrier function. Specifically, these improvements were manifested as enhanced intestinal structure, promotion of digestive enzyme activity, increased VFAs content, stimulation of sIgA secretion, reduced inflammatory factor levels, and inhibition of inflammation pathway gene expression.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/anr.2025.10018.
Acknowledgements
This study was supported by the Natural Science Foundation of Hebei Province (C2024204217 and C2022204218), the earmarked fund for CARS-41, and the Innovation Program of the Chinese Academy of Sciences (CAAS-IFR-ZDRW202401).
Author contributions
Haotian Jiang: data analysis, writing – original draft; Siyuan Zhou and Yujia Wang: data curation, software; Lixiao Gao, Man Zhao and Guohua Liu: review and editing; and Shudong Liu and Baojiang Chen: funding acquisition, project administration, and review and editing. All authors read and approved the final version of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest to this work.
Ethical standards and consent to participate
All the uses of birds and the experimental protocols in this study were approved by the Institutional Animal Care and Use Committee of Hebei Agricultural University, with protocol number 2023110.