Inundative biological control, a type of augmentative biological control, entails releasing natural enemies, often repeatedly and to augment a species already present at low densities, at location points throughout the local distribution of a target pest (Sivinski Reference Sivinski2014; Heimpel and Mills Reference Heimpel and Mills2017). Determining an ideal distribution of release points that maximises coverage by mobile agents, however, is challenging without information on agent dispersal capacity (Sivinski Reference Sivinski2014). The importance of dispersal in biological control programmes is widely recognised, and augmentative biological agents can fail due to inadequate or excessive dispersal (Heimpel and Asplen Reference Heimpel and Asplen2011). Moreover, when releasing augmentative biological control agents that are flightless, including those that are at immature stages, it is helpful to know how the insects might move within and among plants of various sizes (e.g., among row crops versus urban street trees).
Lacewing larvae (Neuroptera: Chrysopidae) are released against various insect pests – scale insects (Hemiptera: Coccoidea), aphids (Hemiptera: Aphidoidea), caterpillars (Lepidoptera), whiteflies (Hemiptera: Aleyrodoidea), etc. – and in diverse ecosystems (Hassanpour et al. Reference Hassanpour, Asadi, Jooyandeh, Madadi, Karimi and Madadi2021). Multiple species are commercially available in different formulations, including as hex frames and vials of buckwheat husks (e.g., https://dmvbeneficials.com, https://www.biobest.com, https://www.koppertus.com, https://insectary.com). Lacewing larvae appear to be active foragers in the laboratory and field, yet it remains unknown how far they are capable of dispersing, especially following shipment. Quantifying dispersal capacity could inform dispersion of release points or at least provide insights into the likelihood that released lacewings will reach prey populations.
We studied the dispersal capacity of larval Chrysoperla rufilabris Burmeister, red-lipped green lacewings (Neuroptera: Chrysopidae). We obtained commercially available C. rufilabris and recorded their movement during five-minute observation periods in the laboratory. We then used these findings to simulate dispersal over extended periods of time. These efforts were aimed at helping inform releases of larvae in which knowledge of prey dispersion, habitat connectivity, or both can be reasonably estimated or inferred.
Second- and third-instar larvae were obtained in November 2024 from Arbico Organics (Oro Valley, Arizona, United States of America) in a 400-cell hex frame (SKU: 1110005) with one larva per cell. Eggs of conspecifics were available on the hex frame during shipment and storage, but otherwise lacewings were not provided with food or water and were stored in the refrigerator (approximately 2–4 °C) for a maximum of five days before being used in dispersal trials. For each trial, sheets of white printer paper (8.5 × 11 inches, or approximately 0.22 × 0.28 m; Staples 30% recycled paper; Item #: 492071; Staples, Framingham, Massachusetts, United States of America) were cut into squares (approximately 0.22 × 0.22 m). The centre of each sheet was marked with a small “x” using a number two pencil. A single lacewing larva was then placed at the x and allowed to move freely for five minutes (300 seconds). After each 10-second interval, we marked the position of the insect with a dot and observation number (i.e., “1” for the first location…, “30” for the final location). Care was taken to wait until the lacewing had moved from a spot before marking it, which reduced disturbance. Although standing near lacewings and marking locations could have increased escape efforts (and therefore dispersal), we note our goal was to measure dispersal capacity regardless of behavioural responses.
Trials (n = 32) continued for five minutes or until the individual crawled off the sheet. In 17 of the trials, the lacewing left the sheet, corresponding to an average of 12 missed increments (out of a possible 30 marked time points) across the 17 trials (range: 1–25 missed increments); handling of these missing increments is described below.
After all trials were complete, each sheet was placed next to a ruler and photographed using a smartphone. We took pictures from the same distance and angle to minimise measurement errors. The images were then loaded into ImageJ software (https://imagej.net/ij/) and analysed by creating a coordinate system for the sheet, such that the bottom left corner was set as (0, 0). We then marked the coordinates (x, y) for all numbered observations, allowing us to determine the approximate location of each lacewing every 10 seconds. We followed the approach of Wright et al. (Reference Wright, Long, McAndrew, Paul, Sabet and Riggins2025) in quantifying dispersal distance. Briefly, we used the bayesmove package (Cullen and Valle Reference Cullen and Valle2021) in R, version 4.5.1 (R Core Team 2025), to quantify the total distance moved (m) by each lacewing. For insects that left the sheet before the assay was complete, we multiplied distance travelled by 300/t, where t equals the time in seconds at the last complete observation before the insect left the paper. For example, if the insect left between observation 29 and 30, t would equal 290 seconds, and the distance travelled would be estimated using dispersal through observation 29.
We used our laboratory data to construct iterative simulations of potential dispersal distances with the aim of informing release guidelines. Time spent dispersing in the field is exceedingly difficult to quantify, and so we evaluated three time-based scenarios in which we assumed insects walked for a minimum of 1 minute up to: (1) 1 hour, (2) 6 hours, and (3) 12 hours after release. Under each scenario, an iteration started by randomly selecting an average dispersal rate (metres per minute) from our laboratory trials using the sample() command in R – each scenario could thus have a different randomly selected dispersal rate per iteration. Then, for each scenario, we multiplied this distance by a dispersal time that was estimated using a random draw from a uniform distribution parameterised with the corresponding time windows above (e.g., for scenario1, minimum and maximum dispersal times were 1 minute and 1 hour, respectively). This process was repeated for 200 iterations per scenario, and thus dispersal times were sampled with replacement. Medians and 95% confidence limits were estimated using the median and middle 95% of simulations per scenario. All data and code are publicly available at github.com/sfward/lw_lab_dispersal.
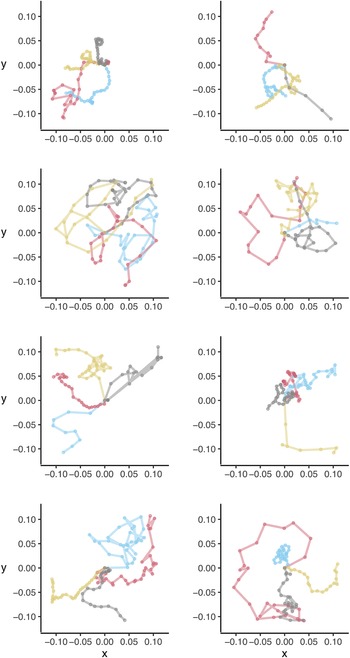
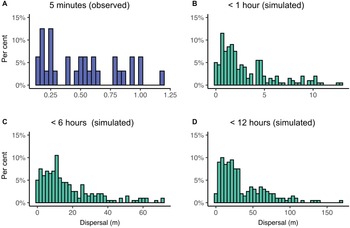
We did not observe any obvious directional or circling patterns during five-minute dispersal assays (Fig. 1), with larvae moving an average of 0.10 m per minute (95% confidence interval: 0.08–0.13, median = 0.10) or 0.52 m per five minutes (95% confidence interval: 0.41–0.63; median = 0.51; Fig. 2A). When using this information to simulate potential crawling distances for up to (1) 1 hour, (2) 6 hours, and (3) 12 hours, we found that lacewings could be capable of walking median distances of 2.0 m (95% confidence interval: 0.1–10.3; Fig. 2B), 12.0 m (0.4–59.6; Fig. 2C), and 25.0 m (2.2–107.1; Fig. 2D), respectively.
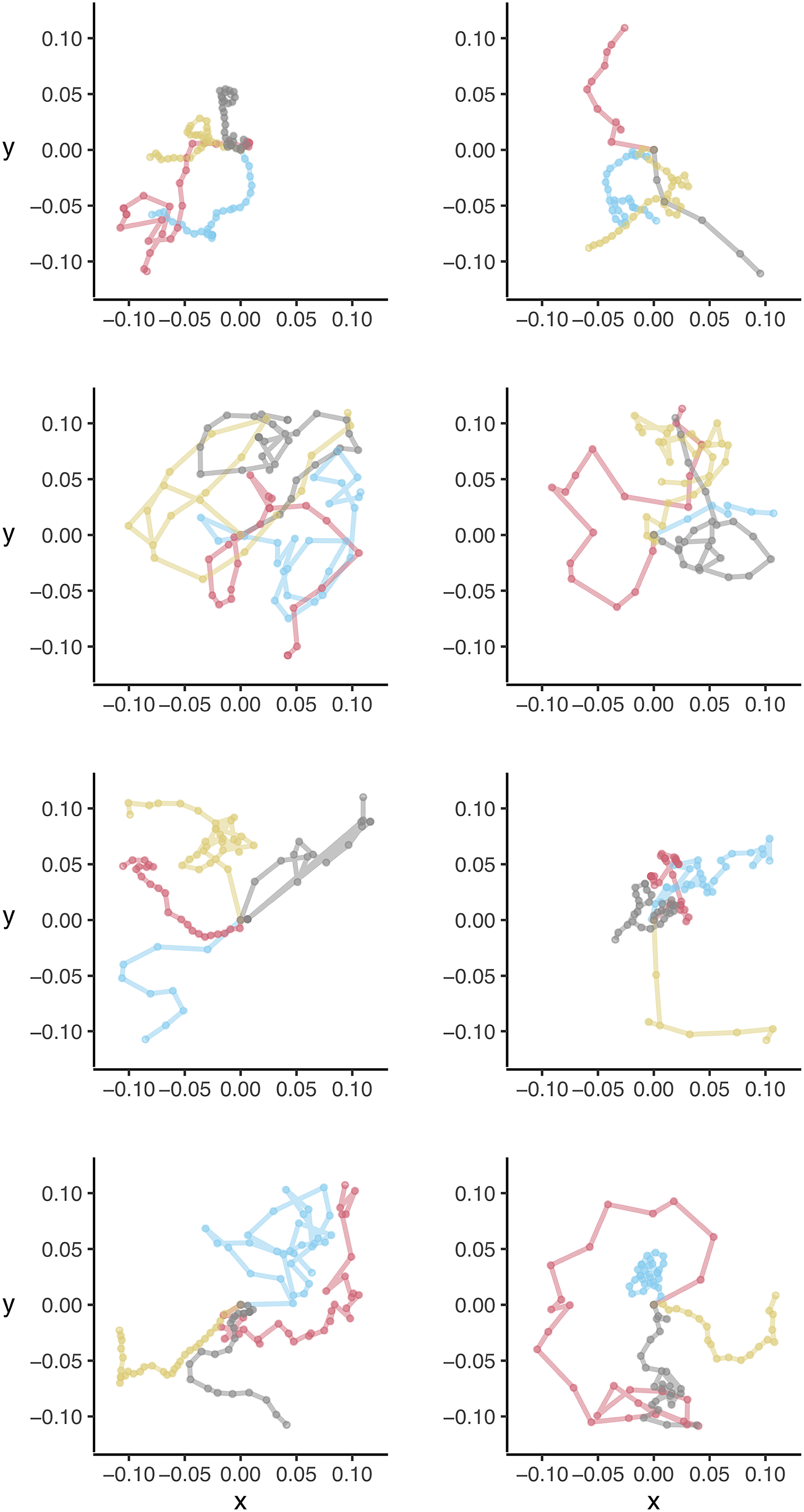
Figure 1. Dispersal paths for individual green lacewings, Chrysoperla rufilabris, in laboratory trials. Each colour indicates an individual larval lacewing, with four individuals displayed per panel. Axes indicate coordinates in metres. Insects were released at the centre of a paper and allowed to move freely for five minutes or until they left the paper.
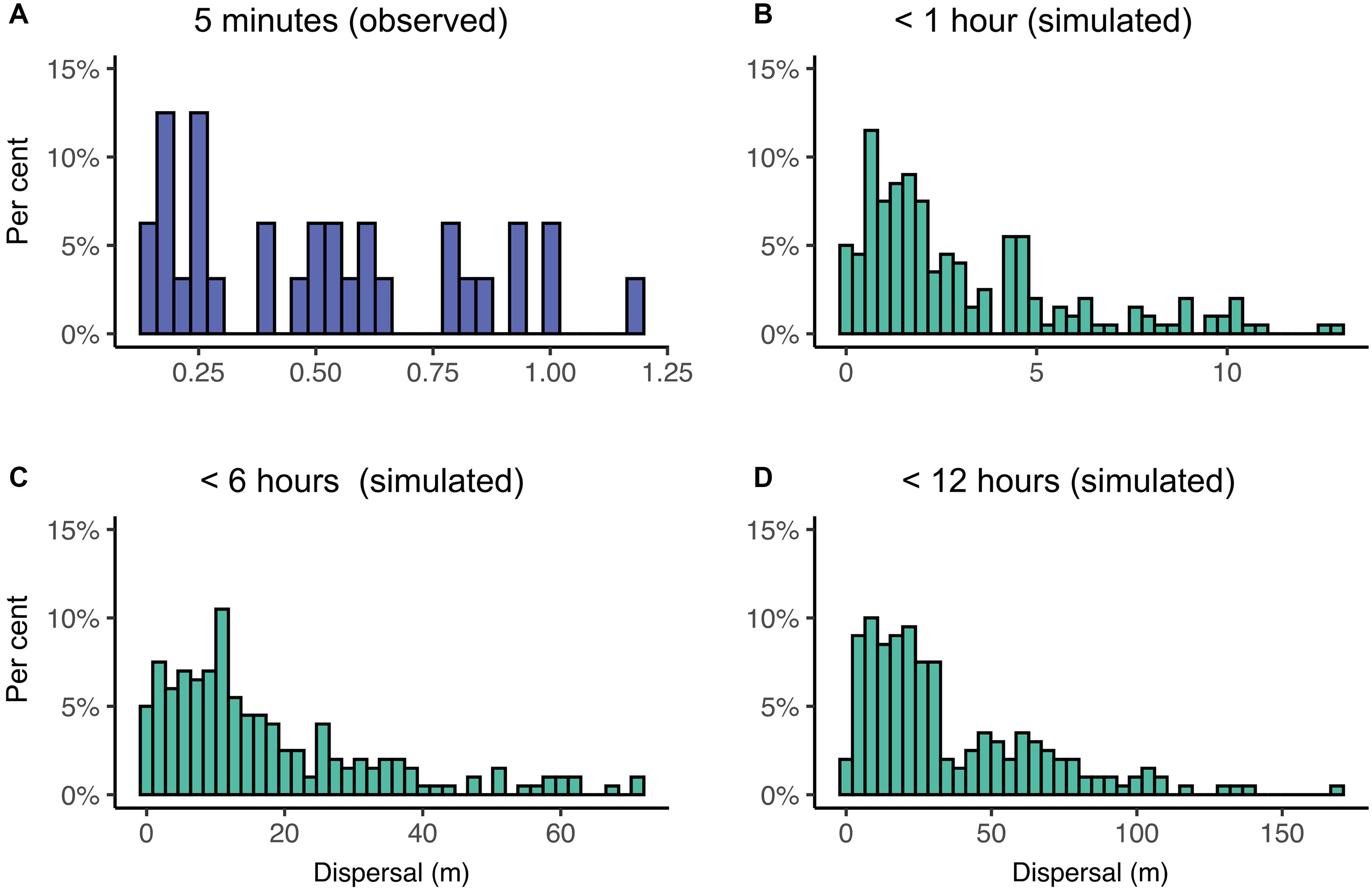
Figure 2. Observed (A, laboratory) and simulated (B–D) dispersal distances (metres) by Chrysoperla rufilabris indicated by the percentage of individuals (y-axes) that moved each distance (x-axes). Three simulations were evaluated in which we assumed insects walked for 1 minute up to: B, 1 hour, C, 6 hours, and D, 12 hours.
Dispersal capacity plays a critical role in the ability of released biological control agents to locate, attack, and control target pest populations. Our study with larval C. rufilabris indicates that this species is capable of dispersing approximately 0.10 m/minute, potentially corresponding to much longer distances in longer periods of time (Fig. 2). This suggests that C. rufilabris larvae should be physiologically capable of dispersing among prey patches – at least those located within individual large plants or smaller plants with contiguous canopies – if deployment methods result in suboptimal placement. We note that dispersal in natural settings, which exhibit substantial physical complexity and different abiotic challenges than our two-dimensional laboratory assays comprised, could be markedly different.
Pre-release conditioning and handling of biological control agents (e.g., field collecting adults, providing food in release cups, or both) can impact their propensity and ability to disperse (Mazzi and Dorn Reference Mazzi and Dorn2012). Larval lacewings can be released using a variety of methods (e.g., by shaking them from a bottle with buckwheat husks or by removing coverings on hex frames and deploying frames on or next to plants). The lacewings used in the present study had access only to conspecific eggs as a food source, and we therefore assumed assayed individuals had fed cannibalistically somewhat recently. Our simulations of dispersal distances indicate that, following shipment and a short period of cold storage, C. rufilabris could potentially disperse tens of metres in the hours following their release (Fig. 2). We caution that these forecasts, although based on laboratory data, have not been empirically validated: indeed, differences among lacewing species, the physiological status of larvae (e.g., age, starvation status, and post-release declines in overall health), multiple cues (e.g., chemical, visual, and both), temperature, time of day, substrate (e.g., pavement versus soil), and habitat complexity (e.g., plant size and dispersion) likely impact dispersal among and between plants. Identifying the optimal conditions for release could help enhance or moderate post-release dispersal efforts over short time scales (e.g., hours) and potentially enhance efficacy. Our laboratory estimates and simulations, however, can help guide or justify dispersion of release points for C. rufilabris and underscore the ability of larval lacewings to move long distances following shipment.
Acknowledgements
This research was supported by state and federal funds appropriated to The Ohio State University, College of Food, Agricultural, and Environmental Sciences, Ohio Agricultural Research and Development Center. The authors thank Amber Stiller and Kevin Chase for providing feedback on an earlier version of the manuscript and two anonymous peer reviewers for their insightful comments.
Competing interests
The authors declare that they have no competing interests.